Abstract
Exposure to environmental chemicals may precipitate autism spectrum disorders (ASD) in genetically susceptible children. Differences in the efficiency of the glucuronidation process may substantially modulate substrate concentrations and effects. To determine whether the efficiency of this pathway is compromised in children with ASD, we measured the efficiency of glucuronidation for a series of metabolites derived from the commonly used plasticizer, diethylhexyl phthalate. Spot urines were collected and analyzed for the fraction of each metabolite conjugated by isotope dilution-liquid chromatography mass spectrometry-mass spectrometry. The degree of glucuronidation was lower with the ASD group. The glucuronidation pathway may differ in some children with ASD.
Keywords: Diethylhexyl phthalate, Phthalates, Autism, Glucuronidation
Introduction
Autism spectrum disorders (ASD) are a family of neurodevelopmental disorders characterized by impaired social interactions, limited verbal and nonverbal communication and repetitive and restricted behavioral patterns. A 2012 report from the CDC estimates the prevalence of ASD could be as high as 1 in 88 children and may be increasing (Baio et al. 2012). Because children with ASD exhibit a wide spectrum of symptoms and severity, it is believed that the etiology of the disease is a consequence of a complex inter-play between genetic and environmental factors (Herbert 2010; Muhle et al. 2004; Landrigan 2010).
There is widespread concern that exposure to environmental chemicals (ECs) can exacerbate or precipitate ASD in genetically susceptible children who have impaired ability to detoxify these compounds (De Cock et al. 2012; Pessah et al. 2008). For most xenobiotics and many endobiotics, glucuronidation is a major route for detoxification and elimination. Glucuronidation makes a large variety of unrelated substances (e.g., drugs and toxins) less toxic and more water-soluble, thereby allowing for their subsequent elimination from the body upon urination. Differences in the efficiency of the glucuronidation process may substantially modulate substrate concentrations and effects (Wells et al. 2004; Argikar et al. 2008).
There is some evidence from the literature suggesting that detoxification of environmental contaminants for some individuals with ASD might be abnormal. One study found 90 % of the autistic children with known food/chemical intolerance showed a deficiency in phenosulfotransferase, a liver enzyme involved in detoxification (O’Reilly and Waring 2003; Waring and Klovrza 2000). Another study reported an increased biomarker for xenobiotics contamination in a test group of autistic children together with evidence of abnormal liver detoxification profiles in all of the autistic children tested (Edelson and Cantor 2000). Drug metabolism may also be compromised. A small study by Alberti et al. found depressed acetaminophen conjugation by sulfation in 20 low functioning autistic children (Alberti et al. 1999). James et al.(2004, 2008) found evidence for abnormal methylation by Glutathione S-transferases (GSTs) in autistic children and their parents. Glutathione S-transferases are enzymes that catalyze conjugation of glutathione to toxic ECs thereby detoxifying them (Armstrong 1997; James et al. 2004, 2008). Subsequently, we and others showed that genetic polymorphisms of GST M1, glutathione peroxidase and GST P1 occur in families with ASD children (Buyske et al. 2006; Williams et al. 2007; Ming et al. 2010).
Most prior studies such as those described above found differences in detoxification for a single compound and inferentially implicated that type of environmental contaminant in the development of ASD. The purpose of this study was to determine whether the effect is general; i.e., multiple compounds metabolized by a given pathway are impacted. The implication is that finding compromised detoxification in a single metabolite study does not necessarily implicate the particular toxic environmental variable being studied, it implicates a compromised detoxification pathway and may apply to multiple unknown potential toxicants, any one(s) of which could be a factor in ASD development.
To investigate whether findings of compromised detoxification are not compound specific but rather a general defect in detoxification, we selected for study a series of metabolites derived from the commonly used plasticizer, Diethylhexyl phthalate (DEHP). Phthalates are widely used as plasticizers for many types of plastics ranging from food wraps, toys and building products. They give plastics characteristics such as flexibility, softness and workability. DEHP usage is particularly widespread in plastics used in food packaging (Rudel et al. 2012; NIEHS 2011).
