Abstract
Many psychophysiologists have noted the striking similarities between the antecedent conditions for the P3 component of the event-related potential and the orienting response: both are typically elicited by salient, unexpected, novel, task-relevant, and other motivationally significant stimuli. Although the close coupling of the P3 and orienting response has been well documented, the neural basis and functional role of this relationship is still poorly understood. Here we propose that the simultaneous occurrence of the P3 and autonomic components of the orienting response reflects the co-activation of the locus coeruleus-norepinephrine system and the peripheral sympathetic nervous system by their common major afferent: the rostral ventrolateral medulla, a key sympathoexcitatory region. A comparison of the functional significance of the locus coeruleus-norepinephrine system and the peripheral sympathetic nervous system suggests that the P3 and orienting response reflect complementary cognitive and physical contributions to the mobilization for action following motivationally significant stimuli.
Keywords: P300, P3, orienting response, norepinephrine, noradrenergic, rostral ventrolateral medulla, arousal, reticular formation
Introduction
In the past decade, the use of functional neuroimaging techniques has greatly aided our understanding of the cortical and subcortical brain structures involved in regulating the autonomic nervous system, and in representing bodily states (Berntson, Sarter, & Cacioppo, 2003; Critchley, 2005). It has long been known that the interplay between the central and autonomic nervous system is not just important for homeostatic regulation, but also an essential component of active, stimulus-driven behavior. As an important example, motivationally significant stimuli1 are typically followed by a phasic response of the autonomic nervous system, often referred to as the orienting response, along with a transient change in brain activity known as the P3 or P300. However, although the close coupling between these two phenomena has been well documented, the functional role and neural basis of this relationship is still poorly understood. Here, we propose an hypothesis that attempts to explain this relationship.
The orienting response is a collection of physiological changes that are elicited by the occurrence of motivationally significant events (Lynn, 1966; Pavlov, 1927; Sokolov, 1963). These changes include a temporary dilation of the pupils, a rise in skin conductance, a momentary change in heart rate, and a range of other short-lived changes in organ activity. Although the orienting response should probably be regarded as a convenient grouping of physiological changes rather than as a unitary construct (e.g., Barry, 1979, 2009; Kahneman, 1973), these changes generally occur together, typically accompanied by a shift of attention toward the eliciting event. While the precise functional significance of the orienting response has been a topic of much debate, there appears to be consensus that it serves to potentiate information processing and to prepare or facilitate a rapid behavioral response to the eliciting stimulus (even if such action is not always undertaken; e.g., Donchin et al., 1984; Lynn, 1966).
An important question that occupied many psychophysiologists in the late 1970s and early 1980s concerned the neural correlates of the orienting response. In particular, they debated whether the electrophysiological P3 should be seen as the central nervous system counterpart to the autonomic components of the orienting response, and how explanations of the functions of these phenomena might be integrated within one theoretical framework (Donchin et al., 1984; Friedman, 1978; Kimmel, Van Olst, & Orlebeke, 1979). It was clear to most researchers that the P3 and orienting response have very similar antecedent conditions (Ritter, Vaughan, & Costa, 1968). These antecedent conditions indicate that both phenomena reflect more closely the motivational significance of eliciting stimuli (as determined by their inherent value, task instructions, recent experience, and other factors) than their physical characteristics per se. To illustrate, both the P3 (Sutton, Tueting, Zubin, & John, 1967) and the orienting response (Sokolov, 1963) can be elicited by the absence of a stimulus when that absence delivers information to the subject. These and other similarities as well as some apparent discrepancies fuelled the discussion about the functional relationship between the P3 and the orienting response.
