Abstract
Background
Perioperative cardio-pulmonary arrests are uncommon and little is known about rates and predictors of in-hospital survival.
Methods
Using the Get-With-The-Guidelines – Resuscitation national cardiopulmonary resuscitation registry, we identified all patients aged 18 years or older who experienced an index, pulseless cardiac arrest in the operating room or within 24 hours postoperatively. The primary outcome was survival to hospital discharge, and the secondary outcome was neurologically intact recovery among survivors. Multivariable logistic regression models using generalized estimating equation models were used to identify independent predictors of survival and neurologically intact survival.
Results
There were 2,524 perioperative cardiopulmonary arrests identified from 234 hospitals. The overall rate of survival to discharge was 31.7% (799/2,524), including 41.8% (254/608) for ventricular tachycardia and ventricular fibrillation, 30.5% (296/972) for asystole, and 26.4% (249/944) for pulseless electrical activity. Ventricular fibrillation and pulseless ventricular tachycardia were independently associated with improved survival. Asystolic arrests occurring in the operating room and post-anesthesia care unit were associated with improved survival when compared to other perioperative locations. Among patients with neurological status assessment at discharge, the rate of neurologically intact survival was 64.0% (473/739). Pre-arrest neurological status at admission, patient age, inadequate natural airway, pre-arrest ventilatory support, duration of event and event location were significant predictors of neurological status at discharge.
Conclusion
Among patients with a perioperative cardiac arrest, 1 in 3 survived to hospital discharge, and good neurological outcome was noted in 2 out of 3 survivors.
Introduction
Perioperative cardio-pulmonary arrests are uncommon events, and their morbidity and mortality has not been well studied. The early postoperative period poses additional risks to patients due the proximate nature of anesthesia and surgical insults. Thus, several features of perioperative cardiac arrests are unique.1 As with other specialized areas, such as the emergency room,2 perioperative events may differ from cardiac arrests elsewhere in the hospital in terms of resuscitation response times and underlying etiology. Thus, survival outcomes may be different in these locations than those seen on general in-patient units.
In prior studies, survival rates from perioperative arrests3–5 were higher than those reported in large multicenter in-hospital arrest studies.6 Other studies have reported on the incidence and risk factors for perioperative cardiac arrests,3–5 but these have typically been single-institution studies with small sample sizes (the largest study population of 223 patients4), raising the question of generalizability. There remain significant limitations in our knowledge of perioperative arrests. For instance, no prior study has described outcomes for cardiac arrests occurring in the early postoperative period or variability of survival in different postoperative locations. Such information may be important for anesthesiologists who are often involved in the decision-making for the postoperative disposition of patients (floor status vs. telemetry vs. intensive care). In addition, the relationship between process-of-care measures (e.g., time to epinephrine, intubation, and defibrillation) and outcomes in the perioperative setting is scant.
To better address these gaps in knowledge related to perioperative cardiac arrests, we set out to study the presentation, management and outcomes of arrests occurring in the operating room and the postoperative period within 24 hours of surgery.
Materials and Methods
Study design
To achieve the study goals, we analyzed data from the multicenter Get With The Guidelines –Resuscitation (GWTG-Resuscitation, formerly known as the National Registry for Cardiopulmonary Resuscitation)7 database, an American Heart Association sponsored prospective, multi-site, observational registry, because of its detailed collection of measures of care and outcomes for in-hospital cardiac arrests. The members of the American Heart Association GWTG-Resuscitation Investigators are listed in appendix 1. The study design of the GWTG-R has been described previously in detail.6 Briefly, a resuscitation event is defined as a pulseless cardiopulmonary arrest that requires chest compressions and/or defibrillation. Data abstraction for each cardiac arrest is performed by trained personnel at each participating institution.7 Data accuracy within the GWTG-R is ensured through periodic chart review, and the mean error rate has been previously reported to be less than 2.4% for all data.8 To allow for comparative analyses across multiple sites, data elements within the registry are standardized using Utstein-style definitions to ensure uniformity of data collection.9 Oversight of data collection and analysis, integrity of the data, and research is provided by the American Heart Association.
The registry is currently the largest repository of information on in-hospital cardiopulmonary arrest from over 400 participating hospitals.10 Because the GWTG-Resuscitation data are de-identified and already exist, need for consent was waived by the Adult Research Task Force of the National Registry of Cardiopulmonary Resuscitation and the Executive Database Steering Committee of the American Heart Association.
Patient population
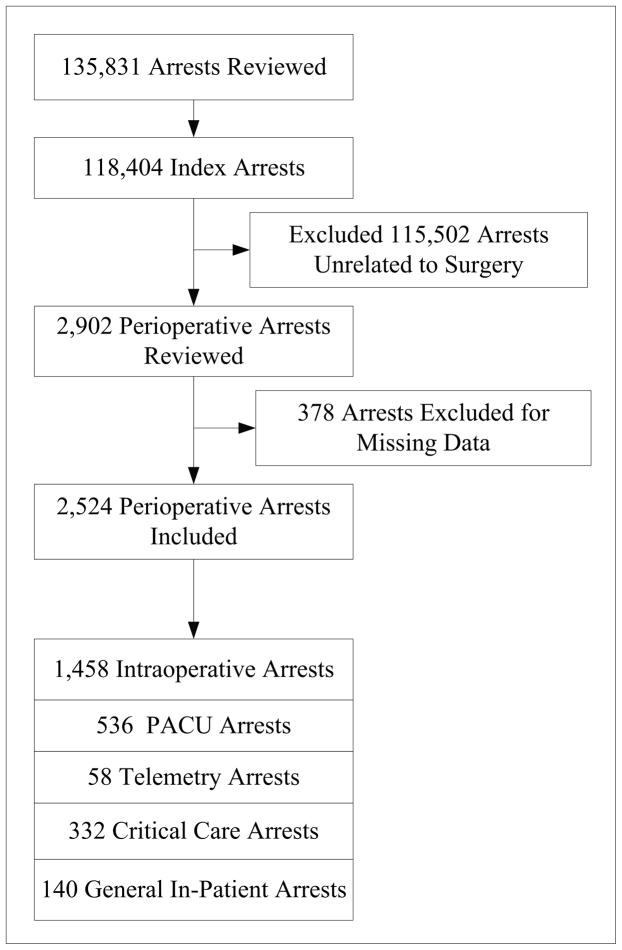
Of 118,404 patients aged 18 years or older who experienced an index, pulseless cardiac arrest from February 24, 2000 to August 3, 2008, we excluded 115,502 patients because their cardiac arrest did not occur in the operating room (OR), post-anesthesia care unit (PACU) or any locations, within 24 hours after leaving the PACU care (Fig 1). An additional 378 were excluded due to missing data on first pulseless rhythm or survival outcomes. Our final study cohort was comprised of 2,524 patients with perioperative cardiac arrests.
Figure 1.
Study outcomes
The primary outcome measure was survival to hospital discharge. We examined as a secondary outcome measure neurologically intact survival among patients surviving to hospital discharge. Neurological outcome was assessed using previously described cerebral performance category (CPC) scores,6 which describes patients as having no major disability (CPC=1), moderate disability (CPC=2), severe disability (CPC=3), and coma or vegetative state (CPC=4). For this study, we dichotomized patients as having neurologically intact survival (CPC=1) or survival with neurological disability (CPC>1). Of the patients who survived to hospital discharge (survivors), only those with both admission and discharge CPC recorded (n=663 [82.9% of survivors]) were included in multivariate analyses of predictors of neurologically intact survival.
Statistical analysis
Baseline patient characteristics were compared between survivors and non-survivors with Pearson’s chi-square test for discrete variables, t-test for normally distributed continuous data and Wilcoxon rank sum test for non-normally distributed variables.
We then constructed separate multivariable models to identify predictors of survival to discharge and neurologically intact survival. Variables with a univariate association with survival (p<0.10) were considered for model inclusion. Candidate patient-level variables included: admitting diagnosis (medical, cardiac; medical, non-cardiac; surgical, cardiac; or surgical, non-cardiac) and presence or absence of coexisting medical conditions at the time of cardiac arrest (respiratory, renal, or hepatic insufficiency; congestive heart failure, metabolic or electrolyte derangements; pneumonia; neurological disorders; shock; sepsis; major trauma, or cancer). Additionally, we controlled for variables related to the cardiac arrest, including initial cardiac rhythm (asystole, pulseless electrical activity [PEA], pulseless ventricular tachycardia [PVT] or ventricular fibrillation [VF]), duration of cardiac arrest, time of cardiac arrest (during work hours or during after-hours periods [i.e., 5 p.m. to 8 a.m.], and weekend events. Consistent with previous literature8,10 shockable rhythms PVT and VF were analyzed together as one rhythm type. For models assessing neurologically intact survival among survivors, we also included as a binary covariate pre-arrest neurological status (baseline CPC score 1 vs. other CPC score). As the data in the GWTG-R database is derived from multiple sites of differing volume, all models used generalized estimating equation methodology with an exchangeable-correlation matrix to control for patient clustering at the facility level. Collinearity was evaluated on all pairs of variables to assess for independence. The magnitudes of the standard errors were used as additional measures of collinearity. The model test of significance was used to investigate model performance. The resultant chi-square statistic value is a measure of the relationship between observed and expected frequencies. A p-value of <0.05 in this test denotes that the null hypothesis is rejected. If the test was significant, linear relationships between predictors and log odds of outcome for continuous variables were further investigated. Model overfitting was limited by ensuring >10 subjects per independent variable included in the model.
Secondary analyses were performed to explore the differences in outcomes relating to initial rhythm type and presenting location (intraoperative, post-anesthesia care unit, telemetry/step-down, intensive care unit or general inpatient area). For this, we assessed the unadjusted relationship between process-of-care measures (time to administration of epinephrine, invasive airway placement and defibrillation), event location and survival. Groups were compared using t-test for normally distributed continuous data and Wilcoxon rank sum test for non-normally distributed variables. Additionally, multivariate models were developed for evaluating risk factors for survival to discharge for each primary rhythm type. Finally, we examined the relationship between the comorbid disease burden (defined as the total number of preoperative comorbidities documented at the time of the arrest) and outcomes (survival and good neurological status, defined as a CPC 1). For this analysis, we compared increasing number of coexisting medical conditions with the binary outcomes of interest using Pearson’s chi-square test and Fischer’s exact test as appropriate. Statistical analyses were performed using Stata 10 (StataCorp LP, College Station, TX).
