Abstract
Studies that investigate the differences between high and low psychopathic persons in brain activity during emotional facial expression processing are rare and commonly focus on males. The current study assessed whether previously reported behavioral differences would be reflected in differential brain activity in a sample of female offenders.
The participants included 23 female forensic inpatients with high and low scores on the Psychopathy Checklist Revised (PCL-R). ERPs were recorded during presentation of emotional facial expressions (i.e., fear, angry, and happy). Results revealed no differences in N170, P3 and late positive potential components between groups, but a significant difference in N2 only for angry and fear facial expressions, with high psychopathic participants showing lower reactivity. This N2 effect was found to be related to Factor 2 but not Factor 1 of the PCL-R. In time frequency analysis, theta activity underlying N2 best reflected these differences.
Findings in this female sample are consistent with a cortical deficit in processing facial expression of negative emotions in psychopathic men. In addition, differences in processing seem to appear relatively early.
Keywords: facial affect, psychopathy, female, time-frequency, event-related potentials
There is a substantial research tradition focusing on the processing of emotional facial expressions in healthy groups, as they are highly relevant social and emotional cues in personal interactions (Frischen, Eastwood, & Smilek, 2008). Previous research has shown that facial expressions can be detected more quickly and elicit different behavioral and psychophysiological responses depending on the emotional content (e.g. Alpers & Gerdes, 2007; Calvo, Avero, & Lundqvist, 2006; Zebrowitz, 2006). Some key underlying mechanisms of attention modulation and perception of faces have been investigated previously. First, the early event-related potential (ERP) N170 component has been found to be sensitive to a number of early face processing parameters, as well as to personally significant objects (Rossion, Curran, & Gauthier, 2002). In addition, an increased later positivity (p2) in response to emotional facial expressions has been found (Ashley, Vuilleumier, & Swick, 2004; Eimer & Holmes, 2007). For example, in a typical face processing task (i.e., face in the crowd task), where a single different emotional facial expression has to be detected in a field of emotional facial expressions, happy faces have been shown to be more readily detected as compared to other emotional facial expressions (Becker, Anderson, Mortensen, Neufeld, & Neel, 2011). This effect has been interpreted as a fast detection of the less ambiguous emotional facial expression. In other studies, however, a rapid detection of angry facial expressions has been related to a time shift in ERPs, namely an earlier N2 for angry compared to happy targets (Feldmann-Wüstefeld, Schmidt-Daffy, & Schubö, 2011). Furthermore, processing of angry facial expressions has been related to an enhanced early posterior negativity and an enhanced late positive potential (LPP) compared to other emotional expressions (Schupp et al., 2004). Thus, previous research supports the inference that emotional face processing can be readily detected in both early and later neurophysiological processes, and that angry faces are associated with a facilitated processing.
There are several disorders that are partially characterized by symptoms of deficient emotional facial expression decoding, such as schizophrenia or autism (Dalton et al., 2005; Fullam & Dolan, 2006). The psychopathic personality has also been linked to a deficient processing of emotional facial expressions (Blair et al., 2004). The capacity for categorization of facial expressions according to their emotional content has been shown to be attenuated in psychopathy, for different negative emotional facial expressions such as disgust (Kosson, Suchy, Mayer, & Libby, 2002), sadness (Eisenbarth, Alpers, Segrè, Calogero, & Angrilli, 2008; Hastings, Tangney, & Stuewig, 2008), and fear (Iria & Barbosa, 2009). Similar impairments can also be found in children or juveniles with personality traits related to psychopathy (e.g. so called callous-unemotional traits; Dadds, El Masry, Wimalaweera, & Guastella, 2008; Sylvers, Brennan, & Lilienfeld, 2011). In addition, it has been shown that this impairment can be temporarily reversed by a short training on directing attention to the eyes of the facial expressions (Dadds et al., 2008). At the same time, some studies have found no differences between high and low psychopathic individuals, although different subject selection approaches and methodological aspects, such as presentation durations or number of emotion categories, represent important potential confounds. In an attempt to deal with the multitude of methods used, a recent meta-analysis on emotional categorization capacity in psychopaths showed that there is an impairment for recognition of negative emotions (Wilson, Juodis, & Porter, 2011), with the highest effect sizes found for verbal response tasks.
There are few studies investigating cerebral processes underlying these impairments. Psychophysiological research using different kinds of emotional pictures found a reduced reactivity mainly to negative stimuli using the startle reflex (Justus & Finn, 2007; Patrick, Bradley, & Lang, 1993; Vaidyanathan, Hall, Patrick, & Bernat, 2011) or functional magnetic resonance tomography (see for a review: Blair, 2006; Yang & Raine, 2009). These effects have been related to Factor 1 (affective and interpersonal aspects; Vaidyanathan et al., 2011) of the Psychopathy Checklist Revised (Hare, 2003), but also to Factor 2 (antisocial and lifestyle aspects; Yang & Raine, 2009). These findings would suggest similar reduced emotion related activity in response to negative facial stimuli, consistent with findings concerning the categorization of negative facial expressions. There are only a few studies targeting this hypothesis. One study, which utilized a visual oddball task with emotional facial expressions, found an earlier P3a component and a later P3b component and a decreased N300 in highly psychopathic students (Campanella, Vanhoolandt, & Philippot, 2005). Another study investigating startle reactions to facial expressions in high and low psychopathic students found a reduced habituation to startle probes in students high on psychopathic traits (Anderson, Wan, Young, & Stanford, 2011) instead of the expected reduced startle modulation. Functional differences do not have to be linked to reduced categorization capacities; however, although high psychopathic participants showed no behavioral differences compared to low psychopathic ones in response to emotional facial expressions, reduced activity in subregions of the frontal cortex and the amygdala were found (Gordon, Baird, & End, 2004). In a flanker task using facial expressions of fear and anger, highly psychopathic students showed a reduced error related negativity (ERN;Munro et al., 2007). During simple watching of facial expressions of fear, psychopathic forensic inpatients show reduced activation of the fusiform face area compared to a control group. These group differences were not present for happy facial expressions (Deeley et al., 2006). A recent study investigated the interaction of inhibitory control and emotional content (affective words) on P3 amplitudes in male psychopaths as compared to criminals with antisocial personality disorder and to criminal control group. They found a reduced reactivity in the psychopathic group to negative words, independent of inhibitory control condition (Verona, Sprague, & Sadeh, 2012). Only few of the reported findings on emotion processing refer to female samples of psychopaths, which might be due to the smaller prevalence of high psychopathic traits in females compared to males (Verona & Vitale, 2006). Gender differences in the appearance of psychopathic traits have been stated mainly for the antisocial component, i.e. antisocial behavior (see Dolan & Voellm, 2009). Current research on emotion detection in female psychopaths points to deficits similar to those known from male samples, such as in emotion categorization (Eisenbarth, Alpers, Segrè, Calogero, & Angrilli, 2008), in startle potentiation during negative emotional content presentation (Vaidyanathan, Patrick, & Bernat, 2009) and in non-moral emotion regulation (Harenski, Kim, & Hamann, 2009). However, it is unclear whether gender differences in emotion processing influence the emotional deficit, as there are only a few studies investigating this relationship.