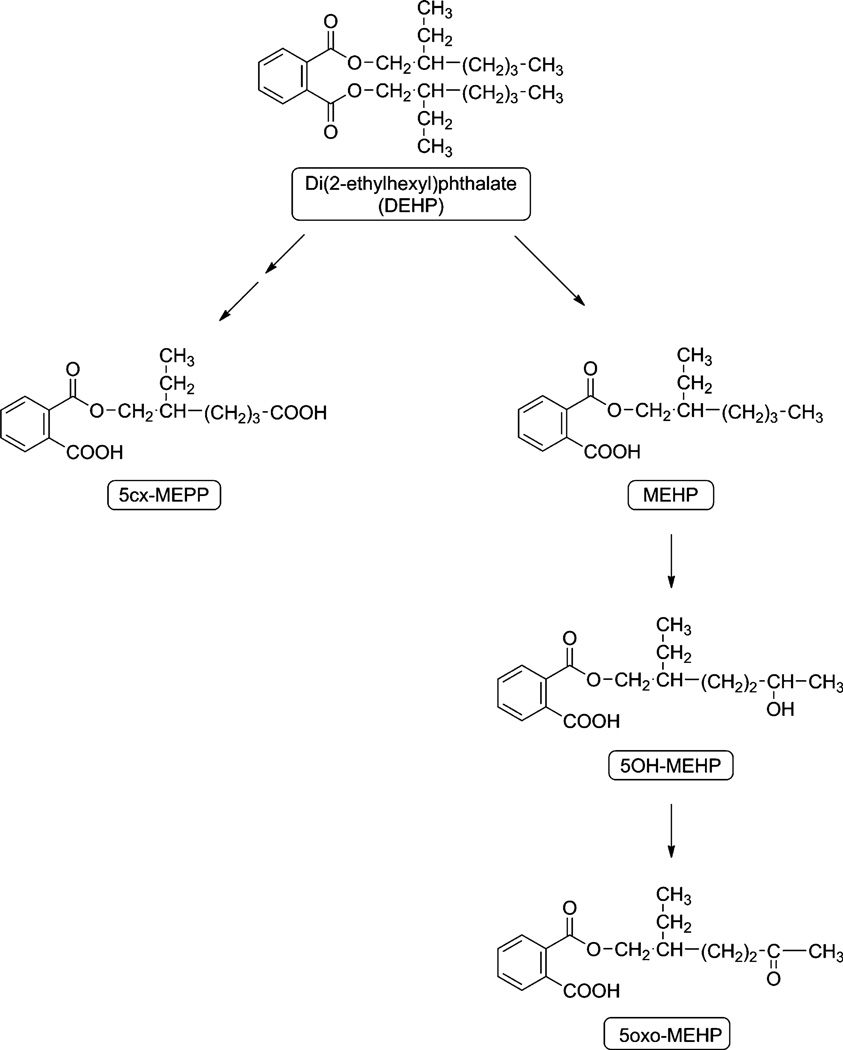
Exposure to phthalates is universal. DEHP enters the human body via the gut but may also enter through the lungs from dust in the air (Hwang et al. 2008; NIEHS 2011). It is then partially hydrolyzed to the monoester, mono-2-ethylhexyl phthalate (MEHP) in the gut and liver (NTP-CERHR 2005; Koch et al. 2006). MEHP is then further metabolized by a series of oxidation reactions in the liver to three secondary metabolites, mono-(2-ethyl-5-oxohexyl) phthalate (5-oxo MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (5-OH MEHP) and mono-(2-ethyl-5-carboxypentyl phthalate (5-CX MEPP, Fig. 1). These metabolites of DEHP are then conjugated to glucuronic acid to reduce toxicity and increase solubility to make them easier to excrete in the urine; most of the remainder is detoxified by sulfation (Koch et al. 2005, 2010). The glucuronidation pathway accounts for about 80+ % of the total DEHP ingested (Koch et al. 2006, 2010). For this study we compared the efficiency of this series of conjugation reactions in a group of children with documented ASD and against a control group of children.
Fig. 1.
Pathways of DEHP metabolism (after Koch et al. 2005)
Materials and Methods
Subjects
Informed consent for these studies was obtained from the care-givers and was approved by the Institutional Review Board of the UMDNJ-New Jersey Medical School. Subjects were recruited from Pediatric Neurology and Pediatrics clinical practices at University of Medicine and Dentistry of New Jersey (UMDNJ), New Jersey Medical School. Random spot urine specimens were collected from 50 children with ASD and 53 age-matched healthy controls between 10:00 a.m. and 4:00 p.m. The samples were frozen and stored at −80 °C within 30 min of collection. All ASD subjects were under the care of the pediatric neurologist (X.M.) and the diagnoses were made by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV TR); 52 % of the subjects were further confirmed by Autism Diagnostic Interview-Revised, and/or Autism Diagnostic Observation Scale-Generic criteria. Medical history and comorbidity data were collected in the ASD subjects. Since medical and psychiatric comorbidities are common among ASD children (Ming et al. 2008), we included ASD children with or without comorbidity. Control children were screened for medical and developmental disorders during their well-child visits in addition to chart review and only those free of any chronic or recurrent medical disorders were considered healthy and included in this study. All subjects were carefully screened for signs of infection or inter-current illness on the day of specimen acquisition, and subjects with acute illness were excluded. The dietary intake history within 24 h of sampling was recorded, including that of medication and vitamin intake.
Analytical Methods
The concentration of free phthalates and total phthalates in the collected urines was measured by isotope dilution-liquid chromatography mass spectrometry–mass spectrometry (ID-LC-MSMS) using minor modifications of the methodologies described by Silva and Koch (Silva et al. 2003,2005; Koch et al. 2005). The metabolites measured were mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-oxohexyl) phthalate (5-oxo MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (5-OH MEHP) and mono-(2-ethyl-5-carboxypentyl phthalate (5-CX MEPP). The LC-MSMS assay measures the free phthalate. In order to measure the total phthalates present in the urine, the glucuronidated phthalate had to be deconjugated. This was done by treating the urine with β-glucuronidase to remove the glucuronic acid residue from the glucuronidated phthalate (Sigma-Aldrich, St. Louis MO).