However, we believe it is fair to say that no satisfactory integrative theoretical framework emerged from these years of discussion, and in the second half of the 1980s interest in the link between P3 and the orienting response quickly diminished. There seem to be at least three reasons for this course of events. First, a fair amount of initial confusion, which impeded theoretical progress, was caused by the fact that the paradigms used to study the P3 generally differed from those traditionally employed to study the autonomic components of the orienting response. Specifically, the former studies tended to focus on the P3 to task-relevant stimuli (which shows very little habituation over the course of an experiment), whereas the latter studies focused on the orienting response to task-irrelevant stimuli in passive observers (which habituates rapidly). Fortunately, several researchers noted the artifactual nature of the ensuing discrepancies in results, and this issue of debate was resolved (for a more detailed summary, see Donchin et al., 1984).
A second challenge for the development of an integrative theoretical framework concerned the gap between prevalent conceptions of the functional role of the orienting response and the most influential theory of the P3: the context-updating hypothesis (Donchin & Coles, 1988). As noted above, a common and natural interpretation of the orienting response is that it serves to prepare or facilitate rapid action in response to the eliciting stimulus. This action-oriented view stands in marked contrast with the context-updating hypothesis, which posits a strategic role for the process underlying the P3: updating of a cognitive schema of the environment. Although, according to these interpretations, the orienting response (action preparation) and the P3 (context updating) may be triggered by very similar antecedent conditions, and hence could be considered correlates of each other, their action-based vs. memory-based contributions to goal-directed behavior are of a distinct nature (Donchin, 1981).
Finally, evidence from intracranial recordings (in humans and animals) and functional imaging suggested the involvement of multiple, and diverse brain areas in generating the P3 (reviewed in Soltani & Knight, 2000), and it proved difficult to integrate this evidence in a comprensive account of the neural basis of the P3. Therefore, researchers were lacking a neurobiological framework with which to correlate the—at the time—sparse knowledge of the brain areas involved in eliciting the orienting response.
Thus, for a long time, the available knowledge and theoretical conceptions of the P3 made it hard to understand the link between this component and the orienting response. However, recent research has led to a new, detailed theory of the neural basis and functional significance of the P3 (Nieuwenhuis, Aston-Jones, & Cohen, 2005). As we will argue, this theory sheds new light on the relationship between the P3 and the orienting response, suggesting a close correspondence between the two phenomena at both the neurobiological and functional levels.
The remainder of this article is organized as follows. We first review the empirical evidence for a close link between the P3 and the orienting response, focusing in particular on the similarity in antecedent conditions. The scope of this review is modest, in particular in the sense that it does not cover many of the subtleties of the orienting response, which are discussed in detail elsewhere (e.g., Sokolov, Spinks, Nätäänen, & Lyytinen, 2002). Furthermore, we limit our discussion to two of the autonomic components of the orienting response that have received the most attention in the context of the P3: the skin conductance response (SCR) and the pupil dilation response (PDR). The SCR is entirely driven by changes in the sympathetic nervous system (SNS) whereas the PDR is subserved by closely coupled SNS and parasympathetic inputs. Although phasic heart rate changes have also been a popular measure of the autonomic orienting response to motivationally significant stimuli (for excellent reviews see Graham & Clifton, 1966; Simons 1988), they mainly reflect parasympathetic (or vagal) inhibitory influences which are strongly modulated by respiratory behavior and baroreflex activation (e.g., Berntson, Cacioppo, & Quigley, 1993). Because these influences complicate interpretation, phasic heart rate changes will not be discussed in our review.
Following this brief review, we will summarize the theory of the P3 recently proposed by Nieuwenhuis, Aston-Jones, and Cohen (2005). According to this theory, the P3 reflects the response of the neuromodulatory locus coeruleus–norepinephrine system to the outcome of stimulus evaluation and perceptual decision making. Furthermore, the theory holds that the observed properties of the P3 reflect an important information processing function of the locus coeruleus–norepinephrine system, which is to potentiate the response to motivationally significant events. In the final sections of the article, we suggest how this theory and knowledge about the anatomy of the locus coeruleus–norepinephrine system can be used to leverage our understanding of the neurobiological and functional relationship between the P3 and the orienting response.