Time-frequency (TF) approaches to ERP analysis have been widely applied in the field, and can provide more selective and sensitive measures by incorporating frequency information simultaneously with more conventional time domain information. TF approaches have been used to assess processes occurring across a broad range of frequencies. The present work is focused on lower frequency delta (e.g. 0–3 Hz) and theta (e.g. 3–7 Hz) activity, which have been widely assessed during conventional time domain components such as P2, N2, and P3. An important research thread in this area has focused on functional inferences about cognitive control related to theta activity, including the error-related negativity (ERN; Cohen, Elger, & Ranganath, 2007; Gehring & Willoughby, 2004; Trujillo & Allen, 2007; Yordanova, Falkenstein, Hohnsbein, & Kolev, 2004), feedback-negativity (FN; Cohen, 2011; Gehring & Willoughby, 2004), and the no-go N2 (Kamarajan et al., 2006). Recent work supports the view that medial-frontal theta observed across such tasks may represent highly related brain activations (Cavanaugh et al., 2011). Additional work has documented a central role for theta in conventional orienting-novelty and oddball tasks (Barry, Heys, & Hasselmo, 2012; Başar, Başar-Eroglu, Karakaş, & Schürmann, 2001; Başar, Başar-Eroğlu, Karakaş, & Schürmann, 1999; Demiralp, Ademoglu, Istefanopulos, Başar-Eroglu, & Başar, 2001; Folstein & Van Petten, 2008). Recent work from our group has focused on separating contributions from activity occurring in the theta and delta frequency ranges during time-domain ERP component measures, including the feedback-negativity (FN) and P3 (Bernat, Nelson, Steele, Gehring, & Patrick, 2011), error-related negativity (ERN; Bernat, Williams, & Gehring, 2005), go/no-go N2 (Harper, Malone, & Bernat, in press), and P3 and slow-wave during affective picture processing (Olson, Bernat, Cole, & Anokhin, 2012). These findings support the view that theta and delta measures generally index separable neurophysiological process that co-occur during conventional component windows, motivating assessment of activity in these bands for the current report.
The present study investigates psychophysiological correlates of face processing in female criminal psychopaths using both time domain and time-frequency domain analytic approaches, in order to assess these processes and their relationship with psychopathic traits in a female offender sample. In line with past behavioral studies on psychopathic traits in both males and females (Blair et al., 2004; Eisenbarth et al., 2008), we hypothesized that there would be a reduction in reactivity to negative facial expressions as compared to positive facial expressions in female criminal psychopaths in the early stages of face processing.
METHOD
Sample
Participants in this study were all female inpatients in a psychiatric facility of the Italian prison system. They were recruited as high and low psychopathic patients, by excluding those with schizophrenic disorders and with low intelligence (score below 70). This recruitment included 28 participants with a mean age of 37.50 years (SD = 6.80, range = 24–52). After artifact tagging, data of only 23 participants were clean and suitable for analysis. This subsample had a mean age of 36.96 years (SD = 6.81, range = 24–52), the mean education was 9.68 years (SD = 2.93, range = 5–15). The mean level of current substance abuse (average of present abuse of drugs, tobacco, or alcohol) was 0.55 (SD = 0.38). The diagnoses from clinical files were personality disorders and depression (see Table 1). Actual criminal acts were murder (34.8%), attempted murder (21.7%), robbery (8.7%), physical injury (21.7%) and others. Thirteen participants (56.5%) had previous convictions.
Table 1.
Descriptives for all participants, for the group of low psychopathic participants and the group of high psychopathic participants
| all participants (N = 23) | low psychopathic group (n = 10) | high psychopathic group (n = 13) | ||
|---|---|---|---|---|
| age (M(SD)) | 36.96 (8.81) | 36.80 (5.47) | 37.08 (7.91) | |
| school education (yrs., M(SD)) | 9.68 (2.93) | 11.00 (2.92) | 8.77 (2.68) | |
| intelligence quotient (M(SD)) | 97.17 (8.94) | 100.20 (6.25) | 94.85 (10.18) | |
| diagnoses (clinical files, N) | Borderline PD | 11 (47.8%) | 0 (0%) | 11 (84.6%) |
| Antisocial PD | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| Schizotypic PD | 1 (4.3%) | 1 (10%) | 0 (0%) | |
| Paranoid PD | 1 (4.3%) | 1 (10%) | 0 (0%) | |
| PD unspecified | 2 (8.7%) | 1 (10%) | 1 (7.7%) | |
| Depression | 4 (17.4%) | 4 (40%) | 0 (0%) | |
| remitted psychosis | 3 (13%) | 3 (30%) | 0 (0%) | |
| existing previous convictions (N) | 13 (56.5%) | 1 (10%) | 12 (92.3%) | |
| actual criminal act | murder | 8 (34.8%) | 5 (50%) | 3 (23.1%) |
| attempted murder | 5 (21.7%) | 4 (40%) | 1 (7.7%) | |
| robbery | 2 (8.7%) | 0 (0%) | 2 (15.4%) | |
| physical injury | 5 (21.7%) | 0 (0%) | 5 (38.5%) | |
| arson | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| maltreatment | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| damage | 1 (4.3%) | 1 (10%) | 0 (0%) | |
| average actual drug use (M(SD)) | 0.55 (0.38) | 0.23 (0.22) | 0.81 (0.25) | |
The mean Psychopathy Checklist – Revised (see below for description) score was 20.70 (SD = 11.95), ranging from 4 to 38 points (see Table 2). Groups were defined by splitting those above a PCL-R score of 20 (median) and below. This led to a group of 10 low psychopathic patients and a group of 13 high psychopathic patients. Groups did not differ for intelligence (t(21) = 1.46, p = .16) or age (t(21) = −0.09, p = .93, see Table 1 for means). The differences between groups in diagnoses (more borderline personality disorder in the high psychopathic group and more depression diagnoses in the low psychopathic group) reflect the known overlap of borderline and psychopathic symptoms in female offenders on the one hand (Sprague, Javdani, Sadeh, Newman, & Verona, 2012) and the reduced rates of depression in psychopaths on the other hand (Cleckley, 1964).
Table 2.