Urine (4 ml) was pipetted into a 16 × 100 screw-cap tube. The tube flushed with Argon and the internal standards added, 50 ng of 13C-MEHP (Cayman Chemicals, Andover MA). The contents of the tube were flushed with Argon, spun down at 1,500g for 2 min and divided into two equal aliquots of 2 ml. Aliquot 1 was treated with glucuronidase to measure the total amount of each phthalates, aliquot 2 was not treated. A stock β-glucuronidase solution was made up fresh daily and kept on ice. 16 µl enzyme (~80,000 units/ml) was added to 1 ml 75 mM phosphate buffer, pH 6.8 and 50 µl of the diluted β-glucuronidase solution added to each tube. Both the ‘treated’ and ‘not treated’ urines were incubated at 37 °C for 90 min.
At the end of the incubation period, 3 ml ethyl acetate and 2 ml saturated KH2PO4 were added to each tube, the tubes vortexed and then centrifuged at 1,500 rpm for 6 min at 4°. The organic layer was then transferred to a 16 × 100 tube containing ~600 mg anhydrous Na2SO4. The mixture was shaken and then centrifuged for 2 min at 1500 rpm. The top layer was transferred to a clean 16 × 100 screw-cap tube and taken to dryness by aerating with Argon. Samples were reconstituted with Acetonitrile and stored under Argon at −20 °C until injected in the LC-MSMS. A mixture of external standards consisting of 50 ng each 13C MEHP, 13C 5-oxo MEHP, 13C 5-OH MEHP and 13C 5-cx MEPP (Cayman Chemicals, Andover MA) were run after every 5 injections to calculated response factors.
LC-MSMS conditions
The analyses were done with an Agilent 1200 Series LC coupled to an Agilent 6410 Triple Quadrupole MS-MS (Agilent Technologies, Wilmington DE) with a Phenomenex Kinetix C18, 2.6 µm column (Phenomenex, Torrance CA). Chromatography was done isothermally at 23 °C. Mobile Phase A was Water: Acetic Acid, (100:0.1), mobile Phase B: Methanol:Acetic Acid, (100:0.1). All reagents were previously sparged with Argon. LC gradient conditions were flow rate 0.2 ml mi−1 and: 0–5 min 65 % A, 35 % B; 5–15 min 35 % A, 65 % B; 15–25 min 0 % A, 100 % B; 25–36 min 65 % A, 35 % B. The triple quad was run in the negative ESI mode. The source parameters were: Collision Gas (N2): 300°, gas flow at 6 l/min, nebulizer: 15 psi Capillary: 3500 v. The transitions used were: MEHP 277.2 → 134.1, 13C-MEHP 281.2 → 137.1, 13C 5-oxo MEHP 295.2 → 124.1, 5-oxo MEHP 291.1 → 121.2, 13C 5-OH MEHP 297.3 → 124.1, 5-OH MEHP 293.2 → 120.8,13C 5-CX MEPP 311.3 → 159.1 and 5 CX MEPP 307.1 → 158.9.
Statistical Analyses
SPSS 20.0 was used to conduct the statistical analyses. Histograms of the frequency distributions for all of the variables were plotted and indices of skewness and kurtosis were calculated for all of the continuous variables. Kolmogorov–Smirnov (K–S) tests were employed to determine whether the frequency distribution for each continuous variable significantly deviated from that expected for a normal distribution. Age (years), BMI, creatinine, % Bound MEHP, and % Bound 5-cx MEPP were normally distributed, but most of the other metabolite indices were positively skewed. Therefore, logarithmic (base 10) transformations were applied to these metabolic indices before using them in parametric analyses; 0.05 was added to the metabolite indices that contained zero values before applying the logarithmic transformation. However, the % Bound 5-OH MEHP and % Bound 5-oxo MEHP were negatively skewed. A log10 transformation based on first subtracting the values for these latter two % Bound indices from 100 and adding .05 was applied before correlating them with the other two indices.
Although the conversion of percentages to arcsines followed by square root transformations have been suggested for parametric statistical analyses with variables based on percentages (Cohen and Cohen 1983), the distributions of the log10 transformations of the percentage Bound indices did not display extreme outliers and skewness warranting arcsine conversions.
A Chi square test for independence with Yates’ correction for continuity was calculated to ascertain whether the percentages of boys and girls in autistic and control samples were comparable, and t tests for independence were performed to determine whether the mean ages, BMIs, and creatinine levels were comparable in both samples. The concentrations and percentages of the untransformed phthalate metabolite indices were also compared using Mann–Whitney U tests. Since the Ns for both samples were >30, the resultant U values were converted into z statistics for interpretive purposes, and effect sizes were estimated with r, i.e., z/square root of the sum of the Ns for both groups. Two-tailed tests of significance were employed for all of the analyses. No statistical correction, such as a Bonferroni adjustment, was made to alpha to control for the familywise error rate that was incurred in conducting the multiple statistical comparisons that were made.