Missing data analyses were performed to compare between cases included and excluded from the general estimating equation models. Survival to hospital discharge (27.7% vs. 32.4%; p-value=0.056), admission CPC (66.9% vs. 63.1%; p-value=0.451) and discharge CPC (67.7% vs. 63.6%; p-value=0.517) were similar in the missing data and included patients’ groups. In order to explore and present the missing data’s potential influence on the estimates of risk, data imputation was performed using the following methodology. The number of missing values was assessed for key variables used in the analysis. Only missing data for the predictors were imputed. We employed multiple imputation with IVEware Version 0.1 (University of Michigan, Ann Arbor, MI) for missing data. IVEware is an imputation and variance estimation software that creates single or multiple imputations of missing values using the Sequential Regression Imputation Method. 11,12 IVEware also creates partial or full synthetic data sets using the sequential regression approach to protect confidentiality and limit statistical disclosure and can combine information from multiple sources by vertically concatenating data sets and multiply imputing the missing portions to create larger rectangular data sets. For our imputation, IVEware was used to impute data through SAS version 9.2 (SAS Inc., Cary, NC) was used. This approach allowed us to handle complex data structures that were created from a large number of variables with mixed formats (dichotomous, categorical, continuous, counts, etc.) For this analysis, 5 imputations were performed and the datasets were assembled into one dataset so analysis could be conducted. After imputation, Stata 10 was utilized to analyze multivariate models for major outcome measures using General Estimation Equation to account for clustering at the facility level. The same syntax was used to recreate the multivariate models after imputation to provide information on the influence of missing data on risk estimates.
Results
Of 2,524 patients from 234 hospitals, 1,458 (57.7%) had a cardiac arrest in the operating room and the rest had arrests in the postoperative setting (Fig 1). Return of spontaneous circulation occurred in 1,485 patients (58.7%), 1,151 patients (45.5%) survived to 24 hours after their cardiac arrest, and 799 (31.7%) survived to hospital discharge. Neurologically intact survival was observed in 473 (64.0%) of 739 survivors with valid CPC scores at discharge.
Results are clustered around key areas relevant to perioperative arrests listed below: primary arrest rhythm, location specific differences, patient-level associations, event-level associations, and neurological status at admission and outcomes. Univariate analyses are presented in Table 1. Multiple adjusted analyses presented in Table 2, Table 3 and appendices 2–7 are provided to compare estimates of risk factors before and after data imputation for missing variables. All estimates presented in the following sections refer to the post-imputation data analyses. Patients excluded due to any missing data were similar in baseline characteristics to patients in the final study cohort, except that the excluded patients had lower rates of previous myocardial infarction (4.1% vs. 6.8%, p=0.045), septicemia (5.4% vs. 9.0%; p=0.019), arrhythmia (50.8% vs. 59.3%; p=0.042) and metabolic derangement (5.1% vs. 10.0%, p= 0.001). Patients excluded for missing data on primary rhythm did not differ significantly from those without missing data with regard to survival to discharge (35.4% vs. 33.3%; p=0.362) and good neurological outcome (67.7% vs. 63.3%; p=0.306).
Table 1.
Baseline characteristics of perioperative cardiopulmonary arrests by survival to discharge status
| Characteristic | Survivors (n=799) | Non-survivors (n=1725) | P value | Unadjusted Odds Ratio [95%CI] | Missing Data n (%) |
|---|---|---|---|---|---|
| Age | 63.2 ± 15.7 | 65.1 ± 18.1 | <0.001 | - | 0 (0) |
| Male Sex | 446 (55.8) | 980 (56.8) | 0.640 | 1.0 [0.9, 1.1] | 6(0.2) |
| White Race | 615 (82.1) | 1253 (77.7) | 0.014 | 0.3 [1.1, 1.6] | 168 (6.6) |
| First documented rhythm | |||||
| Asystole | 296 (37.0) | 676 (39.2) | 0.304 | 0.9 [0.8, 1.1] | 371 (12.8) |
| PEA | 249 (31.2) | 695 (40.3) | <0.001 | 0.7 [0.6, 0.8] | 371 (12.8) |
| PVT/VF | 254 (31.8) | 354 (20.5) | <0.001 | 1.6 [1.3, 2.0] | 371 (12.8) |
| Duration of event | 14.3 ±14.2 | 25.5 ±19.6 | <0.001 | - | 0 (0) |
| Length of hospital stay | 15.4 ±15.1 | 11.7 ±6.3 | <0.001 | - | 9 (0.3) |
| Admitting Diagnosis | |||||
| Medical, cardiac | 64 (8.1) | 98 (5.7) | <0.001 | 1.5 [1.0, 2.0] | 28 (0.9) |
| Medical, noncardiac | 60 (7.6) | 224 (13.1) | <0.001 | 0.5 [0.4, 0.7] | 28 (0.9) |
| Surgical, cardiac | 129 (16.4) | 229 (13.4) | 0.055 | 1.3 [1.0, 1.6] | 28 (0.9) |
| Surgical, noncardiac | 516 (65.6) | 994 (58.0) | 0.001 | 0.3 [1.1, 1.6] | 28 (0.9) |
| Time of cardiac arrest | |||||
| After hours | 67 (8.4) | 324 (18.8) | <0.001 | 0.4 [0.3, 0.5] | 0 (0) |
| Weekend | 88 (11.0) | 381 (22.1) | <0.001 | 0.4 [0.3, 0.6] | 0 (0) |
| Event location | |||||
| Operating room | 455 (56.9) | 1003 (58.1) | 0.571 | 1.0 [0.8, 1.1] | 0 (0) |
| Post-Anesthesia Care Unit | 214 (26.8) | 32 (18.8) | <0.001 | 1.5 [1.2, 1.9] | 0 (0) |
| Intensive care area | 76 (9.5) | 256 (14.8) | <0.001 | 0.6 [0.5, 0.8] | 0 (0) |
| Telemetry/step-down | 20 (2.5) | 38 (2.1) | 0.573 | 0.2 [0.7, 2.1] | 0 (0) |
| General inpatient area | 34 (4.3) | 106 (6.1) | 0.054 | 0.7 [0.5, 1.0] | 0 (0) |
| Coexisting medical conditions | |||||
| Congestive heart failure at admission | 55 (6.9) | 175 (10.1) | 0.008 | 0.7 [0.5, 0.9] | 0 (0) |
| Previous congestive heart failure | 117 (14.6) | 284 (16.4) | 0.245 | 0.9 [0.7, 1.1] | 0 (0) |
| Myocardial infarction at admission | 81 (10.1) | 165 (9.6) | 0.652 | 1.0 [0.8, 1.4] | 0 (0) |
| Previous myocardial infarction | 130 (16.3). | 280 (16.2) | 0.981 | 1.0 [0.8, 1.3] | 0 (0) |
| Respiratory insufficiency | 228 (28.5) | 655 (38.0) | <0.001 | 0.7 [0.5, 0.8] | 0 (0) |
| Renal insufficiency | 151 (18.9) | 483 (28.0) | <0.001 | 0.6 [0.5, 0.7] | 0 (0) |
| Hepatic insufficiency | 20 (2.5) | 81 (4.7) | 0.009 | 0.5 [0.3, 0.9] | 0 (0) |
| Metabolic or electrolyte derangement | 60 (7.5) | 263 (15.2) | <0.001 | 0.5 [0.3, 0.6] | 0 (0) |
| Baseline neurological deficits | 45 (5.6) | 184 (10.7) | <0.001 | 0.5 [0.4, 0.7] | 0 (0) |
| Acute stroke | 19 (2.4) | 44 (2.6) | 0.796 | 0.9 [0.5, 1.6] | 0 (0) |
| Acute non-stroke neurological disorder | 32 (4.0) | 106 (6.1) | 0.028 | 0.6 [0.4, 1.0] | 0 (0) |
| Pneumonia | 26 (3.3) | 82 (4.8) | 0.083 | 0.7 [0.4, 1.1] | 0 (0) |
| Sepsis | 35 (4.4) | 194 (11.2) | <0.001 | 0.4 [0.3, 0.5] | 0 (0) |
| Shock | 188 (23.5) | 708 (41.0) | <0.001 | 0.4 [0.4, 0.5] | 0 (0) |
| Major trauma | 42 (5.3) | 233 (13.5) | <0.001 | 0.4 [0.3, 0.5] | 0 (0) |
| Cancer | 75 (9.4) | 214 (12.4) | 0.027 | 0.7 [0.6, 1.0] | 0 (0) |
| Immediate cause of arrest | |||||
| Active myocardial infarction | 40 (5.0) | 129(7.5) | 0.021 | 0.7 [0.5, 1.0] | 14 (0.5) |
| Arrhythmia | 528 (66.4) | 959 (55.9) | <0.001 | 1.6 [1.3, 1.9] | 14 (0.5) |
| Hypotension/hypoperfusion | 290 (36.5) | 1025 (59.7) | <0.001 | 0.4 [0.3, 0.5] | 14 (0.5) |
| Acute pulmonary edema | 6 (0.8) | 30 (1.7) | 0.051 | 0.4 [0.2, 1.0] | 14 (0.5) |
| Acute pneumothorax | 6 (1.0) | 14 (1.1) | 0.800 | 0.9 [0.3, 2.3] | 744 (25.6) |
| Acute pulmonary embolism | 14 (1.8) | 47 (2.7) | 0.139 | 0.6 [0.4, 1.2] | 14 (0.5) |
| Acute respiratory insufficiency | 227 (28.6) | 513 (29.9) | 0.493 | 0.9 [0.8, 1.1] | 14 (0.5) |
| Inadequate invasive airway | 13 (1.6) | 38 (2.2) | 0.339 | 0.7 [0.4, 1.4] | 14 (0.5) |
| Inadequate natural airway | 36 (4.5) | 37 (2.2) | 0.001 | 2.2 [1.4, 3.4] | 14 (0.5) |
| Invasive airway displacement | 6 (0.8) | 10 (0.6) | 0.614 | 0.3 [0.5, 3.6] | 14 (0.5) |
| Metabolic abnormality | 32 (4.0) | 218 (12.7) | <0.001 | 0.3 [0.2, 0.4] | 14 (0.5) |
| Invasive ventilation during or prior arrest | 713 (89.2) | 1688 (97.9) | <0.001 | 0.2 [0.1, 0.3] | 0 (0) |
| Neurological status at admission | |||||
| No major disability | 566 (78.2) | 865 (57.1) | <0.001 | 2.7 [2.2, 3.3] | 92 (3.1) |
| Moderate disability | 115 (15.9) | 394 (26.0) | <0.001 | 0.5 [0.4, 0.7] | 92 (3.1) |
| Severe disability | 36 (5.0) | 148 (9.8) | <0.001 | 0.5 [0.3, 0.7] | 92 (3.1) |
| Coma or vegetative state | 7 (1.0) | 109 (7.2) | <0.001 | 0.1 [0.1, 0.3] | 92 (3.1) |
CI confidence intervals, PEA pulseless electrical activity, PVT/VF pulseless ventricular tachycardia or ventricular fibrillation
Table 2.