Mean PCL-R scores and SDs for the total score, the 2-factor solution (factor 1 and 2) and the 4-factor solution (interpersonal, affective, lifestyle and antisocial factors)
| PCL-R (M(SD)) | all participants (N = 23) | low psychopathic group (n = 10) | high psychopathic group (n = 13) |
|---|---|---|---|
| total score | 20.70 (11.95) | 8.40 (3.20) | 30.15 (7.91) |
| factor 1 | 8.48 (5.43) | 3.70 (1.77) | 12.15 (4.24) |
| factor 2 | 9.65 (5.45) | 3.90 (1.97) | 14.08 (1.66) |
| interpersonal factor | 3.52 (3.17) | 0.60 (0.84) | 5.77 (2.31) |
| affective factor | 4.96 (2.62) | 3.10 (1.73) | 6.38 (2.29) |
| lifestyle factor | 6.52 (3.25) | 3.30 (1.49) | 9.00 (1.53) |
| antisocial factor | 4.30 (3.39) | 0.70 (1.06) | 7.08 (1.04) |
Participants received 5€ (equivalent to US$7) for compensation. All participants gave informed consent to the experimental procedure and to PCL scoring in collaboration with the institutional staff.
Measures
Key individual differences were measured using the Standard Progressive Matrices (SPM), and the Psychopathy Checklist Revised (PCL-R).
The Standard Progressive Matrices (SPM; Raven, 1958) is a non-verbal measure for general intelligence, which should not be affected by culture and education. It is based on a sequential procedure with increasing difficulty. The norms used for this study are based on a neuropsychiatric sample (Russ, 2003).
The Psychopathy Checklist-Revised (PCL-R) includes 20 items on behavioral and personality features, rated by an expert who draws on information from file review and from a semi-structured interview. Its validity and reliability are proven repeatedly (Hare, 2003). Harpur and colleagues (1989) proposed a two-factor model, which is the prevalent theory of psychopathy and of the PCL-R. The two factors, Affective Interpersonal (Factor 1) and Antisocial Behavior (Factor 2), differentiate between personality related features that are difficult to measure via self report and behavioral features that highly correlate with antisocial personality disorder symptoms and diagnoses. Factor 1 relates to the affective deficit and the interpersonal style aspects, whereas Factor 2 relates to the antisocial and lifestyle aspects of psychopathy. Thus, the PCL-R is a measurement that takes into account the pathological behavior as well as the specific personality style of individuals scoring high on psychopathy.
Procedure
Testing was completed in a quiet room within the psychiatric facility. The stimuli were presented on a 13” Laptop screen with a monitor-to-head-distance of 60 cm, using the software Presentation (Neurobehavioral Systems). Two tasks were presented in this experiment with parallel physiological recording. The first task, a picture-viewing paradigm involving positive, negative and neutral pictures from the International Affective Pictures System (IAPS; Lang, Bradley, & Cuthbert, 2005) will be reported elsewhere. The second task consisted of a mood induction followed by presentation of IAPS pictures, different from the pictures of the first task. The mood induction has been realized by presenting facial expressions of three different affects, fear, anger and happiness from the Karolinska Directed Emotional Faces set (Lundqvist, Flykt, & Ohman, 1998) for 8000ms each after a fixation cross shown for 300ms and followed by an ISI of 200ms, a presentation of an IAPS picture for 1000ms and an ITI of 2000ms. Participants were asked to empathize with the person depicted on the screen and were instructed to use own memories for situations related to the emotion shown by the person on the picture. Ninety trials were shown in a completely randomized order (30 per emotion category). The present data refer only to the mood induction phase.
Physiological data acquisition
EEG activity was recorded from 38 scalp sites using a 32-electrode ElectroCap (Eaton, Ohio) to which six external electrodes were added. Data were stored with a 40-chan NuAmps system, NeuroScan software, version 4.1 (NeuroScan Labs, Sterling, Virginia, USA). For scalp topography all 38 electrodes were used, while for statistics a subgroup of fifteen EEG electrodes in central map position were chosen: F3, FZ, F4, FC3, FCZ, FC4, C3, CZ, C4, CP3, CPZ, CP4, P3, PZ, P4. Impedances were kept below 5 kΩ. All EEG signals were referenced on-line to CZ and digitized on-line at 500 Hz.
Data preprocessing
EEG signals were epoched off-line from 2000 milliseconds before to 11000 milliseconds after stimulus onset and re-referenced to average mastoids, and analyses focused on −1000 to 2000 milliseconds before and after stimulus onset. Trial-level EEG data were corrected for ocular movement artifacts using an algorithm by Gratton, Coles, and Donchin (1983) implemented in Matlab (version 7.11). In order to minimize the impact of artifacts on the TF principal component analysis decomposition described below, a two-step method of artifact rejection was performed. First, in order to exclude residual artifacts remaining after ocular correction, trials in which activity exceeded ±125 μV at Cz in either the pre- (−500 to −1 ms) or post-stimulus (1 to 1000 ms) time windows were excluded. Then, within each trial, individual electrode sites in which activity exceeded ± 100 μV in the same time regions reported above (relative to one another) were also omitted from analysis. Using these criteria, 14.7% of all trials were excluded. The data were then averaged separately within affect mood induction (fear, anger, happy), and ERPs were baseline-corrected for the 300 ms period prior to stimulus onset. Finally, the processed data were down-sampled to 128 Hz. All preprocessing, time-domain, and time-frequency analyses (described below) were conducted using scripts provided by Bernat and colleagues (Bernat et al., 2005).
Data reduction
Time-domain. The time-domain (TD) N2 was defined as the minimum voltage occurring between 187.5 and 390.6 milliseconds (ms). N170 was defined as the minimum voltage occurring between 93.8 and 179.7 ms. P3 was defined as the maximum voltage between 296.9 and 500 ms; whereas, LPP was defined as the mean voltage between 500 and 1000 ms. All ERP components were relative to a pre-stimulus baseline (−101.6 to −7.8 ms), with ms corresponding to bins of a 128 Hz re-sampled signal. The average of a 3×3 grid (F3, FZ, F4, C3, CZ, C4, P3, PZ, P4) entered the TD statistical analyses for all TD components.
Time-frequency domain. A time-frequency transform (TFT) method was applied in order to quantify the frequency characteristics of the ERP signals over time, and to disaggregate components that differ in spectral properties (e.g., delta, theta) but share temporal qualities (Bernat et al., 2005). Time-domain ERP condition averages were first transformed into time-frequency energy distributions (surfaces) using the binomial reduced interference distribution (RID) variant of Cohen's class of time-frequency transforms (for details, see Bernat et al., 2005). Next, the TFT was applied to an area corresponding to the 0 to 750 ms time range and 0 to 14 Hz. Finally, two regions of interest (ROIs) were applied to the TF surface in order to parse the grand average TF surface into relevant TF subcomponents: one corresponding to the 219 to 313ms time region and 1.5 to 5 Hz frequency window (referred to as N2-Theta below), and another in the same time region but with the frequency window set at 0.5 to 1 Hz (N2-Delta). The average of a 3×3 grid (F3, FZ, F4, C3, CZ, C4, P3, PZ, P4) was used for related statistical analyses. Additional analyses of the topographic distribution of the effects within this grid proved unreliable due to the relatively small number of participants (incarcerated female offenders represent a difficult population to recruit and test) and the higher degree of noise.