Pearson product-moment correlations were calculated among the patients’ characteristics and the metabolite indices to evaluate the magnitudes of the associations among these variables. Prior research has found or indicated that sex, age, body mass index (BMI), and creatinine levels might affect the excretion of phthalate metabolites in urine (Hauser et al. 2006; Wolff et al. 2007; Engel et al. 2010; NTP-CERHR 2005; Whiteley et al. 2006; Koch et al. 2006). Therefore, these four variables were simultaneously entered as covariates in multiple regression analyses comparing the autistic and control samples’ adjusted mean metabolite differences. Cohen’s d statistic was chosen to reflect the effect sizes for the adjusted mean difference (Cohen 1993). The multiple regression analyses with the % Bound MEHP and % Bound 5-cx MEPP indices are based on the untransformed indices because these indices’ distributions did not deviate from those expected for normal distributions.
Results
The subject population consisted of 50 children (49 %) diagnosed with ASD and 53 were non-ASD control children. The characteristics for both samples are given in Table 1. Like other ASD studies, our study included a greater proportion of males reflecting the known increased prevalence of ASD in males. However, as the magnitude of the φ correlation in Table 1 indicates, the difference in percentages (21 %) was small according to Cohen’s (1993) interpretative guidelines for effect sizes and did not meaningfully differentiate the two groups indicating that separate comparisons of the both groups’ metabolite data had to be conducted for each sex.
Table 1.
Characteristics of autistic and control samples
| Characteristic | ASD (N = 50) (M ± SD) |
Control (N = 53) (M ± SD) |
Statistic | p | Effect size | |
|---|---|---|---|---|---|---|
| Sex (% boys) | 76 | 55 | .04* | φ = .22 | ||
| Age (years) | 10.26 ± 3.83 | 10.74 ± 4.03 | t(101) = .61 | .54 | d = .12 | |
| BMI | 16.81 ± 2.38 | 16.51 ± 1.86 | t(101) = 73 | .47 | d = .14 | |
| Creatinine (ng ml−1) | 1.23 ± 0.10 | 1.13 ± 0.09 | t(101) = 74 | .46 | d = .15 |
p < .05, two-tailed test
The phthalate excretion data in the tables are presented in two formats; the actual amounts of each metabolite excreted in the free and conjugated states expressed as ng ml−1 urine and the percentage of each metabolite excreted in the bound (glucuronidated) state. Urinary concentration data are frequently normalized to, or controlled for creatinine to control for differences in urine dilution. In our study, we observed a non-significant trend for creatinine to be lower with ASD; in other studies the trend was statistically significant (Whiteley et al. 2006). Controlling for creatinine excretion had only a minor impact on the results. Furthermore, our primary interest is the % of the total phthalate metabolite excreted in the conjugated form, which being a ratio is dimensionless and so should be independent of creatinine.
The medians along with the 25th and 75th percentiles for each metabolite are presented in Table 2. Because the majority of these metabolite indices were positively skewed, Mann–Whitney U tests were first used to test whether the medians of the autistic and control samples were comparable and might have been drawn from the same population. As Table 2 shows the median of the free MEHP was higher in the ASD group was higher than the median in the control group. The medians of the % Bound MEHP, % Bound 5-oxo-MEHP, and % Bound 5-cx-MEPP were higher in the control group than the ASD group. The rs shown in Table 2 indicate that the effects sizes for the aforementioned significant differences were small.
Table 2.
Comparisons of metabolites for autistic and control samples by Mann–Whitney U Tests
| Metabolite | ASD |
Controls |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | 25th | 75th | N | Median | 25th | 75th | U | z | p | r | |
| Total MEHP | 50 | 10.96 | 8.91 | 14.67 | 53 | 13.16 | 9.60 | 18.35 | 1,033 | 1.93 | 0.06 | 0.19 |
| Total 5-OH MEHP | 50 | 5.79 | 2.31 | 13.88 | 52 | 8.19 | 4.36 | 12.47 | 1,069 | 1.55 | 0.12 | 0.15 |
| Total 5-oxo-MEHP | 50 | 3.33 | 1.22 | 5.89 | 53 | 4.21 | 2.48 | 5.77 | 1,133 | 1.27 | 0.21 | 0.12 |
| Total 5-cx MEPP | 48 | 238.58 | 125.82 | 443.75 | 51 | 230.74 | 170.40 | 401.08 | 1,173 | 0.36 | 0.72 | 0.04 |
| Total Metabolites | 48 | 263.42 | 141.16 | 471.92 | 50 | 259.27 | 190.65 | 439.28 | 1,132 | 0.48 | 0.63 | 0.05 |
| Free MEHP | 50 | 7.02 | 6.47 | 7.88 | 53 | 6.72 | 6.37 | 7.12 | 1,015 | 2.05 | 0.04* | 0.20 |
| Free 5-OH MEHP | 50 | 0.33 | 0.00 | 0.95 | 52 | 0.19 | 0.00 | 0.59 | 1,224 | 0.52 | 0.60 | 0.05 |
| Free 5-oxo MEHP | 50 | 0.13 | 0.04 | 0.35 | 53 | 0.08 | 0.00 | 0.17 | 1,055 | 1.79 | 0.07 | 0.18 |