Similarities between the P3 and autonomic components of the orienting response
An extensive review of the P3, SCR, and PDR is beyond the scope of this paper, and can be found elsewhere (e.g., Janisse, 1977; Nieuwenhuis, Aston-Jones et al., 2005; Pritchard, 1981; Roth, 1983). Our goal here is merely to illustrate the point that the P3 shares many properties with phasic sympathetic responses reflected in the SCR and the PDR.
The P3
The P3 is a broad, positive large-amplitude potential with a parieto-central scalp distribution, and a typical peak latency between 300 and 400 ms following presentation of stimuli in any sensory modality (Sutton, Braren, Zubin, & John, 1965). An important factor affecting the amplitude of the P3 is the subjective probability of the eliciting stimulus (Donchin & Coles, 1988). The effect of stimulus probability on P3 amplitude has been thoroughly documented using the oddball task. In this task, low-frequency target stimuli (“oddballs”) are embedded in a train of non-target stimuli (“standards”), and the subject’s task is either to actively respond to each target stimulus, or to passively attend to the stimulus sequence. Using this task, it has been found that the amplitude of the P3 associated with targets and non-target stimuli is inversely related to their probability of occurrence (Duncan-Johnson and Donchin, 1977; Gonsalvez & Polich, 2002). Furthermore, the amplitude of the P3 to the oddball stimulus is proportional to the degree of deviation from the standards (e.g., in terms of tone pitch; Ford, Roth, & Kopell, 1976).
Although both targets (i.e., stimuli requiring a response) and non-target stimuli can elicit a P3, when equated for frequency of occurrence, targets typically elicit somewhat higher P3 amplitudes than nontarget stimuli (e.g., Duncan-Johnson & Donchin, 1977). This indicates that P3 amplitude is also sensitive to the motivational significance of the eliciting stimulus. In laboratory contexts, such as the oddball task, stimuli often derive their motivational significance from a set of, in some sense, arbitrary task instructions. However, the P3 is also sensitive to stimuli with more intrinsic significance. For example, emotionally valent stimuli, whether experienced as positive or negative, are associated with larger P3s than emotionally neutral stimuli (Johnston, Miller, & Burleson, 1986; Yeung & Sanfey, 2004). Moreover, the P3 can be elicited by the absence of a stimulus when that absence delivers important information to the subject (Sutton et al., 1967), which further illustrates that the P3 is sensitive to the significance rather than physical properties of a stimulus.
The effects of subjective probability and motivational significance on P3 amplitude are modulated by a third variable, the amount of attention paid to the stimulus (Johnson, 1993). Specifically, the same stimuli that would under normal circumstances elicit a robust P3, elicit no P3 or a P3 of much smaller amplitude when they are deliberately ignored or when subjects’ attention is occupied by another, secondary task (Duncan-Johnson & Donchin, 1977). A P3 will be observed only if an initially unattended stimulus has sufficient intensity to capture attention and intrude into consciousness (Ritter et al., 1968). Indeed, the only physical property that systematically affects the P3 is stimulus intensity, which is positively correlated with P3 amplitude (Covington & Polich, 1996; Roth, Dorato, & Kopell, 1984).
Highly deviant or salient task-irrelevant stimuli, such as infrequently presented loud sounds, can be regarded as a specific class of motivationally significant, attention-capturing stimuli. The P3 elicited by this class of stimuli (often labeled P3a) has a number of properties that distinguish it from the typical P3 (or P3b) associated with task-relevant stimuli (Friedman, Cycowicz, & Gaeta, 2001; Simons, Graham, Miles, & Chen, 2001; Spencer, Dien, & Donchin, 2001): (i) its scalp distribution has a prominent fronto-central focus; (ii) it peaks 60–80 ms earlier than the P3b; and (iii) its amplitude shows rapid habituation as the novelty or salience of task-irrelevant stimuli decreases with repeated presentations (Courchesne, Hillyard, & Galambos, 1975; Roth, 1973; see Rushby & Barry, 2007 for more stringent habituation criteria), although the evidence for such habituation at long interstimulus intervals (> 30 sec in traditional orienting response studies) is mixed (e.g., Rushby & Barry, 2009; Rust, 1977; Simons et al., 1987).