Data analysis
Analyses of variance were computed including the between-subjects factor group (high vs. low psychopathic patients) and the within-subjects factors emotion (anger vs. fear vs. happy). All post-hoc-tests were Bonferroni corrected for multiple testing. In addition, linear and logistic regression analyses were computed using TD and TF components as predictors and PCL score (linear regression) or group (logistic regression) as dependent variable. Also, TD and TF components were included as dependent variables and PCL Factors 1 and 2 as predictors, to assess for unique contributions. Standardized coefficients are reported (β - weights). All statistical analyses were carried out with SPSS (IBM, Armonk, NY).
RESULTS1
Time domain
ANOVAs with factors Group and Emotion were carried out for N170, N2, P3, and LPP components. Concerning N170, no significant main effect for Group (F(1,21) = 0.05, p = .83, ηp2 = .002),Emotion (F(2,42) = 1.72, p = .20, ηp2 = .001),or their interaction (F(2,42) = 0.02, p = .98, ηp2 = .001) were found. Analysis of N2showed, a significant main effect of Group (F(1,21) = 7.58, p = .01, ηp2 = .27), no main effect of Emotion (F(2,42) = 0.94, p = .39, ηp2 = .04),but a significant interaction of Group × Emotion (F(2,42) = 4.23, p = .03, ηp2 = .17). Simple effects analysis revealed that the interaction is driven by significant group differences in response to angry faces (F(1,21) = 9.94, p = .01, ηp2 = .32) and fear faces (F(1,21) = 9.55, p = .01, ηp2 = .31), but not to happy facial expressions (F(1,21) = 2.05, p = .17, ηp2 = .09), see Figure 2. For P3, there is a significant difference between Groups (F(1,21) = 4.23, p = .05, ηp2 = .17), a trend for an effect for Emotion (F(2,42) = 2.79, p = .09, ηp2 = .12),and no significant interaction of Emotion and Group (F(2,42) = 0.73, p = .44, ηp2 = .03). For the LPP, we found no difference between groups (F(1,21) = 1.22, p = .28, ηp2 = .06), no effect for Emotion (F(2,42) = 0.87, p = .42, ηp2 = .04) and no interaction of Group and Emotion (F(2,42) = 0.48, p = .60, ηp2 = .02).
Figure 2.
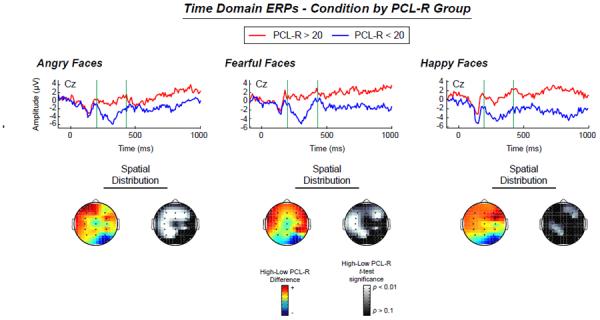
Top: Time domain ERPs for angry, fearful, and happy facial expression conditions, split up by PCL-R group (above and below 20), with green bars marking the boundaries of the interval of N2 activity. Bottom: Colored topographic maps. Scalp topography for the mean High-Low PCL-R group difference for activity in the N2 time window. Greyscale topographic maps. Scalp topography depicting the statistical significance of the High-Low PCL-R group t-test for each channel for activity in the N2 time window.
To assess unique aspects of psychopathy, regression analyses were carried out predicting the N2 component with Factor 1 and Factor 2 scores of the PCL-R in a linear regression. Results indicated a significant overall prediction of N2 amplitude in response to fear faces (R2 = .40, F(2,22) = 6.59, p = .01), where Factor 1 did not add unique predictive value (β = −.02, p = .94) but Factor 2 did (β = .65, p = .04). The same model for anger faces significantly predicted N2 activity (R2 = .40, F(2,22) = 6.36, p = .01), where again, Factor 2 added predictive value (β = .75, p = .02) but Factor 1 did not (β = −.16, p = .59). For happy facial expressions, this model was not significant at all (R2 = .19, F(2,22) = 2.36, p = .12). Results support the inference that PCL-R Factor 2 uniquely predicted ERPs for negative affect (i.e., fear and anger) when controlling for variance in Factor 1 scores, pointing to a more relevant association of Factor 2 to explained variance. Neither Factor 1 nor Factor 2 predicted responses to the positive affect stimuli.
Similarly, linear regression analyses predicting P3 were conducted, including Factors 1 and 2 as predictors. There was a significant overall prediction of P3 amplitude in response to anger faces (R2 = .35, F(2,22) = 5.43, p = .01), where Factor 1 did not add unique predictive value (β = −.23, p = .47) but Factor 2 did (β = .76, p = .02). The same models for fear faces (R2 = .16, F(2,22) = 1.88, p = .18), and for happy facial expressions were not significant (R2 = .19, F(2,22) = 2.36, p = .12).
Time domain components with significant group effects (N2 and P3) were included in logistic regression analyses. Predicting psychopathy group with N2 and P3 measures in response to fear stimuli in a logistic regression model revealed a significant prediction (R2 = .45, χ2 (9) = 9.44, p = .01), with a significant unique prediction of N2 (β = .40, p = .05), but no unique prediction of P3 (β = .08, p = .51). For anger, a logistic regression model (R2 = .52, χ2 (9) = 11.28, p = .004) showed predictive value for group, with no unique prediction of either N2 (β = .37, p = .10) or P3 (β = .19, p = .27), indicating that the shared variance between these components was responsible for the effect.
Time-frequency domain
Based on the observed time-domain effects, time-frequency related analyses were focused on the N2 component. Descriptively, the N2 component includes both higher frequency theta activity and lower frequency delta activity (see Figure 3). The overall relationship between the time-domain N2 and the time-frequency theta and delta components was tested using linear regressions, with N2 as the dependent variable and N2 theta and delta as independent variables. The linear regression model for anger, predicting the time domain N2 with theta and delta measures was significant (R2 = .50, F(2,22) = 9.88, p = .001), where N2-related theta contributed independently to the model (β = −.65, p = .002), while N2 delta did not (β = −.09, p = .62). A similar model for fear faces also predicted significantly time-domain N2 (R2 = .37, F(2,22) = 5.90, p = .01), where N2 related theta activity contributed(β = −.61, p = .01)and N2 delta (β = .47, p = .02)contributed independently to the model. An equivalent model for happy faces also significantly predicted the time-domain N2 (R2 = .55, F(2,22) = 12.40, p< .001), where N2 related theta activity (β = −.47, p = .01) andN2 delta(β = −.43, p = .02) did contribute independently to the model.