| Free 5-cx MEPP | 48 | 162.90 | 103.00 | 331.08 | 51 | 163.14 | 88.60 | 269.70 | 1,121 | 0.72 | 0.47 | 0.07 |
| Total Free Metabolites | 48 | 170.00 | 112.97 | 339.66 | 50 | 167.68 | 95.71 | 280.87 | 1,090 | 0.78 | 0.43 | 0.08 |
| % Bound MEHP | 50 | 36.72 | 25.16 | 47.74 | 53 | 48.66 | 32.43 | 63.55 | 971 | 2.34 | 0.02* | 0.23 |
| % Bound 5-OH MEHP | 50 | 95.58 | 89.03 | 100.00 | 52 | 97.24 | 94.81 | 100.00 | 1,143 | 1.08 | 0.28 | 0.11 |
| % Bound 5-oxo-MEHP | 50 | 96.23 | 90.43 | 98.24 | 53 | 97.49 | 96.46 | 99.77 | 919 | 2.69 | 0.01* | 0.27 |
| % Bound 5-cx MEPP | 48 | 27.30 | 13.05 | 37.97 | 51 | 33.86 | 21.06 | 45.66 | 894 | 2.31 | 0.02* | 0.23 |
Concentrations are in ng ml−1
MEHP monoethylhexylphthalate, 5-OH MEHP 5-hydroxy-methylethylhexyl phthalate, 5-oxo MEHP 5-oxo-methylethylhexyl phthalate, 5-CX MEPP 5-carboxy-methylethylhexyl phthalate, U Mann–Whitney U test statistic, r effect size
p<05,
p < .01, two-tailed tests
Because previous findings (Windham et al. 2006; Engel et al. 2010; Calafat et al. 2008) have suggested that the excretion of the phthalate metabolites might have been affected by gender, age, BMI, and creatinine levels, multiple regression analyses were conducted in which, sex, age, BMI, and creatinine were entered simultaneously as covariates with the metabolite indices. Table 3 shows the adjusted means, standard deviations, F statistics, and effect sizes Cohen’s (1993) d for the multiple regression analyses. The adjusted means for the % Bound MEHP and % Bound 5-oxo-MEHP indices were higher in the control group than in the ASD group, and the adjusted means for the Free MEHP and Free 5-oxo-MEHP for the controls were lower than the adjusted means for these respective metabolic indices in the autistic group. The overall pattern of results from the multiple regression analyses parallel those found with the nonparametric Mann–Whitney U tests for % Bound MEHP and % Bound 5-oxo MEHP.
Table 3.
Adjusted means of the metabolite indices after simultaneously controlling for sex, age, body mass index, and creatinine
| Metabolite | ASD |
Control |
F | (1,df) | p | d | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | |||||
| Total MEHP | 50 | 20.18 | 24.63 | 53 | 15.80 | 24.60 | .47 | 97 | .50 | 0.14 |
| Total 5-OH-MEHP | 50 | 10.84 | 12.94 | 52 | 10.66 | 12.93 | 3.58 | 96 | .06 | 0.39 |
| Total 5-oxo-MEHP | 50 | 5.31 | 5.94 | 53 | 5.00 | 5.93 | 1.88 | 97 | .17 | 0.28 |
| Total 5-cx-MEPP | 48 | 433.89 | 436.57 | 51 | 341.06 | 436.04 | .38 | 93 | .54 | 0.13 |
| Total Metabolites | 48 | 464.86 | 469.42 | 50 | 380.58 | 469.02 | .43 | 92 | .51 | 0.14 |
| Free MEHP | 50 | 7.42 | 1.15 | 53 | 6.79 | 1.14 | 7.62 | 97 | .01* | 0.56 |
| Free 5-OH-MEHP | 50 | .72 | 1.13 | 52 | .56 | 1.13 | .29 | 96 | .59 | 0.11 |
| Free 5-oxo-MEHP | 50 | .32 | .40 | 53 | .18 | .39 | 5.04 | 97 | .03* | 0.46 |
| Free 5-cx-MEPP | 48 | 309.68 | 316.09 | 51 | 247.63 | 315.71 | .24 | 93 | .63 | 0.10 |
| Total free metabolites | 48 | 314.13 | 317.55 | 50 | 260.18 | 317.27 | .06 | 92 | .80 | 0.05 |
| % Bound MEHP | 50 | 37.76 | 19.84 | 53 | 48.11 | 19.82 | 6.73 | 97 | .01** | 0.53 |
| % Bound 5-OH-MEHP | 50 | 90.80 | 12.03 | 52 | 95.11 | 12.01 | .30 | 96 | .59 | 0.11 |
| % Bound 5-oxo-MEHP | 50 | 90.22 | 11.80 | 53 | 96.49 | 11.79 | 4.88 | 97 | .03* | 0.45 |
| % Bound 5-cx-MEPP | 48 | 24.98 | 20.68 | 51 | 30.92 | 20.65 | 1.96 | 93 | .16 | 0.29 |
With the exceptions of the % Bound MEHP and % Bound 5-cx-MEPP indices which were normally distributed, all of the multiple regression analyses are based on log10 transformations of the metabolite values. The % Bound 5-OH-MEHP and % Bound 5-oxo-MEHP were negatively skewed and the log10 transformation applied to them was based on first subtracting the values for these latter two % Bound indices from 100 and adding .05. The F and d statistics are based on the log10 transformed data as noted above where appropriate and apply to adjusted mean differences, but the adjusted Ms and SDs for the log10 transformed metabolite data have been converted back to their untransformed metabolite values to facilitate interpretation
MEHP monoethylhexylphthalate, 5-OH MEHP 5-hydroxy-methylethylhexyl phthalate, 5-oxo MEHP 5-oxo-methylethylhexyl phthalate, 5-CX MEPP 5-carboxy-methylethylhexyl phthalate, d effect size
p < .05,
p<.01 two-tailed tests
Total Metabolites
There was no difference in the sum of the total amount (free plus conjugated) of phthalate metabolites between groups shown in Tables 2 and 3, regardless of statistical analysis employed. This was because there was no difference for the major metabolite, 5-cx MEPP, which accounted for more than 90 % of the total metabolites detected.