Independent predictors of survival to discharge and good neurological outcome in perioperative arrests
| Survival to Discharge | Good Neurological Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-imputation | Post-imputation for missing data | Pre-imputation | Post-imputation for missing data | |||||
| Risk factor | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value |
| Acute non-stroke neurological event | 1.02 [0.68, 1.53] | 0.918 | 1.08 [0.72, 1.62] | 0.712 | 0.90 [0.29, 2.78] | 0.855 | 0.94 [0.35, 2.52] | 0.909 |
| Baseline depression in neurological status | 0.64 [0.41, 1.01] | 0.055 | 0.71 [0.47, 1.06] | 0.095 | 0.46 [0.21, 1.00] | 0.05 | 0.61[0.27, 1.41] | 0.249 |
| Congestive heart failure during admission | 0.58 [0.41, 0.83] | 0.003 | 0.59 [0.41, 0.85] | 0.005 | 1.07 [0.37, 3.04] | 0.905 | 1.03 [0.44, 2.42] | 0.941 |
| Hepatic insufficiency | 0.62 [0.36, 1.08] | 0.092 | 0.66 [0.38, 1.15] | 0.142 | 0.83 [0.15, 4.56] | 0.832 | 0.77 [0.25, 2.36] | 0.653 |
| Hypotension/hypoperfusion | 0.54 [0.43, 0.67] | <0.001 | 0.51 [0.41, 0.63] | <0.001 | 0.71 [0.43, 1.19] | 0.198 | 0.84 [0.56, 1.26] | 0.387 |
| Metastatic or hematological malignancy | 0.53 [0.38, 0.74] | <0.001 | 0.63 [0.47, 0.83] | 0.001 | 0.73 [0.36, 1.47] | 0.378 | 0.86 [0.52, 1.43] | 0.568 |
| Metabolic and electrolyte abnormality | 0.67 [0.44, 1.02] | 0.064 | 0.64 [0.43, 0.96] | 0.029 | 1.90 [0.79, 4.55] | 0.152 | 1.38 [0.79, 2.43] | 0.257 |
| Renal insufficiency | 0.69 [0.53, 0.88] | 0.003 | 0.66 [0.52, 0.85] | 0.001 | 0.63 [0.32, 1.22] | 0.171 | 0.64 [0.38, 1.08] | 0.093 |
| Respiratory failure | 0.82 [0.65, 1.03] | 0.089 | 0.91 [0.73, 1.13] | 0.401 | 0.90 [0.51, 1.59] | 0.712 | 0.94 [0.58, 1.54] | 0.812 |
| Septicemia | 0.46 [0.28, 0.74] | 0.002 | 0.45 [0.28, 0.72] | 0.001 | 0.24 [0.07, 0.84] | 0.026 | 0.47 [0.16, 1.40] | 0.177 |
| Active or evolving myocardial infarction | 0.67 [0.42, 1.07] | 0.097 | 0.58 [0.36, 0.93] | 0.025 | 1.19 [0.49, 2.84] | 0.704 | 1.05 [0.49, 2.26] | 0.899 |
| Inadequate natural airway | 2.39 [1.20, 4.76] | 0.013 | 1.79 [1.00, 3.22] | 0.051 | 0.48 [0.20, 1.19] | 0.113 | 0.44 [0.21, 0.92] | 0.029 |
| Arrhythmia | 1.42 [1.17, 1.73] | <0.001 | 1.37 [1.13, 1.66] | 0.001 | 0.85 [0.54, 1.35] | 0.5 | 0.78 [0.54, 1.13] | 0.195 |
| After-hours | 0.56 [0.42, 0.88] | 0.001 | 0.61 [0.52, 0.8] | <0.001 | 1.32 [0.43, 4.00] | 0.626 | 0.68 [0.28, 1.62] | 0.38 |
| Arrest Rhythm (PEA reference) | ||||||||
| Asystole | 1.00 [0.77, 1.31] | 0.993 | 0.97 [0.78, 1.21] | 0.78 | 0.79 [0.43, 1.45] | 0.449 | 0.94 [0.63, 1.39] | 0.75 |
| PVT/VF (Shockable rhythms) | 1.60 [1.16, 2.20] | 0.004 | 1.54 [1.20, 1.98] | 0.001 | 0.91 [0.49, 1.70] | 0.76 | 1.04 [0.67, 1.61] | 0.866 |
| White vs. non-white | 1.22 [0.94, 1.57] | 0.135 | 1.05 [0.84, 1.33] | 0.663 | 1.73 [0.94, 3.18 | 0.081 | 1.52 [0.94, 2.47] | 0.088 |
| Event location (General inpatient unit – reference) | ||||||||
| Operating room | 1.38 [0.83, 2.30] | 0.215 | 1.07 [0.69, 1.66] | 0.758 | 5.77 [1.74, 19.10] | 0.004 | 1.64 [1.16, 4.39] | 0.041 |
| PACU | 2.03 [1.16, 3.56] | 0.013 | 1.57 [0.97, 2.52] | 0.064 | 5.64 [1.77, 17.95] | 0.003 | 1.72 [1.07, 4.55] | 0.049 |
| Telemetry | 1.22 [0.50, 2.99] | 0.658 | 1.02 [0.46, 2.27] | 0.958 | 2.21 [0.62, 7.95] | 0.223 | 0.93 [0.23, 3.74] | 0.919 |
| Intensive care area | 0.96 [0.52, 1.77] | 0.894 | 0.70 [0.40, 1.21] | 0.2 | 6.79 [1.77, 26.05] | 0.005 | 1.72 [0.51, 5.85] | 0.383 |
| Weekend | 0.62 [0.45, 0.86] | 0.004 | 0.53 [0.39, 0.71] | <0.001 | 1.65 [0.73, 3.74] | 0.229 | 1.15 [0.64, 2.08] | 0.637 |
| Invasive ventilation in place pre- arrest | 0.28 [0.17, 0.45] | <0.001 | 0.44 [0.33, 0.59] | <0.001 | 0.08 [0.03, 0.21] | <0.001 | 0.59 [0.35, 0.99] | 0.046 |
| Illness Category (Medical non cardiac - reference value) | ||||||||
| Medical cardiac | 2.04 [1.13, 3.72] | 0.019 | 1.91 [1.15, 3.19] | 0.013 | 1.66 [0.58, 4.79] | 0.349 | 2.27 [0.95, 5.39] | 0.064 |
| Surgical cardiac | 1.65 [1.03, 2.65] | 0.039 | 1.86 [1.22, 2.84] | 0.004 | 1.88 [0.70, 5.03] | 0.209 | 1.88 [0.87, 4.08] | 0.11 |
| Surgical non cardiac | 1.44 [1.00, 2.08] | 0.048 | 1.50 [1.09, 2.06] | 0.013 | 1.88 [0.83, 4.25] | 0.128 | 1.65 [0.87, 3.11] | 0.122 |
| Trauma | 0.20 [0.09, 0.46] | <0.001 | 0.25 [0.12, 0.51] | <0.001 | 4.56 [0.26, 81.53] | 0.302 | 3.69 [0.73, 18.70 | 0.115 |
| Age at hospital admission | 0.98 [0.97, 0.99] | <0.001 | 0.98 [0.97, 0.99] | <0.001 | 0.98 [0.96, 0.99] | 0.001 | 0.98 [0.97, 0.99] | 0.003 |
| Duration of event | 0.96 [0.95, 0.96] | <0.001 | 0.95 [0.95, 0.96] | <0.001 | 0.99 [0.97, 1.00] | 0.047 | 0.98 [0.97, 0.99] | 0.001 |
| Admission CPC score 1 | Not included | - | Not included | - | 48.84 [23.53, 101.38] | <0.001 | 21.69 [12.52, 37.57] | <0.001 |
CI confidence intervals, CPC cerebral performance category, PACU post-anesthesia care unit, PEA pulseless electrical activity, PVT/VF pulseless ventricular tachycardia or ventricular fibrillation
Table 3.
Summary of study end points and adjusted survival rates by primary arrest rhythm type
| End-point | PEA | Asystole | PVT/VF | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency (%) | AOR (95%CI) | Frequency (%) | AOR (95%CI) | p-value | Frequency (%) | AOR (95%CI) | p-value | |
| ROSC¶ | 533/944 (56.5%) | Reference | 551/972 (56.7%) | 1.0 (0.8, 1.2) | 0.901 | 401/608 (65.9%) | 1.2 (1, 1.6) | 0.106 |
| Survival to 24 hours | 395/944 (41.8%) | Reference | 436/972 (44.9%) | 1.0 (0.9, 1.3) | 0.772 | 320/608 (52.6%) | 1.3 (1.0, 1.7) | 0.032 |
| Survival to discharge | 249/944 (26.3%) | Reference | 296/972 (30.5%) | 1.0 (0.8, 1.2) | 0.78 | 254/608 (41.8%) | 1.5 (1.2, 2.0) | 0.001 |
| Good neurological outcome | 144/234 (61.5%) | Reference | 178/272 (65.4%) | 0.9 (0.6, 1.4) | 0.75 | 151/233 (64.8%) | 1.0 (0.7, 1.6) | 0.866 |
Adjusted Odds ratios are adjusted for acute non-stroke neurological event, baseline depression in neurological status, congestive heart failure during admission, hepatic insufficiency, hypotension, metastatic or hematological malignancy, metabolic and electrolyte abnormality, renal insufficiency, respiratory failure, septicemia, active or evolving myocardial infarction, inadequate natural airway, invasive ventilation, arrhythmia, time of day, weekend, location of arrest, admission diagnosis and age. Admission Cerebral Performance Category score was included in adjusted model for neurological outcome. Model comparisons for neurological outcomes were made between survivors discharged with no major disability and those with a moderate degree of disability or worse. AOR adjusted odds ratio, CI confidence intervals, PEA pulseless electrical activity, PVT/VF pulseless ventricular tachycardia or ventricular fibrillation, ROSC return of spontaneous circulation. See appendices 2–4 for full model outputs. Multiple imputations and bootstrapping were performed to uncover hidden estimates for covariates included in the model. Estimates provided in this table are derived from post-imputation adjusted analyses.
Primary Arrest Rhythm
Asystole was the most commonly encountered rhythm, but survivors were more likely to have a shockable initial cardiac arrest rhythm (31.8% vs. 20.5%; P<0.001) compared with non-survivors (Table 1). After adjusting for several patient-level and event-level variables, PVT/VF alone was significantly associated with survival to 24 hours post-arrest and survival to discharge (Table 2 and Table 3, appendices 2–3). Adjusted analyses of survival outcomes stratified by first documented pulseless rhythm are described in Table 3 (and appendices 4–6). There were no rhythm-specific differences in neurological outcomes on adjusted models (Table 2, appendix 7). In adjusted sub-analyses, arrest location, increasing age and longer duration of arrest were common independent associations with worse survival to discharge across all the three primary rhythms (appendices 4–6).
Location-Specific Differences
There were significant differences in survival and neurological outcome by hospital location of arrest. The majority of arrests occurred in the OR (1458/2524). Survival rates were highest in PACU arrests (214/536; 39.8%) followed by telemetry (20/58; 35.1%), OR arrests (455/185; 31.2%), general in-patient areas (34/140; 24.3%) and intensive care unit (ICU) locations (76/332; 23.0%). Arrests in the OR, PACU and ICU were associated with significantly shorter time to epinephrine, whereas OR and PACU arrests were associated with significantly shorter times to invasive airway placement. There were no location specific differences in the time to defibrillation (Table 4).
Table 4.