Figure 3.
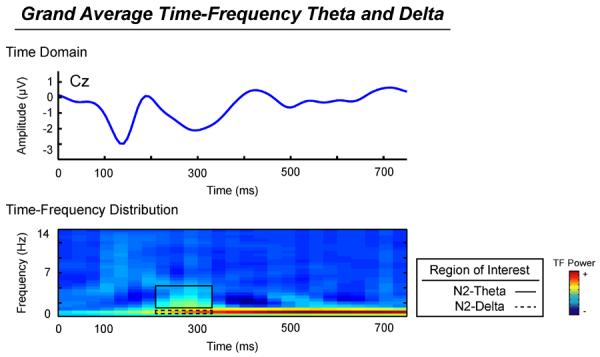
Top: Grand average time domain ERP, frequency-filtered using a 3rd order Butterworth filter to isolate activity below 14Hz. Bottom: Grand average time-frequency distribution, depicting the higher frequency theta (solid box) and lower frequency delta (perforated box) underlying the N2 component.
Next, the effects of group relative to the TF measures were assessed. First, an analysis of variance for N2 theta including Group (high vs. low psychopathic) and Emotion (fear vs. anger vs. happy) revealed a main effect of Group (F(1,21) = 5.01, p = .04, ηp2 = .19), no main effect of Emotion (F(1,21) = 0.22, p = .79, ηp2 = .01) and no interaction of Group by Emotion (F(2,42) = 1.74, p = .19, ηp2 = .08). An analysis of variance for N2 delta including the same factors showed no effects for Group (F(1,21) = 1.67, p = .21, ηp2 = .07), Emotion (F(2,42) = 0.05, p = .89, ηp2 = .002) or Group by Emotion interaction (F(2,42) = 0.38, p = .61, ηp2 = .02).
But, a logistic regression model predicting Group by TF components for anger faces was significant (R2 = .63, χ2 (3) = 14.44, p = .002), with no independent contribution of N2 theta (β = −.08, p = .16) and N2 delta (β = .07, p = .21). For fear faces, a similar model was not significant (R2 = .27, χ2 (3) = 5.09, p = .17).
DISCUSSION
This study investigated reactions of high and low psychopathic female forensic inpatients to emotional facial expressions showing afraid, angry, and happy mood. ERP components, previously demonstrated to be sensitive to facial expressions (N170, P3), did not differ between groups, but significant differences were found for the N2 component. The N2, with a latency around 250ms, was originally found to represent the earliest component which differentiates emotional content of stimuli (Palomba, Angrilli, & Mini, 1997), a maximum can be located at posterior parieto-occipital sites (Junghoefer, Bradley, Elbert, & Lang, 2001). This early component is an automatic response related to increased activation of visual-associative posterior regions during emotional visual stimulation (Junghoefer et al., 2001) and is also modulated by feedback projections from temporal pole cortex and amygdala which enhance visual processing of biological relevant emotional stimuli (Lane, Chua, & Dolan, 1999). This group difference for the N2 component was associated primarily with Factor 2 of the PCL-R (antisocial behavior and impulsivity) but not with Factor 1 (emotional detachment). In terms of the underlying time-frequency activity, the observed N2 effects were related mainly to theta activity, which points to an activity that has been related to orienting as well as fear processing. These results add to past studies describing emotion recognition behavioral deficits in psychopaths (Blair et al., 2004; Eisenbarth et al., 2008) and provide the first evidence for a significant reduction of cortical activity during emotional face processing in female criminal psychopaths.
Theories of psychopathy
Several etiological theories of psychopathic personality emphasize a primary emotion processing deficit, e.g., the dual process theory (Fowles & Dindo, 2006) or the low-fear-hypothesis (Lykken, 1995). Also, the triarchic model of psychopathy (Patrick, Fowles, & Krueger, 2009) states an emotion processing deficit as an important basis of psychopathic personality. All these theories postulate the relevance of reduced reactions to negative emotional content, at both physiological and subjective experience levels. Related neurophysiological circuits are the limbic system, mainly the amygdala as primary emotion processing structure and the prefrontal cortices as emotion interpretation and regulating areas (Blair, 2006). This emotion processing deficit has also been linked to an attentional deficit hypothesis (Baskin-Sommers, Curtin, & Newman, 2011), which has been proposed to be responsible for the emotion processing deficits. The poor performance of psychopaths in tasks involving secondary or contextual information compared to single-task performance could be relevant also for several emotion processing tasks which necessarily involve motivation-related attentional demand (Hiatt & Newman, 2006). In terms of the PCL-R (Hare, 2003), Factor 2 has been shown to primarily index externalizing (Patrick et al., 2005), e.g. impulse and attentional dysregulation, while Factor 1 is associated with the affective and interpersonal aspects of psychopathy. Debate exists about the primacy of affective deficits as the unique factor in psychopathy, relative to the attentional deficits.
Mapping current results onto the presented constructs
Our results can be interpreted within both frameworks, the primary emotion deficit framework and the attention modulation framework. The reduced processing for negative emotional facial stimuli is consistent with previous behavioral results related to facial expression processing in males, and the few studies conducted on females, indicating reduced categorization capacity for negative emotional facial expressions in highly psychopathic participants (see Wilson, Juodis, & Porter, 2011). Findings are also consistent with psychophysiological measures in psychopaths, demonstrating reduced reactivity in response to broadly negative emotional content (e.g., Blair, 2006; Patrick et al., 1993; Verona et al., 2012). The group differences for the N2 time-domain component were found for both anger and fear, but not for happy faces, suggesting that the deficit may be related to negative emotional content more broadly, rather than to aggression per se. One previous study, which assessed emotional face processing in male psychopathy, similarly found no group differences for the classical face processing components such as N170, P300 or LPP (e.g., Flor, Birbaumer, Hermann, Ziegler, & Patrick, 2002). The consistency between these studies supports the view that primary face processing mechanisms indexed by these components may be working appropriately, while a part of the processing indexed by the N2 component is reduced.