Various lines of evidence indicate that the P3 is intimately related to task performance. For example, under the attention-demanding circumstances presented by signal-detection tasks, P3 amplitude varies directly with detection and recognition performance on signal-present trials (e.g., Hillyard, Squires, Bauer, & Lindsay, 1971). More specifically, stimuli that elicit a large P3 have a higher chance of being accurately discriminated. Similar findings have been obtained with the attentional blink paradigm (cf. Nieuwenhuis, Gilzenrat, Holmes, & Cohen, 2005). Furthermore, in speeded two-choice reaction time tasks, P3 latency and reaction time generally covary across trials (Makeig et al., 2004; Pfefferbaum, Ford, Roth, & Kopell, 1980; Ritter, Simson, & Vaughan, 1972) with the peak of the P3 generally occurring around the time of the response. They also covary across task conditions when these affect the duration of stimulus encoding or the decision process (for a review and some exceptions, see Verleger, 1997). Finally, P3 amplitude and reaction time are negatively correlated across trials in the oddball task (Holm, Rantaaho, Sallinen, Karjalainen, & Müller, 2006; Li, Keil, & Principe, 2009; Suwazono et al., 1994). Together, these results are consistent with the notion that the P3 process serves to facilitate behavioral responses (Nieuwenhuis, Aston-Jones et al., 2005). The P3 process may also facilitate internal “responses” such as the encoding of information into long-term memory. For example, P3 amplitude to a stimulus is predictive of later recall of that stimulus (Karis, Fabiani, & Donchin, 1984).
To summarize, the process underlying the P3 is driven by the motivational significance and frequency of task-relevant stimuli while being relatively insensitive to their physical attributes. In addition, a P3 with a slightly earlier timing and a more frontal scalp distribution occurs to task-irrelevant stimuli that are salient by virtue of novelty or intensity. Finally, the P3 is closely associated with the speed and accuracy of responding.
The skin conductance response
The eccrine sweat glands are innervated by efferent neurons from the sympathetic axis of the autonomic nervous system. The primary function of most eccrine sweat glands is thermoregulation, but the eccrine glands located on the palms and soles of the feet may be more concerned with grasping behavior than with evaporative cooling (Edelberg, 1972). Furthermore, it has been suggested that these glands are more responsive to emotional stimuli than to thermal stimuli (Dawson, Schell, & Filion, 2000). Transient changes in SNS activity are reflected in measurable changes in skin conductance at the surface, where the activity modulates the conductance of an applied current to the skin. The SCR to external stimuli consists of a rise in conductance beginning more than 1 second following stimulation, a peak around 1 second following onset, and a slow recovery to baseline. This pattern can be readily measured on single trials.
The antecedent conditions for the SCR have been thoroughly investigated. In this research, subjects are typically passive observers, and the intervals between consecutive stimuli are relatively long so that the SCR to one stimulus has time to evolve before the next stimulus appears. No study has systematically examined the relationship between stimulus probability and SCR amplitude, presumably because of the large number of (long-duration) trials required. However, one robust finding is that unexpected stimulus change is sufficient to produce a reliable SCR (Siddle, O’Gorman, & Wood, 1979). For example, in the study of Siddle, Remington, and Churchill (1984) subjects watched a sequence of 41 stimuli, spaced at random intervals, all longer than 20 seconds. Half of the subjects saw 40 letter stimuli (H and F), followed by a shape stimulus (diamond or triangle). The other half of the subjects saw 40 shape stimuli followed by a letter. The data indicated a substantially increased SCR on the change trial compared to the two preceding non-change trials, and this increase was independent of the identity of the change stimulus (letter or shape). Other studies have found that the size of the SCR is proportional to the degree of (e.g., physical or semantic) mismatch between the standards and the oddball (e.g., Siddle & Heron, 1976).