Relationship of event location, process of care measures and outcomes
| Time to epinephrine | Time to defibrillation | Time to invasive airway | |
|---|---|---|---|
| Event location | |||
| General Inpatient | 4 [0, 7] | 4 [1, 7] | 7 [5, 10] |
| OR | 0 [0, 2] | 1 [0, 3] | 2 [0, 5] |
| PACU | 1 [0, 3] | 1 [0, 4] | 3 [1, 6] |
| Telemetry | 3 [1, 5] | 2 [1, 3] | 8 [3, 10] |
| ICU | 0 [0, 3] | 1 [0, 2] | 8 [4, 10] |
| p-value | <0.001* | 0.06 | <0.001* |
| Event survival | |||
| Survived event | 0 [0, 3] | 0 [0, 3] | 4 [1, 7] |
| Non, survivors | 1 [0, 3] | 1 [1, 5] | 4 [1, 8] |
| p-value | 0.48 | 0.01* | 0.25 |
| 24 hour survival | |||
| Survived 24 hours | 0 [0, 3] | 0 [0, 3] | 3 [1, 7] |
| Non survivors | 1 [0, 3] | 1 [1, 5] | 5 [1, 8] |
| p-value | 0.28 | 0.01* | 0.03* |
| Survival to discharge | |||
| Survived to discharge | 0 [0, 3] | 0 [0, 3] | 3 [1, 7] |
| Non, survivors | 1 [0, 3] | 1 [1, 4] | 4 [1, 8] |
| p-value | 0.56 | 0.01* | 0.15 |
| Neurological outcome | |||
| Good neurological status | 1 [0, 3] | 1 [0, 2] | 3 [1, 6] |
| Neurological injury | 1 [0, 3] | 1 [0, 5] | 5 [2, 7] |
| p-value | 0.24 | 0.76 | 0.03* |
Median (25th, 75th centiles) values are displayed. Good neurological outcome refers to Cerebral Performance Score of 1. Asymptotic significances are displayed.
The significance level is 0.05. ICU intensive care unit, OR operating room, PACU post anesthesia care unit.
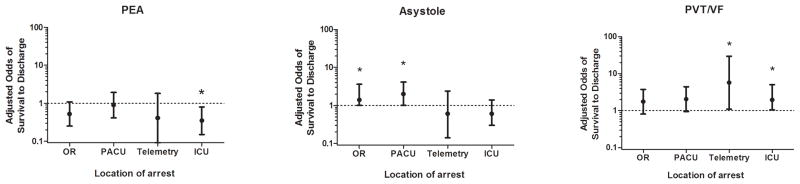
On adjusted analyses, arrest location was not associated with survival to discharge. However, significant location specific differences were observed in adjusted sub-analyses of survival to discharge within each arrest rhythm. Among patients exhibiting asystole as the first documented pulseless rhythm (appendix 5), intraoperative (adjusted odds ratio 1.4; 95% CI 1.0 to 2.6; p-value 0.047) and PACU (adjusted odds ratio 2.0; 95% CI 1.0 to 4.1; p-value 0.044) locations were significantly better survival to discharge compared to general inpatient locations (Fig 2). ICU location was associated with worse survival following PEA arrests (adjusted odds ratio 0.4; 95% CI 0.2 to 0.8; p-value 0.012). In contrast, telemetry (adjusted odds ratio 5.7; 95% CI 1.1 to 29.6; p-value 0.038) and ICU arrests (adjusted odds ratio 1.9; 95% CI 1.1 to 5.1; p-value 0.041) were significantly associated with improved survival following PVT/VF arrests in comparison with general inpatient arrests
Figure 2.
Intraoperative (adjusted odds ratio 1.6; 95% CI 1.2 to 4.4; p-value 0.041) and PACU (adjusted odds ratio 1.1; 95% CI 1.1 to 4.6; p-value 0.05) were significantly associated with better neurological outcomes in comparison to the events occurring in the general inpatient location (Table 2). On sensitivity analyses, after exclusion of patients with CPC scores >1, location was not associated with neurological outcome, suggesting that patient factors likely play a large role in neurological recovery from perioperative arrest.
Patient-Level Associations
The following variables were each associated with lower survival rates to discharge on univariate analyses (Table 1): Older age, congestive heart failure during admission (23.9% survival vs. 32.4% control survival; p=0.008), respiratory insufficiency (25.8% survival vs. 34.8% control survival; p<0.001), renal insufficiency (23.8% survival vs. 32.1% control survival; p=0.028), hepatic insufficiency (19.8% survival vs. 32.2% control survival; p=0.009), baseline depression in neurological status (19.7% survival vs. 32.9% control survival; p<0.001), acute non-stroke neurological event (23.2% survival vs. 32.1% control survival; p=0.028), septicemia (15.3% survival vs. 33.3% control survival; p<0.001), shock (21.0% survival vs. 37.5% control survival; p<0.001), major trauma (15.3% survival vs. 33.7% control survival; p<0.001), metabolic abnormality (18.6% survival vs. 33.6% control survival; p<0.001), and metastatic or hematological malignancy (26.0% survival vs. 32.4% control survival; p=0.027). While myocardial infarction at admission showed no relationship with survival, the survival rate was lower in those cases in which the arrest was specifically attributed to the myocardial infarction (23.7% survival vs. 32.2% control survival; p=0.021).
Arrests attributed to arrhythmia (35.5% survival vs. 26.1% control survival; p<0.001), inadequate natural airway (49.3% survival vs. 31.1% control survival; p=0.01), and white race (32.9% survival vs. 27.1% control survival; p<0.001) had higher rates of survival.
After multivariable adjustment, several patient-level characteristics and medical conditions were associated with lower survival from perioperative arrests (Table 2) including: age (adjusted odds ratio per additional year 0.98; 95% CI 0.96 to 0.99), congestive heart failure during current admission (adjusted odds ratio 0.6; 95% CI 0.4 to 0.9), shock (adjusted odds ratio 0.5; 95% CI 0.4 to 0.6), metabolic abnormality (adjusted odds ratio 0.6; 95% CI 0.4 to 0.9), metastatic or hematological malignancy (adjusted odds ratio 0.6; 95% CI 0.4 to 0.9), renal insufficiency (adjusted odds ratio 0.7; 95% CI 0.5 to 0.9), sepsis (adjusted odds ratio 0.5; 95% CI 0.3 to 0.7), active or evolving myocardial infarction (adjusted odds ratio 0.6; 95% CI 0.4 to 0.9) and trauma (adjusted odds ratio 0.3; 95% CI 0.1 to 0.5). Arrhythmic cause of arrest (adjusted odds ratio 1.4; 95% CI 1.1 to 1.7) was associated with higher adjusted odds of survival to discharge. Age was the only patient-level risk factor associated with both reduced survival to discharge and poor neurological outcome. Inadequate natural airway and admission neurological status were the other patient-level factors associated with neurological recovery (Table 2).
Event-Level Associations
Of the process of care measures, time to epinephrine administration was not related to any of the outcome measures (Table 4). Time to defibrillation was related to all survival end points except neurological outcomes in survivors. Time to invasive airway placement was related to 24-hour survival and neurological outcomes, but not survival to discharge.
After multivariable adjustment, the event-level variables associated with survival to discharge included duration of event (adjusted odds ratio 0.96; 95% CI 0.95 to 0.96; p-value <0.001), weekend arrests (adjusted odds ratio 0.5; 95% CI 0.4 to 0.7), after-hours arrests (adjusted odds ratio 0.6; 95% CI 0.5 to 0.8), and need for invasive ventilation (adjusted odds ratio 0.4; 95% CI 0.3 to 0.6). Duration of event (adjusted odds ratio 0.98; 95% CI 0.97 to 0.99) and need for invasive ventilation (adjusted odds ratio 0.6; 95% CI 0.4 to 0.9) were both associated with neurological outcomes on adjusted analyses.
Neurological Status at Admission and Outcomes
Admission CPC score of 1 was noted in 1,431 patients (among 2,240 patients with recorded admission CPC scores), of whom, 566 survived to hospital discharge. Of these survivors with admission CPC1, 445 patients (80%) had CPC1 at discharge. Thus 31.1% of patients who were admitted to hospital with CPC1 were discharged in a neurologically intact state following perioperative cardiac arrests. The strongest patient-level risk factor associated with neurologically intact survival was admission CPC score of 1 (Table 2- adjusted odds ratio 21.7; 95% CI 12.5 to 37.6).
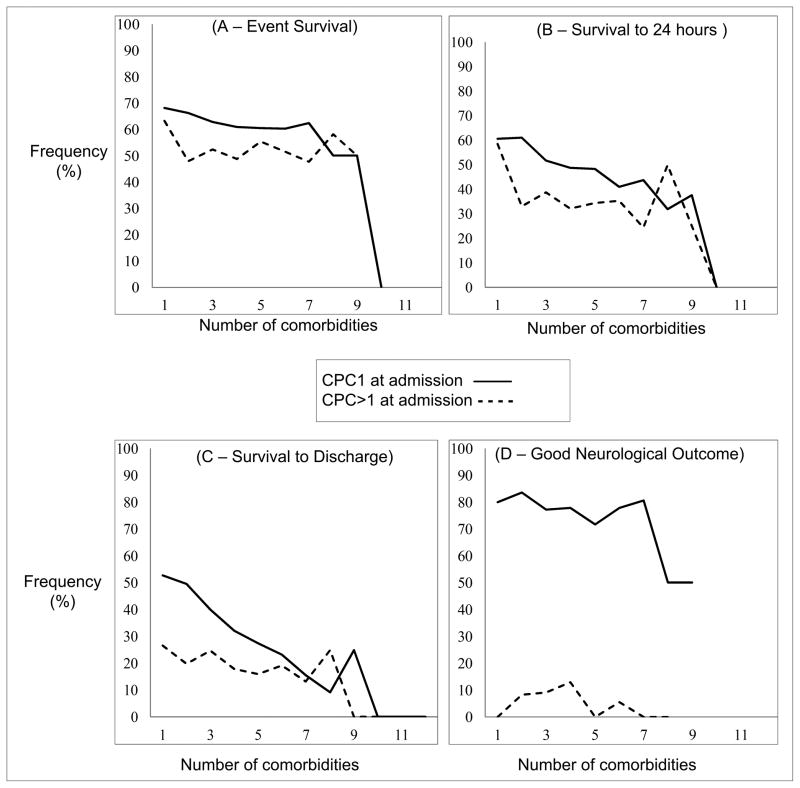
The relationship between the number of preoperative coexisting medical conditions and outcomes is described in Fig 3. Survival to discharge decreased with increasing neurologic disability at admission, from 39.6% (566/1,431) for those with no major disability, 22.6% (115/509) for those with moderate disability, 19.6% (36/184) for those with severe disability and 6% (7/116) for those in a coma or vegetative state (p-value <0.001). In patients with good baseline neurological status, number of coexisting medical conditions was associated with worse survival to discharge (p-value <0.001), worse survival at 24 hours (p-value 0.001), but not survival of event (p-value = 0.42) or neurological outcome among those surviving to hospital discharge (p-value = 0.64). In patients with pre-event neurological deficits, number of coexisting medical conditions was not associated with any of the outcome measures (event survival, p-value 0.76; 24 hour survival, p-value 0.19; survival to discharge, p-value = 0.39; good neurological outcome, p-value = 0.77).
Figure 3.