Because many factors have been shown to modulate activity in the N2 range during affective, face, and other stimulus processing, and because this effect is novel with regard to female psychopathy, inferences about the processes associated with the observed effects must be tentative. Nonetheless, as described in the introduction, substantial work has related theta occurring in the range of the N2 to control processing (Bernat et al., 2011; Cohen et al., 2007; Gehring & Willoughby, 2004; Harper et al., in press). This is particularly relevant because disrupted control processing is a hallmark of externalizing disorders involved in psychopathy (Patrick, Hicks, Krueger, & Lang, 2005). Activity in the N2 range has also been associated with arousal effects in picture presentation paradigms (Schupp, Junghoefer, Weike, & Hamm, 2003) and to rapid affective processing associated with amygdala activity (Olofsson, Nordin, Sequeira, & Polich, 2008). Relative to the present results, this is consistent with the idea of reduced affective processing. N2 activity has also been closely associated with orienting responses (Folstein & Van Petten, 2008), consistent with the idea of inhibited orienting to the negative faces for psychopaths. Finally, N2-related activity is short by definition (generally less than 350–450 ms after stimulus presentation), and thus it is useful to note that these effects occur after initial perceptual and attentional processing (e.g. P1 and N1 components) but attenuates during sustained cognitive processing (e.g. P300 and later slow wave).
Taking a closer look to the relationship of psychopathy and the N2-P3 components, our results point to a stronger relationship of this deficit with Factor 2 of the PCL-R as compared to Factor 1. Thus, group differences in processing negative facial affect seem to be more related to antisocial and lifestyle aspects (Factor 2) than to affective and interpersonal aspects (Factor 1) of psychopathy. This does not seem intuitive because Factor 1 indexes the affective aspect of psychopathy (e.g. reduced empathy and emotionality as well as fearlessness). In contrast, Factor 2 has been found to be strongly related to externalizing (Patrick et al., 2005), a common impulse dysregulation factor related to conduct disorder, adult antisocial behavior, alcohol use/abuse, and drug abuse, combined with impulsivity and disinhibited personality traits (Bogaerts, Polak, Spreen, & Zwets, 2012; Kendler, Aggen, & Patrick, 2012). Thus, relative to the findings reported in the current study, the relationship of an emotion processing deficit with Factor 2 should be most related to impulse dysregulation (cf. externalizing). Such a mechanism could involve disruptions in attentional focus to the negative faces during the orienting process (e.g. more dysregulated affective responding), rather than directly indexing an affect processing deficit per se, although additional work would be needed to make this inference. Bolstering this idea, a high percentage of inmates affected by Antisocial Personality Disorder did suffer from Attention Deficit Hyperactivity Disorder in childhood (Faraone, Biederman, Jetton, & Tsuang, 1997; Mannuzza, Klein, Bessler, Malloy, & LaPadula, 1993), thus attention deficits, at clinical level, are often associated with poor frontal control of impulsivity and antisocial behavior. It is worth noting that the most consistent ERP effect reported related to externalizing disorders has been reduced amplitude of the P300 component in oddball tasks (e.g., Iacono, Carlson, Malone, & McGue, 2002; Patrick, 2006), including Attention Deficit Hyperactivity Disorder (Yoon, Iacono, Malone, Bernat, & McGue, 2008). Although, these effects have been associated more clearly with delta than theta (Bernat et al., 2011; Gilmore, Malone, Bernat, & Iacono, 2010). It is also important to note that Factor 2 is also the main factor which differentiates more between male and female psychopaths (Vitale, Smith, Brinkley, & Newman, 2002), and thus our results suggest that gender differences should be investigated in greater detail, especially when it comes to physiological correlates.
A further issue concerns the different aspects of emotional processing. Most emotion paradigms can be subdivided in two large categories. In perceptual paradigms, participants are passively presented emotional stimuli (e.g., slides, movies, sounds, words, etc.), while in active paradigms, subjects are invited to imagine, produce, or modulate emotions. In the present mixed paradigm, with slide presentation, the reduced processing of negative emotional faces was found in psychopaths at mid-latency components (N2 and partially at P3), but not in the late components (LPP). Therefore, the main effects were found in those components more associated with perceptual attention-related processes.
Although at first glance this seems to be mainly a cortical-cognitive epiphenomenon associated to psychopathy, the altered N2 may mark a reduced feedback connectivity from amygdala afferents to the cortex, an interpretation supported by the selective effect involving the two negative emotions (fear and anger) typically processed by amygdala and by the known key psychopaths' reduced amygdala sensitivity to fear/aversive stimuli (Patrick & Bernat, 2009). In light of this, the present results are highly consistent also with the model advanced by Blair and Mitchell (2009) in which emotional attention deficits play a central role in psychopathy. In addition, the results can be interpreted also in light of the triarchic conceptualization of psychopathy as presented by Patrick and colleagues (Patrick et al., 2009), in which Disinhibition in combination with Boldness or Meanness build the psychopathic personality. Since the PCL-R used in the present research is more related with the Disinhibition and Meanness factors of TriPM, while Boldness is poorly represented, Disinhibition is the main dimension associated with PCL Factor 2, characterized by antisocial behavior and poor impulse control (Patrick et al., 2009). Especially the externalizing factors Disinhibition and Meanness have been shown to be related to a reduced P3 in male prisoner samples (Patrick et al., in press). Relating this construct to our results, Disinhibition, and to a minor extent Meanness, probably account for the observed N2 deficits. Subjects with high levels of Disinhibition may show a reduced emotional attention and limited capacity to process fundamental anger/fear faces (N2) necessary for inhibiting impulsive aggression towards others.
Limitations and Future Directions
The current study has some limitations regarding the sample and the procedure. More specifically, the small sample size led to a smaller number of trials included in the analysis, which is due to a generally very low number of incarcerated women (20 times less compared to men). Also, due to the procedure involving interchanging presentations of facial expressions and IAPS pictures, we cannot exclude priming effects on the facial expressions by the IAPS pictures presented on the previous trial. However, the randomized presentation order of facial expressions of different valence and of positive and negative IAPS pictures would not justify a specific effect of angry and fearful facial expressions. Another limitation of the study is the combination of the pure facial expression presentation with the empathizing instruction. Addressing these limitations will be a focus of future work, by aiming at larger sample size and a higher number of trials. In addition, future studies on processing of facial affect may include ratings of the facial expressions for emotional valence and arousal by the participants, for replication and extension. Also, separate investigation of facial expression processing and empathizing tasks would be helpful. A valuable additional extension would be to directly assess gender differences and similarities.
In sum, this is the first study investigating facial affect processing in female psychopaths using ERPs. We provided evidence for a negative affect related arousal difference of high vs. low psychopathic patients, reflected in the N2 component and driven by theta activity.
HIGHLIGHTS
Highly psychopathic female forensic inpatients show reduced reactivity to emotional facial expressions in early ERP components (N2)
This reduced reactivity is present only for negative emotional facial expressions, not for positive ones
Time-frequency analyses reveal theta activity to reflect this N2 effect
Results match previous findings in male psychopathic patients and underline arousal based deficits in emotion processing of psychopaths
Figure 1.