Free Metabolite Excreted
Free MEHP was significantly greater for the ASD group in both the parametric and nonparametric analyses. Otherwise, there were no differences in either the amounts of the other free metabolites excreted or the total amount of unconjugated phthalate metabolite excreted.
Percent (%) of Bound (Conjugated) Metabolite Excreted
The % of metabolite conjugated and indicated by the % Bound indices is the parameter of interest for this study. To reiterate, it is a derived index calculated by subtracting the % free metabolite from 100. The present data clearly show that for three of the four phthalate metabolites, conjugation is less with the ASD group. The same pattern, albeit not significant is found with the fourth metabolite, 5-OH MEHP. In contrast the total phthalate metabolite excretion between the two groups is similar. The efficiency of conjugation as measured by the medians for the autistic group shown in Table 2 ranges from above 95 % (5-OH MEHP and 5-oxo MEHP) to about 37 % for MEHP and 27 % for 5-cx MEPP.
Because the glucuronidation should follow the same pathways in the ASD and control groups the data have been pooled together to investigate whether there were any relationships between the steps in the putative pathway (see Fig. 1). Table 4 shows the Pearson-product moment correlations among the four % Bound indices for each sample. Step 1 in the pathway is the hydrolysis of DEHP to MEHP. There was a trend for step 2 MEHP → 5-OH MEHP to correlate, (r = 0.19, p > 0.05). For step 3, the % of bound 5-OH MEHP and bound 5-oxo-MEHP were strongly correlated (r = .51, p < .001). This represents conjugation of 5-oxo MEHP and 5-OH MEHP. The final step in the pathway, oxidation to the carboxylic acid 5-cx-MEPP does not correlate with any prior steps.
Table 4.
Intercorrelations among % bound metabolite values
| Metabolite | N | % Bound MEHP | % Bound 5-OH-MEHP | % Bound 5-oxo-MEHP | % Bound 5-cx-MEPP |
|---|---|---|---|---|---|
| % Bound MEHP | 103 | 1.00 | |||
| % Bound 5-OH MEHP | 102 | 0.19 | 1.00 | ||
| % Bound 5-oxo MEHP | 103 | −0.04 | 0.51* | 1.00 | |
| % Bound 5-cx MEPP | 99 | −0.12 | 0.12 | 0.14 | 1.00 |
The % Bound MEHP, and % Bound 5-cx-MEPP indices were normally distributed, but the % Bound 5-OH-MEHP and % Bound 5-oxo-MEHP were negatively skewed. A log10 transformation based on first subtracting the values for these latter two % Bound indices from 100 and adding .05 was applied before correlating them with the other two indices
MEHP monoethylhexylphthalate, 5-OH MEHP 5-hydroxy-methylethylhexyl phthalate, 5-oxo MEHP 5-oxo-methylethylhexyl phthalate, 5-CX MEPP 5-carboxy-methylethylhexyl phthalate
p < .001, two-tailed test
Discussion
The principal findings from this study are an association between phthalate metabolism and ASD. There is a decreased capacity for detoxification via glucuronidation of compounds in the DEHP metabolic pathway in the ASD group. In most cases the decrease is statistically significant. It is believed that toxicity from DEHP is not due to DEHP per se, but to the monoesters derived from DEHP (NTP-CERHR 2005; Koch et al. 2006).
Most likely the effect is limited to a subset of the ASD population. ASD is a heterogeneous, multifactorial disease. In the present study we found no evidence for any obvious differences between the two groups other than the fact that one group had ASD. A larger study might have revealed heterogeneities.
This study demonstrated significant group differences between relatively small samples and would need to be replicated for the extent of any biological significance to these findings to be ascertained.
A number of other pieces of research support the biological plausibility of such a relationship:
A study in New York City found a relationship between increased maternal prenatal exposure to phthalates and behavioral problems in their children some years later (Engel et al. 2010). Autism per se was not documented, but would have been included under the umbrella term ‘behavioral problems’. Phthalate exposure was estimated from maternal urine phthalate metabolite levels.
In a large scale Swedish epidemiological study, the presence of PVC flooring material in the family home when the child was 1–3 years of age, was associated with ASD 5 years later (Larsson et al. 2009).