Discussion
In a large cohort of patients with perioperative cardiac arrests, we found that 1 in 3 patients survived to hospital discharge, representing a significantly higher survival rate than previous studies on in-hospital arrests on general hospital floors.6,13,14 Survival after arrests with shockable rhythms are significantly better than asystole and PEA arrests. Asystolic arrests in the OR and PACU were associated with better survival than asystolic arrests in other locations. Notably, location-specific differences in process-of-care measures were observed, suggesting variability in response times that may contribute to survival and neurological outcomes for perioperative patients with cardiac arrest.
The overall survival-to-discharge rates in this study are significantly higher than previous reports of in-hospital arrests from within the GWTG-Resuscitation database (15.3% –17%),2,6,13,14 but confirms previous reported rates on survival from perioperative arrests.4 One putative reason for this overall survival benefit seen in perioperative arrests is because surgical causes for cardiac arrest are more likely to be reversible. Alternatively, the immediate availability of physician led care in the operating room and the PACU could improve survival from cardiac arrest by influencing the speed and quality of response.2
Primary Arrest Rhythms
There were significantly higher survival rates across all primary rhythm types (asystole 30.5%, PEA 26.4%, and PVT/VF 41.8 %) compared to previous in-hospital GWTG-Resuscitation data (asystole 10%, PEA 10%, VF 34%, and PVT 35%).6 In particular, the high survival rates from asystolic perioperative arrests are a unique finding of the current study, reflecting potentially reversible causes. In a previous sub-analysis of 24 patients with anesthesia-attributed arrests, asystole was associated with a much higher survival rate (80% survival to discharge),4 related to a predominance of medication and airway causes. In addition, location specific differences in survival outcomes with asystole appear to support the view that immediate availability of anesthesia providers in the intraoperative and PACU locations may contribute significantly to improved outcomes in comparison with other locations. This association may be important especially in the context of the modifiable risk such as arrhythmic cause of asystolic arrest, which was observed to have improved survival. Thus, while asystole may indicate a dismal prognosis for out-of-hospital arrest, perioperative asystole in the perioperative setting should be treated aggressively. Intact survival appears to depend less on the presenting rhythm, and more on the etiology of arrest, timing of interventions, and quality of advanced cardiac life support.
Location Specific Differences
Significant location-specific differences have been described for time to invasive airway placement in overall in-hospital arrest literature.14 The first 24 hours after surgery are associated with a heightened risk of respiratory failure needing emergent airway intervention,13,15 suggesting that delays in definitive airway management may be of greater importance in perioperative arrest outcomes. In the current study, time to invasive airway placement was related to 24-hour survival and neurological outcomes, but not survival to discharge. The faster times to defibrillation, epinephrine administration and invasive airway placement seen in the operating room and PACU support the hypothesis that ready availability of skilled perioperative care directly impacts clinical response to cardiac arrest. It has been previously shown by others that defibrillation within 2 minutes of the arrest improves survival from PVT/VF arrests (39.3% vs. 22.2% for delayed defibrillation).8 In our study, only ~20% of the arrests were PVT/VF and survivors had shorter time to defibrillation. However, since trauma and shock were independently associated with worse survival to discharge with PVF/VT arrests, intraoperative PVT/VF arrests associated with these risk factors were less likely to be responsive to defibrillation. Thus, the operating room may be the one location where speed of defibrillation offers little benefit for the patient suffering from surgical exsanguination.10 This may also explain the higher survival seen with PVT/VF arrests in postoperative locations (telemetry, ICU) as they are generally less likely to encounter major exsanguination, and are more likely to have other causes of PVT/VF arrest.
Decisions on location of postoperative care may be extremely important, and our study provides some insight into the location-specific differences in outcomes of high-risk patients. The finding that neurological outcomes are independently better in telemetry or ICU patients compared to general inpatient areas supports the value of increased intensity of monitoring in high-risk patients in the postoperative period. There is evolving evidence that patient surveillance systems16 on general care units, but not just continuous pulse oximetry monitoring 17 may reduce morbidity. Our data suggests that the presence or immediate availability of skilled care (seen with PACU, ICU and to a lesser extent, telemetry beds) may be crucial in modifying survival from early postoperative cardiac arrests. Additional supportive evidence for this is the significantly shorter time to epinephrine administration and invasive airway placement seen in the operating room and PACU in comparison to general care locations.
Patient-Level and Event-level Associations
Previously, Peberdy et al found that survival outcomes were substantially lower during the night hours and the weekend,7 with the greatest effect-size noted for arrests in the operating room and postoperative care unit. Chan and colleagues also demonstrated that survival rates from in-hospital cardiac arrest were lower during nights and weekends, even when adjusted for potentially confounding patient-related, event-related, and hospital characteristics.8 In the current study, we identified lower odds of survival during night-time hours and weekends. It is possible that, in common with in-hospital arrests from other locations, other unmeasured system factors contribute to the observed time and day related differences in survival outcomes. This finding illustrates the limitations of therapy despite fairly intense one-on-one management with faculty anesthesiologist and surgeons.
Measures of acuity of response in the current study, namely time to invasive airway placement and epinephrine administration were associated with better survival rates. This finding suggests that anesthesiologists have the opportunity to modify several factors that can directly influence outcomes from arrests. On the other hand, the lower survival out of hours may reflect the lack of breadth of clinician resources and limited choices for delaying surgery due to greater severity of the primary surgical condition. These findings confirms Sprung’s data4 and mirror findings from the National Confidential Enquiry into Patient Outcome and Deaths.18 However, this finding needs to be viewed in the context of emerging evidence that elective general surgery is safe during late hours with careful provision for appropriate perioperative care19. Thus, it may be possible to modify survival outcomes in high-risk emergent surgical patients by preferentially scheduling such cases during more routine hours, ensuring more appropriate perioperative provider expertise, or both when feasible.20
Baseline Neurological Status and Outcomes
Finally, there are significant controversies that exist around suspension of do-not-resuscitate orders in patients presenting for surgery, as limited data exist on the relationship between preoperative comorbidities and perioperative cardiac arrest survival.21–23 In the current study, survival to discharge fell steeply with increasing neurologic disability at admission, from 39.6% for those with no major disability, 22.6% for those with moderate disability, 19.6% for those with severe disability and 6% for those in a coma or vegetative state. Among patients with baseline neurological deficits, the number of co-existing medical diagnoses did not change survival outcomes. In contrast, among patients with normal baseline neurological status, the total number of co-existing diagnoses was associated with worse survival to discharge, and worse survival at 24 hours, but the comorbid load bore no apparent relationship with neurological outcome among those surviving to hospital discharge. In other words, the risk of patient harm (facilitating survival, but with new neurologic deficits) is not increased among those patients with normal baseline neurologic status, even with an increasing comorbid disease burden. Therefore, the decision to suspend or retain do-not-resuscitate orders in the perioperative period should consider baseline neurologic status to a greater degree than the total number of coexisting medical diagnoses. The policy of routinely suspending do-not-resuscitate orders in the perioperative period may not be appropriate for all surgical patients, particularly those who are already in a coma or persistent vegetative state.
Our study findings should be interpreted in the context of the several limitations described in detail previously.10 First, the registry hospitals may not be representative of all United States hospitals and therefore our findings may not be generalizable, although they represent the best summary evidence available. Second, the GWTG-Resuscitation does not collect certain data that would have been informative for our analyses including: preoperative medications, intraoperative medications, physiologic data, and postoperative interventions. The variables designating cause of arrest were abstracted from the medical record by GWTG-Resuscitation research coordinators not involved in clinical care of the patient, and therefore, their accuracy has not been validated. The registry does not capture events that do not elicit a hospital response the use of a crash cart, and thus cardiac procedural and operative arrests may be treated as routine and may not be part of this registry.
In conclusion, 1 in 3 patients survived to hospital discharge after perioperative cardiac arrests and 2 of 3 survivors had neurologically intact survival. The relatively high rates of survival and good neurological outcomes following intraoperative and PACU arrests suggests that perioperative factors and immediate availability of skilled anesthesia care have direct influence on improved survival outcomes.
Summary Statement.
What We Already Know about This Topic
Perioperative cardiopulmonary arrests are uncommon events, and their morbidity and mortality has not been well studied
Using the Get-With-The-Guidelines –Resuscitation national cardiopulmonary resuscitation registry, this study determined the presentation, management and outcomes of arrests occurring in the operating room and the postoperative period within 24 h of surgery
What This Article Tells Us That Is New
Among patients with a perioperative cardiac arrest, one in three survived to hospital discharge, and good neurological outcome was noted in two out of three survivors
Acknowledgments
Financial support: Supported by the Department of Anesthesiology, The University of Michigan Health System, Ann Arbor, Michigan. The American Heart Association, Dallas, Texas, USA provided funding for the collection and management of the National Registry of Cardiopulmonary Resuscitation database. The Adult Research Task Force of the National Registry of Cardiopulmonary Resuscitation and the Executive Database Steering Committee of the American Heart Association approved the study protocol and the final version of the manuscript. The senior author (Dr. Chan) is a member of the Get With The Guidelines-Resuscitation Investigators and is funded by the National Institute of Health, Bethesda, Maryland, USA through K23#L102224.
Appendix 1
Along with Paul S. Chan, MD, Assistant Professor of Cardiology, Saint Luke’s Mid America Heart Institute, Kansas City, Missouri, USA the American Heart Association Get with the Guidelines (formerly NRCPR) Investigators are:
Emilie Allen, MSN, RN, Manager of Clinical Education, Parkland Health & Hospital System, Dallas, Texas, USA;
Robert A. Berg, MD, Professor of Anesthesiology and Critical Care Medicine and Pediatrics, the Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA;
Scott Braithwaite, MD, MSc, Associate Professor of Department of Population Health, New York University School of Medicine, New York, New York, USA;
Dana P. Edelson, MD, MS, Assistant Professor of Hospital Medicine, University of Chicago Medical Center, Chicago, Illinois, USA;
Kathy Duncan, RN, Faculty, Institute for Healthcare Improvement, Cambridge, Massachusetts, USA;
Brian Eigel, PhD, Member of Statistics Committee, American Heart Association, Dallas, Texas, USA;
Romergryko G. Geocadin, MD, Associate Professor of Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA;
Elizabeth A. Hunt, MD, MPH, PhD, Assistant Professor of Anesthesiology and Critical Care Medicine, Johns Hopkins Medicine Simulation Center, Baltimore, Maryland, USA;
Timothy J. Mader, MD, Clinical Professor of Emergency Medicine, Tufts University, Boston, Massachusetts, USA;
Karl B. Kern, MD, Professor of Medicine, University of Arizona Medical Center, Tucson, Arizona, USA; Mary E. Mancini, RN, PhD, Professor & Chairperson-College of Nursing, University of Texas at Arlington, Texas, USA;
Vincent N. Mosesso Jr., MD, Associate Professor of Emergency Medicine, University of Pittsburgh, Pennsylvania, USA;
Vinay M. Nadkarni, MD, Associate Professor of Anesthesia and Pediatrics, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA;
Graham Nichol, MD, MPH, Professor of Medicine, University of Washington, Seattle, Washington, USA;
Samuel A. Warren, MD, Hospitalist Physician, University of Washington, Seattle, Washington, USA;
Comilla Sasson, MD, MS, Assistant Professor of Emergency Medicine, University of Colorado, Aurora, Colorado, USA;
Mary Ann Peberdy, MD, Professor of Cardiology, Virginia Commonwealth University Health System, Richmond, Virginia, USA;
Joseph P. Ornato, MD, Professor of Emergency Medicine, Virginia Commonwealth University Health System, Richmond, Virginia, USA.