Motivational significance (e.g., task relevance) is also an important determinant of SCR amplitude (Bernstein, 1979; Maltzman, 1979a, b). Instructing the subject to perform a voluntary response to a stimulus generally increases the corresponding SCR compared to when the stimulus is not associated with any task (Bernstein & Taylor, 1979; Siddle et al., 1979). Bernstein, Taylor, and Weinstein (1975) reported enhanced SCRs when subjects were required to respond to a designated class of auditory stimulus in a sequence of tones, compared to when they were asked to merely listen to the sequence. In addition, when subjects were instructed to respond only when the target was presented to one of their ears, the target-evoked SCR was larger for targets presented to the task-relevant compared to the task-irrelevant ear. Enhanced SCRs have also been observed in response to conditioned stimuli that indicate a high probability or intensity of physical punishment (Öhman, Bjorkstrand, & Ellstrom, 1973), and to other stimuli with learned significance, such as one’s own name (Siddle et al., 1979). Finally, the unexpected omission of an unconditioned stimulus can elicit a SCR, again illustrating that it is the meaning rather than the physical properties of an event that elicits the orienting response.
Like P3 amplitude, SCR amplitude increases monotonically with stimulus intensity (Barry, 1975; Jackson, 1974; Turpin & Siddle, 1979). This effect appears independently of whether subjects are passively observing or actively responding to the stimuli (Roth et al., 1984). The initial SCR to salient, task-irrelevant stimuli (often referred to as involuntary orienting response; Maltzman, 1979a) habituates with repeated stimulus presentations (Barry, Feldman, Gordon, Cocker, & Rennie, 1993), with the rate of habituation being slower for more salient stimuli (Raskin, Kotses, & Bever, 1969). In contrast, the SCR to task-relevant stimuli (the voluntary orienting response) usually shows little or no habituation (e.g., Van Olst, Heemstra, & Ten Kortenaar, 1979). The amplitude of the SCR response upon initial presentation of a stimulus is directly related with the probability of long-term recall of that stimulus (Kleinsmith & Kaplan, 1964; Maltzman, Kantor, & Langdon, 1966), mirroring the relationship between P3 amplitude and recall.
Although the antecedent conditions for the P3 and SCR are highly similar across different experiments, the few studies that directly compared these measures within the same experiment offer mixed results. Such direct comparisons have been uncommon because they are limited by methodological factors. The low signal-to-noise ratio of ERPs demands that they are averaged across many trials. ERPs are rapid (<1 sec) and interstimulus intervals are usually short to allow for many repeated trials. In contrast, the SCR does not require averaging and is typically investigated using long interstimulus intervals (>10 sec) to allow for its protracted time course, thus limiting the total number of trials that can be obtained.
Verbaten (1983) measured the P3 and SCR to repeated presentations of schematic pictures while requiring the subjects to either passively watch stimuli or memorize them. Regardless of the instruction, the amplitude of the frontocentral P3 and the SCR showed a significant decrease over multiple stimulus presentations, whereas the posterior P3 did not. Two other studies, both using an active auditory oddball task, compared the P3 across trials with and without a SCR (Bahramali et al., 1997; Halgren & Marinkovic, 1995). In both studies the P3 was reliably larger for SCR-present than for SCR-absent trials, but only at frontocentral electrodes; at posterior electrodes the P3 showed very little difference between these trial groups. Lyytinen, Blomberg, and Näätänen (1992) have reported similar results with a passive auditory oddball task. Roth, Blowers, Doyle, and Kopell (1982) obtained single-trial estimates of P3 amplitude (after low-pass filtering) and SCR amplitude using a passive auditory oddball paradigm, and found no significant correlation between these measures. However, as we will discuss below, this null result might be attributable to extraneous sources of variance inherent to both signals. Finally, two recent studies have compared habituation of the SCR and P3 to repetitive task-irrelevant stimuli in a typical orienting response paradigm with long interstimulus intervals of 8 seconds (Rushby, Barry, & Doherty, 2005) and 2 minutes (Rushby & Barry, 2009). In Rushby et al. (2005), SCR amplitude and P3 amplitude both showed clear habituation, response recovery (to a change stimulus) and enhanced responding (or dishabituation) to a re-presentation of the original stimulus. In Rushby and Barry (2009), SCR amplitude showed habituation over the first few trials of the stimulus train while the P3 showed a nonsignificant decreasing trend across all 12 presented tones. In both studies, principal component analysis was used to investigate habituation of subcomponents of the P3. The 3 extracted phasic subcomponents of the P3 in each study differed widely in terms of their correlation with the SCR across trials. Poor correlations were found between the SCR and a subcomponent corresponding with the P3a (both studies); moderate correlations between SCR and a subcomponent corresponding with the P3b (Rushby et al., 2005); and high correlations between the SCR and a relatively late, frontally distributed subcomponent that the authors labeled “novelty P3” (both studies).