A 2010 study examined perinatal exposure to air pollutants, though not specifically phthalates, as defined in the National Air Toxics Assessment (NATA) program. Exposure levels were associated with the subsequent prevalence of ASD in 8-year-old children (Kalkbrenner et al. 2010).
Phthalate measurements on spot urine specimens that have controlled for creatinine are often used as proxy estimates of total phthalate exposure (Engel et al. 2010; Whiteley et al. 2006; Wolff et al. 2007; Wittassek et al. 2007. In our study no relationship between exposure levels, (as estimated from the total phthalate metabolite excretion) and ASD was found (Tables 2, 3).
The present study is the first to provide information on the potential mechanisms by which phthalate exposure might be linked to ASD. Our findings suggest that the glucuronidation pathway may be somehow compromised in ASD. This could be either at the enzyme or substrate level. Glucuronidation involves the co-factor UDP-glucuronic acid acting as the glucose donor with actual transfer being catalyzed by UDP-glucuronyl transferases (UGT’s, Argikar et al. 2008; Tukey and Strassburg 2000). The most likely source is a difference in UGT transferase activity. Genetic polymorphism of other enzymes such as GST M1 and GST P1 has been found in families with ASD children (Buyske et al. 2006; Williams et al. 2007; James et al. 2004).
Although this study shows a compromised phthalate metabolite glucuronidation pathway, this does not necessarily mean that phthalates are directly linked to ASD. The link between compromised glucuronidation and ASD could be to any compound metabolized via the glucuronidation pathway. These could include environmental pollutants, food additives, drugs, endogenous hormones and products of intermediary metabolism. Since the glucuronidation is responsible for the detoxification of many compounds, which one(s) are responsible for ASD cannot be said at this time.
Limitations of This Study
There are a number of inherent limitations to this study. (1) The relatively small number of subjects precludes examining for heterogeneities in the data set. Yet ASD is known to be a multi-factorial disease. (2) The study assumes that home and socio-economic environments were comparable across the two groups. Although the subjects were drawn from the same catchment area, no socio-economic data were collected and so the data were not adjusted for socio-economic status. (3) Reliance on single spot urine specimens is always problematic. Although not often done, a second urine would have provided supporting data (Teitelbaum et al. 2008). Other studies have shown that for estimating exposure to environmental pollutants, a single spot urine was acceptable for similar epidemiological studies (Engel et al. 2010; Herr et al. 2009; Ye et al. 2008). (4) Although all urines were collected between 10 AM and 4 PM there might have been a possible differences between morning and afternoon urine specimens. (5) With regard to Table 4, the data base was too small to explore the possibility of pathway differences within groups. (6) While we did screen during the selection process for drug use, actual measurement would have been preferable. Drug use could interfere with the phthalate pathway. No genetic data on subjects or their parents are available. As a genetic polymorphism causing altered UGT transferase activity is a likely factor producing these results, this limits the conclusions that can be drawn.
Contributor Information
T. Peter Stein, Email: tpstein@umdnj.edu, Department of Surgery, School of Osteopathic Medicine, University of Medicine and Dentistry of New Jersey, 2 Medical Center Drive, Stratford, NJ 08084, USA.
Margaret D. Schluter, Department of Surgery, School of Osteopathic Medicine, University of Medicine and Dentistry of New Jersey, 2 Medical Center Drive, Stratford, NJ 08084, USA
Robert A. Steer, Department of Psychiatry, School of Osteopathic Medicine, University of Medicine and Dentistry of New Jersey, 2 Medical Center Drive, Stratford, NJ 08084, USA
Xue Ming, Department of Neurology, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark, NJ 07103, USA; Department of Neurology, The New Jersey Neuroscience Institute, JFK Medical Center, Edison, NJ 08817, USA.