Appendix 2: General Estimating Equation models to predict event survival with and without data imputation for missing data
| Pre-imputation | Post-imputation for missing data | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | AOR | 95%CI | p-value | AOR | 95%CI | p-value | ||
| Acute non-stroke neurological event | 0.80 | 0.51 | 1.26 | 0.335 | 0.90 | 0.55 | 1.46 | 0.662 |
| Baseline depression in neurological status | 0.65 | 0.46 | 0.93 | 0.018 | 0.71 | 0.51 | 0.98 | 0.037 |
| Congestive heart failure during admission | 0.80 | 0.57 | 1.12 | 0.189 | 0.74 | 0.54 | 1.02 | 0.065 |
| Hepatic insufficiency | 0.64 | 0.41 | 1.00 | 0.051 | 0.72 | 0.49 | 1.06 | 0.099 |
| Hypotension/hypoperfusion | 0.83 | 0.68 | 1.01 | 0.068 | 0.77 | 0.64 | 0.92 | 0.005 |
| Metastatic or hematological malignancy | 0.94 | 0.68 | 1.30 | 0.714 | 1.01 | 0.76 | 1.33 | 0.968 |
| Metabolic and electrolyte abnormality | 1.17 | 0.89 | 1.55 | 0.263 | 1.20 | 0.95 | 1.52 | 0.129 |
| Renal insufficiency | 1.03 | 0.83 | 1.28 | 0.79 | 1.03 | 0.84 | 1.26 | 0.804 |
| Respiratory failure | 1.13 | 0.88 | 1.43 | 0.337 | 1.13 | 0.92 | 1.40 | 0.236 |
| Septicemia | 0.93 | 0.65 | 1.31 | 0.665 | 0.90 | 0.64 | 1.27 | 0.558 |
| Active or evolving myocardial infarction | 0.64 | 0.46 | 0.90 | 0.009 | 0.67 | 0.49 | 0.92 | 0.012 |
| Inadequate natural airway | 1.25 | 0.70 | 2.22 | 0.446 | 0.98 | 0.62 | 1.55 | 0.938 |
| Arrhythmia | 1.14 | 0.94 | 1.39 | 0.175 | 1.23 | 1.04 | 1.45 | 0.015 |
| Day hours | 1.91 | 1.47 | 2.48 | <0.001 | 1.63 | 1.29 | 2.04 | <0.001 |
| Arrest Rhythm (PEA reference value) | ||||||||
| Asystole | 1.07 | 0.85 | 1.35 | 0.572 | 0.99 | 0.81 | 1.20 | 0.901 |
| PVT/VF (Shockable rhythms) | 1.37 | 1.02 | 1.83 | 0.036 | 1.23 | 0.96 | 1.59 | 0.106 |
| White vs. non-white | 1.05 | 0.83 | 1.32 | 0.675 | 0.97 | 0.80 | 1.19 | 0.8 |
| Event location (General inpatient unit reference) | ||||||||
| Operating room | 1.10 | 0.75 | 1.61 | 0.642 | 1.28 | 0.88 | 1.86 | 0.2 |
| PACU | 2.10 | 1.34 | 3.29 | 0.001 | 2.13 | 1.42 | 3.19 | 0 |
| Telemetry | 1.56 | 0.74 | 3.26 | 0.239 | 1.51 | 0.77 | 2.97 | 0.233 |
| ICU | 1.49 | 0.94 | 2.36 | 0.088 | 1.46 | 0.94 | 2.27 | 0.094 |
| Weekend | 0.83 | 0.64 | 1.06 | 0.133 | 0.84 | 0.68 | 1.04 | 0.117 |
| Invasive ventilation in place pre-arrest | 0.64 | 0.38 | 1.08 | 0.095 | 0.69 | 0.50 | 0.94 | 0.019 |
| Illness Category (Medical non cardiac reference value) | ||||||||
| Medical cardiac | 1.52 | 0.94 | 2.46 | 0.091 | 1.57 | 1.03 | 2.39 | 0.035 |
| Surgical cardiac | 1.44 | 0.97 | 2.15 | 0.071 | 1.49 | 1.05 | 2.10 | 0.025 |
| Surgical non cardiac | 1.73 | 1.29 | 2.33 | <0.001 | 1.45 | 1.13 | 1.86 | 0.003 |
| Trauma | 0.75 | 0.49 | 1.14 | 0.181 | 0.72 | 0.46 | 1.15 | 0.171 |
| Age at hospital admission | 0.99 | 0.99 | 1.00 | 0.029 | 0.99 | 0.99 | 1.00 | 0.003 |
| Duration of event | 0.98 | 0.97 | 0.98 | <0.001 | 0.98 | 0.97 | 0.98 | <0.001 |
AOR adjusted odds ratio, CI confidence intervals, ICU Intensive Care Unit areas, PACU Post Anesthesia Care Unit, PEA pulseless electrical activity, PVT/VF pulseless ventricular tachycardia or ventricular fibrillation.
Appendix 3. General Estimating Equation models to predict survival to 24 hours post-arrest with and without data imputation for missing data
| Pre-imputation | Post-imputation for missing data | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | AOR | 95%CI | p-value | AOR | 95%CI | p-value | ||
| Acute non-stroke neurological event | 0.92 | 0.62 | 1.37 | 0.696 | 1.00 | 0.67 | 1.49 | 0.995 |
| Baseline depression in neurological status | 0.72 | 0.52 | 1.00 | 0.048 | 0.75 | 0.54 | 1.02 | 0.07 |
| Congestive heart failure during admission | 0.99 | 0.72 | 1.37 | 0.969 | 0.96 | 0.71 | 1.29 | 0.776 |
| Hepatic insufficiency | 0.84 | 0.51 | 1.37 | 0.478 | 0.88 | 0.57 | 1.36 | 0.561 |
| Hypotension/hypoperfusion | 0.56 | 0.46 | 0.69 | <0.001 | 0.55 | 0.45 | 0.67 | <0.001 |
| Metastatic or hematological malignancy | 0.81 | 0.62 | 1.07 | 0.132 | 0.90 | 0.70 | 1.17 | 0.441 |
| Metabolic and electrolyte abnormality | 0.75 | 0.55 | 1.02 | 0.062 | 0.70 | 0.53 | 0.93 | 0.013 |
| Renal insufficiency | 1.01 | 0.81 | 1.27 | 0.905 | 0.98 | 0.80 | 1.22 | 0.888 |
| Respiratory failure | 0.91 | 0.72 | 1.15 | 0.427 | 1.02 | 0.82 | 1.26 | 0.859 |
| Septicemia | 0.71 | 0.49 | 1.03 | 0.069 | 0.66 | 0.46 | 0.94 | 0.02 |
| Active or evolving myocardial infarction | 0.62 | 0.43 | 0.89 | 0.01 | 0.55 | 0.37 | 0.81 | 0.002 |
| Inadequate natural airway | 2.37 | 1.27 | 4.43 | 0.007 | 1.57 | 1.00 | 2.48 | 0.052 |
| Arrhythmia | 1.39 | 1.13 | 1.71 | 0.002 | 1.32 | 1.09 | 1.59 | 0.005 |
| Day hours | 1.77 | 1.29 | 2.45 | <0.001 | 1.56 | 1.19 | 2.05 | 0.002 |
| White vs. non-white | 1.08 | 0.86 | 1.37 | 0.497 | 1.02 | 0.82 | 1.27 | 0.853 |
| Arrest Rhythm (PEA reference value) | ||||||||
| Asystole | 1.08 | 0.86 | 1.36 | 0.497 | 1.03 | 0.85 | 1.25 | 0.772 |
| PVT/VF (Shockable rhythms) | 1.35 | 1.01 | 1.81 | 0.042 | 1.30 | 1.02 | 1.66 | 0.032 |
| Event location (General inpatient unit – reference) | ||||||||
| Operating room | 1.18 | 0.77 | 1.79 | 0.448 | 1.20 | 0.78 | 1.82 | 0.405 |
| PACU | 1.76 | 1.12 | 2.76 | 0.015 | 1.73 | 1.13 | 2.65 | 0.011 |
| Telemetry | 0.92 | 0.47 | 1.81 | 0.808 | 0.90 | 0.47 | 1.75 | 0.761 |
| ICU | 0.82 | 0.52 | 1.28 | 0.377 | 0.74 | 0.46 | 1.19 | 0.21 |
| Weekend | 0.67 | 0.51 | 0.88 | 0.004 | 0.61 | 0.48 | 0.77 | <0.001 |
| Invasive ventilation in place pre-arrest | 0.42 | 0.25 | 0.70 | 0.001 | 0.50 | 0.38 | 0.67 | <0.001 |
| Illness Category (Medical non cardiac reference value) | ||||||||
| Medical cardiac | 1.74 | 1.03 | 2.94 | 0.039 | 1.62 | 1.02 | 2.56 | 0.041 |
| Surgical cardiac | 1.84 | 1.22 | 2.78 | 0.004 | 1.81 | 1.24 | 2.64 | 0.002 |
| Surgical non cardiac | 1.71 | 1.26 | 2.31 | <0.001 | 1.42 | 1.07 | 1.87 | 0.015 |
| Trauma | 0.31 | 0.17 | 0.56 | <0.001 | 0.29 | 0.17 | 0.50 | <0.001 |
| Age at hospital admission | 0.99 | 0.98 | 0.99 | <0.001 | 0.99 | 0.98 | 0.99 | <0.001 |
| Duration of event | 0.96 | 0.95 | 0.97 | <0.001 | 0.96 | 0.95 | 0.96 | <0.001 |
AOR adjusted odds ratio, CI confidence intervals, ICU Intensive Care Unit areas, PACU Post Anesthesia Care Unit, PEA pulseless electrical activity, PVT/VF pulseless ventricular tachycardia or ventricular fibrillation.