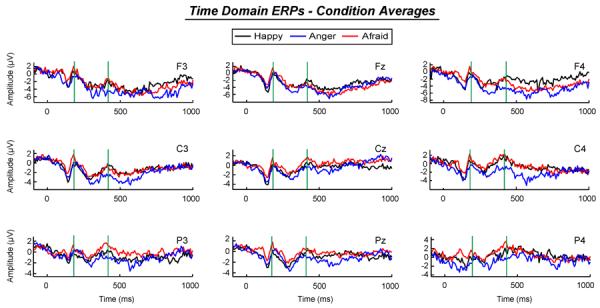
Time domain ERPs, averaged within facial expressions of anger, fear, and happy conditions, where green bars represent the N2 component, green bars mark the investigated N2 component.
ACKNOWLEDEGMENTS
This study was supported by a grant from CARIPARO Foundation to A.A. (project # PARO117731), and NIMH grant K08MH080239 to Edward Bernat. We thank the participants from the prison hospital for their participation.
The first author was funded by a Research Fellowship Training Award of the Society for Psychophysiological Research (SPR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Due to the relatively small sample size used in the current report, steps were taken to identify subjects who may have been highly influential on the results. We evaluated the linear regression models for highly influential subjects using Cook's D statistic, which measures the changes in the model estimates due to a particular data point. Two participants were identified across the linear models, indicating that these subjects were either not fit well by the models, or were unduly influencing the model. PCL scores for both subjects were PCL Total = 23 and 9, F1 = 6 and 6, F2 = 15 and 3. To ensure that these two participants were not responsible for the reported effects, key comparisons between PCL group were conducted for the N2 ERP and N2 TF Theta values from anger and fear faces (i.e., those effects identified as significant in the ANOVA models) for both the total sample and the reduced sample excluding the two out lying subjects. Results indicated that the effects were left functionally equivalent, but nominally stronger, when excluding these subjects (two-sample t-test comparing Low - High Psychopathy groups: Fearful N2 peak ts(21, 19) = −3.09 and −3.42, ps = .006 and .003, Anger N2 peak ts(21, 19) = −3.15 and −3.22, ps = .005 and .005, Anger N2 TF Theta ts(21, 19) = 3.01 and 3.14, ps = .007 and 0.006, for the total and reduced sample, respectively). These results indicate that it is unlikely that these two subjects are driving or exaggerating our effects.
REFERENCES
- Anderson NE, Wan L, Young KA, Stanford MS. Psychopathic traits predict startle habituation but not modulation in an emotional faces task. Personality and Individual Differences. 2011;50(5):712–716. doi: 10.1016/j.paid.2010.12.023. [Google Scholar]
- Barry C, Heys JG, Hasselmo ME. Possible role of acetylcholine in regulating spatial novelty effects on theta rhythm and grid cells. Frontiers in neural circuits. 2012;6 doi: 10.3389/fncir.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakaş S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology. 2001;39(2):241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neuroscience Letters. 1999;259(3):165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Curtin JJ, Newman JP. Specifying the Attentional Selection That Moderates the Fearlessness of Psychopathic Offenders. Psychological Science. 2011;22:226–234. doi: 10.1177/0956797610396227. doi: 10.1177/0956797610396227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DV, Anderson US, Mortensen CR, Neufeld SL, Neel R. The Face in the Crowd Effect Unconfounded: Happy Faces, Not Angry Faces, Are More Efficiently Detected in Single- and Multiple-Target Visual Search Tasks. Journal of Experimental Psychology: General. 2011;140(4):637–659. doi: 10.1037/a0024060. doi: 10.1037/a0024060. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain–loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology. 2011;120(2):352. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time–frequency energy using PCA. Clinical Neurophysiology. 2005;116(6):1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Mitchell DG. Psychopathy, attention and emotion. Psychological Medicine. 2009;39(4):543–555. doi: 10.1017/S0033291708003991. doi: 10.1017/S0033291708003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Subcortical brain systems in psychopathy: The amygdala and associated structures. In: Patrick CJ, editor. Handbook of psychopathy. The Guilford Press; New York: 2006. pp. 296–312. [Google Scholar]
- Blair RJR, Mitchell DGV, Peschardt KS, Colledge E, Leonard RA, Shine JH, Perrett DI. Reduced sensitivity to others' fearful expressions in psychopathic individuals. Personality and Individual Differences. 2004;37(6):1111–1122. [Google Scholar]
- Bogaerts S, Polak M, Spreen M, Zwets A. High and low aggressive narcissism and anti-social lifestyle in relationship to impulsivity, hostility, and empathy in a group of forensic patients in the Netherlands. Journal of Forensic Psychology Practice. 2012;12(2):147–162. doi: 10.1080/15228932.2012.650144. [Google Scholar]
- Campanella S, Vanhoolandt ME, Philippot P. Emotional deficit in subjects with psychopathic tendencies as assessed by the Minnesota Multiphasic Personality Inventory-2: an event-related potentials study. Neuroscience Letters. 2005;373(1):26–31. doi: 10.1016/j.neulet.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. Journal of Neuroscience. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. The mask of sanity. The Mosby Company; Saint Louis: 1964. [Google Scholar]
- Cohen MX. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. Neuroimage. 2011;55(3):1373–1383. doi: 10.1016/j.neuroimage.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, El Masry YB, Wimalaweera SM, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(4):455–463. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, Murphy DG. Facial emotion processing in criminal psychopathy: Preliminary functional magnetic resonance imaging study. British Journal of Psychiatry. 2006;189(6):533–539. doi: 10.1192/bjp.bp.106.021410. doi: 10.1192/bjp.bp.106.021410. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Başar-Eroglu C, Başar E. Wavelet analysis of oddball P300. International Journal of Psychophysiology. 2001;39(2):221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Dolan M, Voellm B. Antisocial personality disorder and psychopathy in women: A literature review on the reliability and validity of assessment instruments. International Journal of Law and Psychiatry. 2009;32(1):2–9. doi: 10.1016/j.ijlp.2008.11.002. doi: 10.1016/j.ijlp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Eisenbarth H, Alpers GW, Segrè D, Calogero A, Angrilli A. Categorization and evaluation of emotional faces in psychopathic women. Psychiatry Research. 2008;159(1–2):189–195. doi: 10.1016/j.psychres.2007.09.001. doi: 10.1016/j.psychres.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Jetton JG, Tsuang MT. Attention deficit disorder and conduct disorder: longitudinal evidence for a familial subtype. Psychological Medicine. 1997;27(2):291–300. doi: 10.1017/s0033291796004515. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive pavlovian conditioning in psychopaths: peripheral and central correlates. Psychophysiology. 2002;39:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC, Dindo L. Dual-deficit model of psychopathy. In: Patrick CJ, editor. Handbook of Psychopathy. The Guilford Press; New York, NY: 2006. pp. 14–34. [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. Errors, conflicts, and the brain. Current opinions on performance monitoring. 2004:14–20. [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010;47(1):123–132. doi: 10.1111/j.1469-8986.2009.00876.x. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56(7):516–521. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hare RD. Manual for The Hare Psychopathy Checklist-Revised. 2nd ed. Multi-Health Systems; Toronto: 2003. [Google Scholar]