References
- Alberti A, Pirrone P, Elia M, Waring RH, Romano C. Sulphation deficit in “low-functioning” autistic children: A pilot study. Biological Psychiatry. 1999;46:420–424. doi: 10.1016/s0006-3223(98)00337-0. [DOI] [PubMed] [Google Scholar]
- Argikar UA, Iwuchukwu OF, Nagar S. Update on tools for evaluation of uridine diphosphoglucuronosyltransferase polymorphisms. Expert Opinion On Drug Metabolism & Toxicology. 2008;4:879–894. doi: 10.1517/17425255.4.7.879. [DOI] [PubMed] [Google Scholar]
- Armstrong RN. Structure, catalytic mechanism and evolution of the glutathione transferase. Chemical Research in Toxicology. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- Baio J, et al. Prevalence of autism spectrum disorders— autism and developmental disabilities monitoring network, 14 sites, United States, 2008 Surveillance summaries. Morbidity and Mortality Weekly Report (MMWR) 2012;61:1–19. [PubMed] [Google Scholar]
- Buyske S, Williams TA, Mars AE, Stenroos ES, Ming SX, Wang R, et al. Analysis of case-parent trios at a locus with a deletion allele: Association of GSTM1 with autism. BMC Genetics. 2006;7:8. doi: 10.1186/1471-2156-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental Health Perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1993;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1983. pp. 265–266. [Google Scholar]
- de Cock M, Maas YG, van de Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatrica. 2012;101:811–818. doi: 10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- Edelson SB, Cantor DS. The neurotoxic etiology of the autistic spectrum disorder: A replicative study. Toxicology and Industrial Health. 2000;16:239–247. [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental Health Perspectives. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Current Opinion in Neurology. 2010;23:103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- Herr C, Zur Nieden A, Koch HM, Schuppe HC, Fieber C, Angerer J, et al. Urinary di(2-ethylhexyl)phthalate (DEHP)—metabolites and male human markers of reproductive function. International Journal of Hygiene and Environmental Health. 2009;212:648–653. doi: 10.1016/j.ijheh.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Hwang HM, Park EK, Young TM, Hammock BD. Occurrence of endocrine-disrupting chemicals in indoor dust. Science of the Total Environment. 2008;404:26–35. doi: 10.1016/j.scitotenv.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. American Journal of Clinical Nutrition. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Hubamks A, Rose S, Gaylor DW. Abnormam methyllation-transulfuration metabolism and DNA hypomethylation among parents of children with autism. Journal of Autism and Developmental Disorders. 2008;38:1966–1975. doi: 10.1007/s10803-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21:631–641. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM. Exposure assessment to phthalates by human biomonitoring. PVC plasticizers 2010 . Brussels: Crain Communications. 2010;15:11–17. [Google Scholar]
- Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Archives of Toxicology. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. Di(2-ethyl-hexyl)phthalate (DEHP): Human metabolism and internal expo-sure—an update and latest results. International Journal of Andrology. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. What causes autism? Exploring the environmental contribution. Current Opinion in Pediatrics. 2010;22:219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Zimmerman-Bier B, Chaaban J, Wagner GC. Autism spectrum disorders: Concurrent disorders. Journal of Child Neurology. 2008;23:6–13. doi: 10.1177/0883073807307102. [DOI] [PubMed] [Google Scholar]
- Ming X, Johnson WG, Stenroos ES, Mars A, Lambert GH, Buyske S. Genetic variant of glutathione peroxidase 1 in autism. Brain and Development. 2010;32:105–109. doi: 10.1016/j.braindev.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- NIEHS. Report on carcinogens. 12th ed. Research Triangle Park, North Carolina: U.S Department of Health and Human Services Public Health Service National Toxicology Program; 2011. pp. 156–158. [Google Scholar]
- NTP-CERHR. NTP-CERHR expert panel update on the reproductive and developmental toxicity of Di)2-ethylhexyl) phthalate. NIEHS, Research Triangle Park, NC: National Toxicology Program—US Dept. of Health and Human Services; 2005. [DOI] [PubMed] [Google Scholar]
- O’Reilly B, Waring RH. Enzyme and sulphur oxidation deficiencies in autistic children with known food and chemical intolerances. Journal of Orthomolecular Medicine. 2003;4:198–200. [Google Scholar]
- Pessah IN, Seegal RF, Lein PJ, LaSalle J, Yee BK, Van De Water J, et al. Immunologic and neurodevelopmental susceptibilities of autism. Neurotoxicology. 2008;29:532–545. doi: 10.1016/j.neuro.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: Findings from a dietary intervention. Environmental Health Perspectives. 2012;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, et al. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. Journal of Chromatography, B: Analytical Technologies in the Biomedical and Life Sciences. 2003;789:393–304. doi: 10.1016/s1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Samander E, Herbert A, Needham LL, Calafat AM. Detection of phthalate metabolites in human saliva. Archives of Toxicology. 2005;79:647–652. doi: 10.1007/s00204-005-0674-4. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental Research. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annual Review of Pharmacology and Toxicology. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- Waring RH, Klovrza L. Sulphur metabolism in autism. Journal of Nutritional & Environmental Medicine. 2000;10:25–32. [Google Scholar]
- Wells PG, Mackenzie PI, Chowdhury JR, Guillemette C, Gregory PA, Ishii Y, et al. Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metabolism and Disposition. 2004;32:281–290. doi: 10.1124/dmd.32.3.281. [DOI] [PubMed] [Google Scholar]
- Whiteley P, Waring R, Williams L, Klovrza L, Nolan F, Smith S, et al. Spot urinary creatinine excretion in pervasive developmental disorders. Pediatrics International. 2006;48:292–297. doi: 10.1111/j.1442-200X.2006.02207.x. [DOI] [PubMed] [Google Scholar]
- Williams TA, Mars AE, Buyske SG, Stenroos ES, Wang R, Factura-Santiago MF, et al. Risk of autistic disorder in affected offspring of mothers with a glutathione S-transferase Pl haplotype. Archives of Pediatrics and Adolescent Medicine. 2007;16:356–361. doi: 10.1001/archpedi.161.4.356. [DOI] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environmental Health Perspectives. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Heger W, Koch HM, Becker K, Angerer J, Kolossa-Gehring M. Daily intake of di(2-ethyl-hexyl)phthalate (DEHP) by German children—A comparison of two estimation models based on urinary DEHP metabolite levels. Int. Journal of Hygiene and Environmental Health. 2007;210:35–32. doi: 10.1016/j.ijheh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environmental Health Perspectives. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environmental Research. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]