Appendix 4. General Estimating Equation models to predict survival to discharge in patients with pulseless ventricular tachycardia or ventricular fibrillation arrests with and without data imputation for missing data
| Pre-imputation | Post-imputation for missing data | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | AOR | 95%CI | p-value | AOR | 95%CI | p-value | ||
| Acute non-stroke neurological event | 0.99 | 0.47 | 2.07 | 0.981 | 0.99 | 0.55 | 1.78 | 0.979 |
| Baseline depression in neurological status | 0.64 | 0.24 | 1.69 | 0.365 | 0.68 | 0.31 | 1.52 | 0.348 |
| Congestive heart failure during admission | 0.58 | 0.29 | 1.19 | 0.137 | 0.66 | 0.36 | 1.18 | 0.162 |
| Hepatic insufficiency | 1.19 | 0.32 | 4.48 | 0.792 | 0.99 | 0.32 | 3.09 | 0.992 |
| Hypotension/hypoperfusion | 0.34 | 0.22 | 0.54 | <0.001 | 0.40 | 0.27 | 0.60 | <0.001 |
| Metastatic or hematological malignancy | 0.83 | 0.43 | 1.60 | 0.569 | 0.70 | 0.44 | 1.13 | 0.15 |
| Metabolic and electrolyte abnormality | 0.56 | 0.29 | 1.10 | 0.095 | 0.60 | 0.31 | 1.16 | 0.132 |
| Renal insufficiency | 0.58 | 0.35 | 0.97 | 0.039 | 0.52 | 0.34 | 0.81 | 0.004 |
| Respiratory failure | 0.72 | 0.48 | 1.08 | 0.111 | 0.81 | 0.57 | 1.15 | 0.242 |
| Septicemia | 0.83 | 0.32 | 2.13 | 0.692 | 0.65 | 0.29 | 1.48 | 0.308 |
| Active or evolving myocardial infarction | 1.21 | 0.56 | 2.61 | 0.636 | 0.97 | 0.48 | 1.98 | 0.944 |
| Inadequate natural airway | 2.15 | 0.28 | 16.52 | 0.461 | 1.57 | 0.44 | 5.66 | 0.49 |
| Arrhythmia | 1.07 | 0.66 | 1.76 | 0.775 | 1.04 | 0.70 | 1.55 | 0.84 |
| Day hours | 1.55 | 0.83 | 2.90 | 0.169 | 1.48 | 0.95 | 2.32 | 0.084 |
| Event location | ||||||||
| Operating room | 2.32 | 0.82 | 6.56 | 0.112 | 1.75 | 0.81 | 3.79 | 0.158 |
| PACU | 2.51 | 0.85 | 7.41 | 0.096 | 2.05 | 0.95 | 4.39 | 0.066 |
| Telemetry | 5.44 | 0.65 | 45.79 | 0.119 | 5.72 | 1.10 | 29.66 | 0.038 |
| ICU | 3.51 | 1.12 | 10.95 | 0.031 | 1.95 | 1.06 | 5.07 | 0.049 |
| Weekend | 0.73 | 0.38 | 1.40 | 0.345 | 0.63 | 0.38 | 1.04 | 0.071 |
| Invasive ventilation in place pre-arrest | 0.64 | 0.24 | 1.68 | 0.362 | 0.69 | 0.36 | 1.32 | 0.267 |
| White vs. non-white | 1.32 | 0.74 | 2.36 | 0.346 | 1.05 | 0.64 | 1.70 | 0.854 |
| Illness Category | ||||||||
| Medical cardiac | 7.34 | 2.06 | 26.13 | 0.002 | 3.64 | 1.52 | 8.72 | 0.004 |
| Surgical cardiac | 2.30 | 0.90 | 5.88 | 0.083 | 2.42 | 1.18 | 4.95 | 0.015 |
| Surgical non cardiac | 1.66 | 0.76 | 3.64 | 0.204 | 1.80 | 1.01 | 3.20 | 0.045 |
| Trauma | 0.12 | 0.03 | 0.48 | 0.002 | 0.22 | 0.07 | 0.71 | 0.012 |
| Age at hospital admission | 0.97 | 0.96 | 0.99 | 0.001 | 0.98 | 0.97 | 0.99 | 0.001 |
| Duration of event | 0.96 | 0.94 | 0.97 | <0.001 | 0.96 | 0.95 | 0.97 | <0.001 |
AOR adjusted odds ratio, CI confidence intervals, ICU Intensive Care Unit areas, PACU Post Anesthesia Care Unit.
Appendix 5. General Estimating Equation models to predict survival to discharge in patients with asystolic arrests with and without data imputation for missing data
| Pre-imputation | Post-imputation for missing data | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | AOR | 95%CI | p-value | AOR | 95%CI | p-value | ||
| Acute non-stroke neurological event | 0.70 | 0.28 | 1.71 | 0.428 | 0.79 | 0.38 | 1.63 | 0.519 |
| Baseline depression in neurological status | 0.79 | 0.38 | 1.64 | 0.526 | 0.95 | 0.55 | 1.63 | 0.847 |
| Congestive heart failure during admission | 0.50 | 0.25 | 1.00 | 0.05 | 0.58 | 0.32 | 1.05 | 0.072 |
| Hepatic insufficiency | 0.62 | 0.24 | 1.56 | 0.308 | 0.73 | 0.33 | 1.62 | 0.435 |
| Hypotension/hypoperfusion | 0.31 | 0.20 | 0.47 | <0.001 | 0.34 | 0.24 | 0.50 | <0.001 |
| Metastatic or hematological malignancy | 0.33 | 0.17 | 0.62 | 0.001 | 0.48 | 0.29 | 0.78 | 0.003 |
| Metabolic and electrolyte abnormality | 1.18 | 0.51 | 2.71 | 0.696 | 0.74 | 0.36 | 1.53 | 0.414 |
| Renal insufficiency | 0.77 | 0.49 | 1.24 | 0.284 | 0.84 | 0.57 | 1.24 | 0.387 |
| Respiratory failure | 0.87 | 0.57 | 1.34 | 0.534 | 1.01 | 0.71 | 1.44 | 0.952 |
| Septicemia | 0.31 | 0.12 | 0.77 | 0.012 | 0.39 | 0.19 | 0.80 | 0.01 |
| Active or evolving myocardial infarction | 0.57 | 0.23 | 1.43 | 0.229 | 0.57 | 0.23 | 1.40 | 0.22 |
| Inadequate natural airway | 3.36 | 0.72 | 15.72 | 0.124 | 3.02 | 0.92 | 9.85 | 0.068 |
| Arrhythmia | 1.65 | 1.17 | 2.33 | 0.004 | 1.60 | 1.18 | 2.18 | 0.003 |
| Day hours | 2.31 | 1.34 | 3.97 | 0.003 | 2.22 | 1.37 | 3.59 | 0.001 |
| Event location | ||||||||
| Operating room | 2.52 | 1.13 | 5.63 | 0.024 | 1.38 | 1.02 | 2.58 | 0.047 |
| PACU | 3.36 | 1.39 | 8.13 | 0.007 | 2.04 | 1.02 | 4.09 | 0.044 |
| Telemetry | 0.57 | 0.13 | 2.40 | 0.442 | 0.59 | 0.14 | 2.39 | 0.456 |
| ICU | 0.99 | 0.39 | 2.49 | 0.979 | 0.60 | 0.26 | 1.38 | 0.228 |
| Weekend | 0.54 | 0.32 | 0.94 | 0.028 | 0.49 | 0.30 | 0.79 | 0.003 |
| Invasive ventilation in place pre-arrest | 0.17 | 0.07 | 0.40 | <0.001 | 0.41 | 0.25 | 0.67 | <0.001 |
| White vs. non-white | 1.35 | 0.80 | 2.28 | 0.264 | 1.18 | 0.79 | 1.75 | 0.427 |
| Illness Category | ||||||||
| Medical cardiac | 1.78 | 0.68 | 4.69 | 0.24 | 1.44 | 0.61 | 3.41 | 0.411 |
| Surgical cardiac | 1.03 | 0.44 | 2.40 | 0.947 | 1.62 | 0.80 | 3.29 | 0.183 |
| Surgical non cardiac | 1.88 | 0.93 | 3.79 | 0.078 | 1.78 | 1.03 | 3.08 | 0.038 |
| Trauma | 0.25 | 0.07 | 0.93 | 0.039 | 0.46 | 0.17 | 1.26 | 0.13 |
| Age at hospital admission | 0.98 | 0.97 | 0.99 | 0.003 | 0.98 | 0.97 | 0.99 | <0.001 |
| Duration of event | 0.95 | 0.93 | 0.97 | <0.001 | 0.95 | 0.93 | 0.96 | <0.001 |
AOR adjusted odds ratio, CI confidence intervals, ICU Intensive Care Unit areas, PACU Post Anesthesia Care Unit.
Appendix 6. General Estimating Equation models to predict survival to discharge in patients with pulseless electrical activity arrests with and without data imputation for missing data
| Pre-imputation | Post-imputation for missing data | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | AOR | 95%CI | p-value | AOR | 95%CI | p-value | ||
| Acute non-stroke neurological event | 1.46 | 0.77 | 2.78 | 0.246 | 1.49 | 0.77 | 2.91 | 0.239 |
| Baseline depression in neurological status | 0.68 | 0.35 | 1.35 | 0.275 | 0.61 | 0.30 | 1.23 | 0.168 |
| Congestive heart failure during admission | 0.60 | 0.32 | 1.13 | 0.116 | 0.68 | 0.38 | 1.20 | 0.184 |
| Hepatic insufficiency | 0.64 | 0.24 | 1.69 | 0.364 | 0.54 | 0.19 | 1.51 | 0.239 |
| Hypotension/hypoperfusion | 0.81 | 0.55 | 1.19 | 0.28 | 0.78 | 0.52 | 1.16 | 0.212 |
| Metastatic or hematological malignancy | 0.70 | 0.42 | 1.18 | 0.183 | 0.64 | 0.39 | 1.06 | 0.082 |
| Metabolic and electrolyte abnormality | 0.53 | 0.27 | 1.04 | 0.063 | 0.46 | 0.24 | 0.89 | 0.021 |
| Renal insufficiency | 0.72 | 0.49 | 1.05 | 0.091 | 0.66 | 0.45 | 0.97 | 0.033 |
| Respiratory failure | 0.81 | 0.54 | 1.21 | 0.299 | 0.92 | 0.61 | 1.40 | 0.707 |
| Septicemia | 0.38 | 0.16 | 0.92 | 0.031 | 0.32 | 0.14 | 0.78 | 0.011 |
| Active or evolving myocardial infarction | 0.09 | 0.01 | 0.62 | 0.015 | 0.19 | 0.05 | 0.69 | 0.011 |
| Inadequate natural airway | 1.91 | 0.80 | 4.57 | 0.144 | 1.53 | 0.69 | 3.39 | 0.297 |
| Arrhythmia | 1.30 | 0.92 | 1.82 | 0.132 | 1.34 | 0.98 | 1.84 | 0.067 |
| Day hours | 1.64 | 0.80 | 3.33 | 0.174 | 1.52 | 0.86 | 2.71 | 0.153 |
| Event location | ||||||||
| Operating room | 0.66 | 0.28 | 1.58 | 0.355 | 0.52 | 0.25 | 1.08 | 0.079 |
| PACU | 1.19 | 0.47 | 2.99 | 0.71 | 0.89 | 0.41 | 1.93 | 0.773 |
| Telemetry | 0.64 | 0.14 | 2.91 | 0.568 | 0.41 | 0.09 | 1.83 | 0.242 |
| ICU | 0.51 | 0.20 | 1.32 | 0.166 | 0.35 | 0.15 | 0.80 | 0.012 |
| Weekend | 0.67 | 0.37 | 1.19 | 0.169 | 0.49 | 0.28 | 0.85 | 0.011 |
| Invasive ventilation in place pre-arrest | 0.31 | 0.14 | 0.70 | 0.005 | 0.53 | 0.32 | 0.87 | 0.012 |
| White vs. non-white | 1.07 | 0.68 | 1.69 | 0.769 | 0.88 | 0.59 | 1.32 | 0.551 |
| Illness Category | ||||||||
| Medical cardiac | 1.51 | 0.53 | 4.31 | 0.437 | 1.55 | 0.64 | 3.79 | 0.334 |
| Surgical cardiac | 1.99 | 0.92 | 4.29 | 0.08 | 1.64 | 0.79 | 3.38 | 0.182 |
| Surgical non cardiac | 1.31 | 0.74 | 2.33 | 0.351 | 1.29 | 0.76 | 2.19 | 0.345 |
| Trauma | 0.27 | 0.09 | 0.79 | 0.017 | 0.20 | 0.07 | 0.57 | 0.002 |
| Age at hospital admission | 0.98 | 0.97 | 0.99 | 0.001 | 0.98 | 0.97 | 0.99 | <0.001 |
| Duration of event | 0.96 | 0.94 | 0.97 | <0.001 | 0.95 | 0.94 | 0.97 | <0.001 |
AOR adjusted odds ratio, CI confidence intervals, ICU Intensive Care Unit areas, PACU Post Anesthesia Care Unit.