- Harenski CL, Kim SH, Hamann S. Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(1):1–15. doi: 10.3758/CABN.9.1.1. doi: 10.3758/cabn.9.1.1. [DOI] [PubMed] [Google Scholar]
- Harper J, Malone SM, Bernat EM. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD, Hakstian AR. Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment. 1989;1(1):6–17. [Google Scholar]
- Hiatt KD, Newman JP. The Cognitive Side. In: Patrick CJ, editor. Handbook of psychopathy. The Guilford Press; New York, NY: 2006. pp. 334–352. [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59(8):750. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Junghoefer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38(2):175–178. [PubMed] [Google Scholar]
- Justus AN, Finn PR. Startle modulation in non-incarcerated men and women with psychopathic traits. Personality and Individual Differences. 2007;34(8):2057–2071. doi: 10.1016/j.paid.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biological Psychiatry. 2006;59(7):625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K, Aggen SH, Patrick CJ. Familial influences on conduct disorder reflect 2 genetic factors and 1 shared environmental factor. Archives of General Psychiatry. 2012 doi: 10.1001/jamapsychiatry.2013.267. doi: 10.1001/jamapsychiatry.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37(9):989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-6. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. Karolinska directed emotional faces (KDEF) Dept. of Neurosciences; Stockholm, Schweden: 1998. [Google Scholar]
- Lykken DT. The antisocial personalities. Lawrence Erlbaum; Hillsdale: 1995. [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Archives of General Psychiatry. 1993;50(7):565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- Munro GES, Dywan J, Harris GT, McKee S, Unsal A, Segalowitz SJ. ERN varies with degree of psychopathy in an emotion discrimination task. Biological Psychology. 2007;76(1–2):31–42. doi: 10.1016/j.biopsycho.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LA, Bernat EM, Cole A, Anokhin A. Genetic influences of neural correlates of emotional processing: Affective picture-related ERPs in female twins. International Journal of Psychophysiology. 2012;85(3):401. [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27(1):55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Back to the future: Cleckley as a guide to the next generation of psychopathy research. In: Patrick CJ, editor. Handbook of psychopathy. Guilford Press; New York: 2006. pp. 605–617. [Google Scholar]
- Patrick CJ, Bernat EM. Neurobiology of psychopathy: A two-process theory. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. John Wiley & Sons; New York: 2009. pp. 1110–1131. [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102(1):82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009;21(Special Issue 03):913–938. doi: 10.1017/S0954579409000492. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Hicks BM, Krueger RF, Lang AR. Relations between psychopathy facets and externalizing in a criminal offender sample. Journal of Personality Disorders. 2005;19(4):339–356. doi: 10.1521/pedi.2005.19.4.339. doi: 10.1521/pedi.2005.19.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A Construct-Network Approach to Bridging Diagnostic and Physiological Domains: Application to Assessment of Externalizing Psychopathology. Journal of Abnormal Psychology. doi: 10.1037/a0032807. in press. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC. Standard Progressive Matrices. H.K.Lewis & Co; London: 1958. [Google Scholar]
- Russ MO. Klinische Diagnostik mit dem Frankfurter Neurologischen Testprofil (FNTP) Klinik fuer Neurologie im Klinikum der J. W. Goethe-Universität; Frankfurt am Main: 2003. [Google Scholar]
- Schupp HT, Junghoefer M, Weike AI, Hamm AO. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. [Journal Peer Reviewed Journal] Neuroreport: For Rapid Communication of Neuroscience Research. 2003;14(8):1107–1110. doi: 10.1097/00001756-200306110-00002. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Ohman A, Junghoefer M, Weike AI, Stockburger J, Hamm AO. The Facilitated Processing of Threatening Faces: An ERP Analysis. Emotion. 2004;4(2):189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Sprague J, Javdani S, Sadeh N, Newman JP, Verona E. Borderline personality disorder as a female phenotypic expression of psychopathy? Personality Disorders: Theory, Research, and Treatment. 2012;3(2):127–139. doi: 10.1037/a0024134. doi: 10.1037/a0024134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvers PD, Brennan PA, Lilienfeld SO. Psychopathic Traits and Preattentive Threat Processing in Children. Psychological Science. 2011;22(10):1280–1287. doi: 10.1177/0956797611420730. doi: 10.1177/0956797611420730. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJB. Theta EEG dynamics of the error-related negativity. Clinical Neurophysiology. 2007;118(3):645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Hall JR, Patrick CJ, Bernat EM. Clarifying the Role of Defensive Reactivity Deficits in Psychopathy and Antisocial Personality Using Startle Reflex Methodology. Journal of Abnormal Psychology. 2011;120(1):253–258. doi: 10.1037/a0021224. doi: 10.1037/a0021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009;46(1):75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Sprague J, Sadeh N. Inhibitory Control and Negative Emotional Processing in Psychopathy and Antisocial Personality Disorder. Journal of Abnormal Psychology. 2012;121(2):498–510. doi: 10.1037/a0025308. doi: 10.1037/a0025308. [DOI] [PubMed] [Google Scholar]
- Verona E, Vitale JE. Psychopathy in Women. In: Patrick CJ, editor. Handbook of Psychopathy. The Guilford Press; New York: 2006. pp. 415–436. [Google Scholar]
- Vitale JE, Smith SS, Brinkley CA, Newman JP. The reliability and validity of the Psychopathy Checklist-Revised in a sample of female offenders. Criminal Justice and Behavior. 2002;29(2):202–231. doi: 10.1177/0093854802029002005. [Google Scholar]
- Wilson K, Juodis M, Porter S. Fear and Loathing in Psychopaths: a Meta-Analytic Investigation of the Facial Affect Recognition Deficit. Criminal Justice and Behavior. 2011;38(7):659–668. doi: 10.1177/0093854811404120. [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Research: Neuroimaging. 2009;174(2):81–88. doi: 10.1016/j.pscychresns.2009.03.012. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, Bernat EM, McGue M. The effects of childhood disruptive disorder comorbidity on P3 event-related brain potentials in preadolescents with ADHD. Biological Psychology. 2008;79(3):329–336. doi: 10.1016/j.biopsycho.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;22(2):590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]