Appendix 7. General Estimating Equation models to predict good neurological outcome in patients with good neurological status at admission, before and after data imputation for missing data
| Pre-imputation | Post-imputation for missing data | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | AOR | 95%CI | p-value | AOR | 95%CI | p-value | ||
| Acute non-stroke neurological event | 0.76 | 0.21 | 2.75 | 0.671 | 1.00 | 0.27 | 3.78 | 0.995 |
| Baseline depression in neurological status | 0.69 | 0.24 | 2.04 | 0.504 | 1.02 | 0.25 | 4.13 | 0.981 |
| Congestive heart failure during admission | 1.07 | 0.38 | 3.04 | 0.897 | 1.09 | 0.41 | 2.94 | 0.857 |
| Hepatic insufficiency | 0.66 | 0.12 | 3.54 | 0.627 | 0.48 | 0.18 | 1.27 | 0.138 |
| Hypotension/hypoperfusion | 0.71 | 0.41 | 1.23 | 0.219 | 0.90 | 0.59 | 1.39 | 0.642 |
| Metastatic or hematological malignancy | 0.75 | 0.37 | 1.51 | 0.417 | 0.82 | 0.47 | 1.44 | 0.487 |
| Metabolic and electrolyte abnormality | 1.55 | 0.60 | 3.96 | 0.363 | 1.03 | 0.51 | 2.07 | 0.933 |
| Renal insufficiency | 0.72 | 0.35 | 1.47 | 0.366 | 0.73 | 0.40 | 1.33 | 0.311 |
| Respiratory failure | 0.88 | 0.51 | 1.54 | 0.658 | 0.91 | 0.57 | 1.46 | 0.704 |
| Septicemia | 0.28 | 0.08 | 0.94 | 0.04 | 0.43 | 0.14 | 1.35 | 0.146 |
| Active or evolving myocardial infarction | 0.96 | 0.39 | 2.36 | 0.929 | 1.31 | 0.55 | 3.15 | 0.545 |
| Inadequate natural airway | 0.44 | 0.17 | 1.13 | 0.087 | 0.52 | 0.23 | 1.18 | 0.118 |
| Arrhythmia | 0.83 | 0.50 | 1.37 | 0.461 | 0.89 | 0.58 | 1.35 | 0.572 |
| Day hours | 1.70 | 0.70 | 4.14 | 0.241 | 1.09 | 0.48 | 2.52 | 0.831 |
| White vs. non-white | 1.63 | 0.88 | 3.02 | 0.124 | 1.23 | 0.72 | 2.11 | 0.451 |
| Arrest Rhythm (PEA reference value) | ||||||||
| Asystole | 0.83 | 0.43 | 1.59 | 0.572 | 1.09 | 0.68 | 1.77 | 0.718 |
| PVT/VF (Shockable rhythms) | 1.04 | 0.55 | 1.98 | 0.906 | 1.07 | 0.66 | 1.74 | 0.773 |
| Event location (General inpatient unit – reference) | ||||||||
| Operating room | 5.72 | 1.75 | 18.65 | 0.004 | 1.66 | 0.71 | 3.86 | 0.242 |
| PACU | 5.17 | 1.62 | 16.45 | 0.005 | 1.88 | 0.80 | 4.47 | 0.15 |
| Telemetry | 2.46 | 0.59 | 10.21 | 0.215 | 0.80 | 0.21 | 3.00 | 0.742 |
| ICU | 6.19 | 1.52 | 25.25 | 0.011 | 1.61 | 0.51 | 5.10 | 0.419 |
| Weekend | 1.34 | 0.58 | 3.10 | 0.498 | 1.20 | 0.63 | 2.28 | 0.578 |
| Invasive ventilation in place pre-arrest | 0.03 | 0.00 | 0.29 | 0.002 | 0.60 | 0.33 | 1.08 | 0.086 |
| Age at hospital admission | 0.98 | 0.97 | 0.99 | 0.005 | 0.98 | 0.96 | 0.99 | 0.001 |
| Duration of event | 0.98 | 0.97 | 1.00 | 0.029 | 0.98 | 0.96 | 0.99 | 0.001 |
| Illness Category (Medical non cardiac reference value) | ||||||||
| Medical cardiac | 1.23 | 0.37 | 4.15 | 0.733 | 1.77 | 0.71 | 4.43 | 0.223 |
| Surgical cardiac | 1.31 | 0.43 | 3.99 | 0.63 | 1.50 | 0.72 | 3.09 | 0.276 |
| Surgical non cardiac | 1.49 | 0.59 | 3.75 | 0.395 | 1.44 | 0.45 | 4.64 | 0.536 |
| Trauma | 1.16 | 0.25 | 5.47 | 0.853 | 0.99 | 0.98 | 1.00 | 0.086 |
AOR adjusted odds ratio, CI confidence intervals, ICU Intensive Care Unit areas, PACU Post Anesthesia Care Unit, PEA pulseless electrical activity, PVT/VF pulseless ventricular tachycardia or ventricular fibrillation.
References
- 1.Runciman WB, Morris RW, Watterson LM, Williamson JA, Paix AD. Crisis management during anaesthesia: Cardiac arrest. Qual Saf Health Care. 2005;14:e14. doi: 10.1136/qshc.2002.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kayser RG, Ornato JP, Peberdy MA. Cardiac arrest in the Emergency Department: A report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2008;78:151–60. doi: 10.1016/j.resuscitation.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Braz LG, Modolo NS, do Nascimento P, Jr, Bruschi BA, Castiglia YM, Ganem EM, de Carvalho LR, Braz JR. Perioperative cardiac arrest: A study of 53,718 anaesthetics over 9 yr from a Brazilian teaching hospital. Br J Anaesth. 2006;96:569–75. doi: 10.1093/bja/ael065. [DOI] [PubMed] [Google Scholar]
- 4.Sprung J, Warner ME, Contreras MG, Schroeder DR, Beighley CM, Wilson GA, Warner DO. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: A study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99:259–69. doi: 10.1097/00000542-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Newland MC, Ellis SJ, Lydiatt CA, Peters KR, Tinker JH, Romberger DJ, Ullrich FA, Anderson JR. Anesthetic-related cardiac arrest and its mortality: A report covering 72,959 anesthetics over 10 years from a US teaching hospital. Anesthesiology. 2002;97:108–15. doi: 10.1097/00000542-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane-Trultt T. Cardiopulmonary resuscitation of adults in the hospital: A report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 7.Peberdy MA, Ornato JP, Larkin GL, Braithwaite RS, Kashner TM, Carey SM, Meaney PA, Cen L, Nadkarni VM, Praestgaard AH, Berg RA. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–92. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 8.Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after inhospital cardiac arrest. N Engl J Med. 2008;358:9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–97. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 10.Mhyre JM, Ramachandran SK, Kheterpal S, Morris M, Chan PS. Delayed time to defibrillation after intraoperative and periprocedural cardiac arrest. Anesthesiology. 2010;113:782–93. doi: 10.1097/ALN.0b013e3181eaa74f. [DOI] [PubMed] [Google Scholar]
- 11.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 12.Raghunathan TE, Reiter JP, Rubin DB. Multiple Imputation for Statistical Disclosure Limitation. J Off Stat. 2003;19:1–16. [Google Scholar]
- 13.Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190:752–6. doi: 10.1016/j.amjsurg.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Wong ML, Carey S, Mader TJ, Wang HE. Time to invasive airway placement and resuscitation outcomes after inhospital cardiopulmonary arrest. Resuscitation. 2010;81:182–6. doi: 10.1016/j.resuscitation.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran SK, Haider N, Saran KA, Mathis M, Kim J, Morris M, O’Reilly M. Life-threatening critical respiratory events: A retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth. 2011;23:207–13. doi: 10.1016/j.jclinane.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: A before-and-after concurrence study. Anesthesiology. 2010;112:282–7. doi: 10.1097/ALN.0b013e3181ca7a9b. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen T, Moller AM, Hovhannisyan K. Pulse oximetry for perioperative monitoring. Cochrane Database Syst Rev. 2009:CD002013. doi: 10.1002/14651858.CD002013.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Gray A. United Kingdom national confidential enquiry into perioperative deaths. Minerva Anestesiol. 2000;66:288–92. [PubMed] [Google Scholar]
- 19.Sessler DI, Kurz A, Saager L, Dalton JE. Operation timing and 30-day mortality after elective general surgery. Anesth Analg. 2011;113:1423–8. doi: 10.1213/ANE.0b013e3182315a6d. [DOI] [PubMed] [Google Scholar]
- 20.Kelz RR, Freeman KM, Hosokawa PW, Asch DA, Spitz FR, Moskowitz M, Henderson WG, Mitchell ME, Itani KM. Time of day is associated with postoperative morbidity: An analysis of the national surgical quality improvement program data. Ann Surg. 2008;247:544–52. doi: 10.1097/SLA.0b013e31815d7434. [DOI] [PubMed] [Google Scholar]
- 21.Obolensky L, Clark T, Matthew G, Mercer M. A patient and relative centred evaluation of treatment escalation plans: A replacement for the do-not-resuscitate process. J Med Ethics. 2010;36:518–20. doi: 10.1136/jme.2009.033977. [DOI] [PubMed] [Google Scholar]
- 22.Waisel DB, Simon R, Truog RD, Baboolal H, Raemer DB. Anesthesiologist management of perioperative do-not-resuscitate orders: A simulation-based experiment. Simul Healthc. 2009;4:70–6. doi: 10.1097/SIH.0b013e31819e137b. [DOI] [PubMed] [Google Scholar]
- 23.Waisel D, Jackson S, Fine P. Should do-not-resuscitate orders be suspended for surgical cases? Curr Opin Anaesthesiol. 2003;16:209–13. doi: 10.1097/00001503-200304000-00016. [DOI] [PubMed] [Google Scholar]