The pupil dilation response
The stimulus-evoked PDR reflects contributions of the SNS and parasympathetic nervous system, which act in a relatively straightforward, reciprocal manner, with SNS activation closely coupled to parasympathetic inhibition (Beatty & Lucero-Wagoner, 2000). The SNS enlarges the pupil by direct stimulation of the dilator muscles. The contribution of the parasympathetic pathway is mediated by central inhibition of the Edinger–Westphal complex, resulting in relaxation of the sphincter muscles and hence dilation. The PDR has a typical onset latency between 200–500 ms after the stimulus, peaks about 1 second later, and terminates rapidly upon completion of stimulus processing. This phasic, stimulus-evoked activity can be distinguished from the more protracted pupil effects of mental processing load, which will not be considered here (for review, see Beatty, 1982).
Though considerably smaller in size, the literature on the antecedent conditions for the PDR is generally consistent with the P3 and SCR literatures summarized above. Like the P3 and SCR, the PDR is highly sensitive to stimulus probability. For example, Qiyuan and colleagues systematically manipulated stimulus probability in an active auditory oddball task and found that the magnitude of the PDR to both targets and non-target stimuli was inversely proportional to their probability (Qiyuan, Richer, Wagoner, & Beatty, 1985). The unexpected absence of a stimulus also evoked a reliable PDR. Friedman, Hakerem, Sutton, and Fleiss (1973) recorded both pupil diameter and the electroencephalogram (EEG) in a passive auditory oddball task in which the relative probability of the two stimulus categories was systematically varied across conditions. Subjects were either told (certain condition) or were asked to guess (uncertain condition) which stimulus would occur on a given trial. Both the PDR and the P3 increased in amplitude as the probability of the eliciting stimulus decreased, but only in the uncertain condition. Steinhauer and Hakerem (1992) report a similar inverse relation between stimulus probability and the amplitudes of the P3 and PDR, both when subjects were counting auditory oddballs, and when they were responding to both stimulus categories (see also Steinhauer & Zubin, 1982).
Regarding the effects of task relevance and stimulus value on the PDR, the available evidence is limited. Peavler (1974) measured the pupillary response while subjects were listening to a string of digits. Significant dilation occurred only when subjects were told they would later be tested for recall, and not when no task was associated with the stimuli. However, it should be noted that the reported pattern (monotonic increase in pupil dilation with each presented digit) was not phasic in nature but instead resembled the tonic dilation effects associated with mental processing load (Beatty, 1982). Van Olst et al. (1979) found larger PDRs to targets than to non-target stimuli in an active auditory oddball task, even though the two stimulus categories were equiprobable (but see Qiyuan et al., 1985). Pleasant stimuli, such as erotic pictures, and stressors typically cause large pupil dilation (Bradley, Miccoli, Escrig, & Lang, 2008, Janisse, 1977), suggesting an important influence of stimulus value.
The evidence concerning the effect of stimulus intensity on the PDR is also sparse. One study has reported that PDR amplitude increases with the intensity of auditory noise (80–100 dB; Antikainen & Niemi, 1983). In another study it was found that stimulus intensity (60, 75, or 100 dB) did not affect PDR amplitude. However, it is worth noting that in this study each level of intensity was presented to a different group of subjects, precluding context-sensitive scaling of pupil responses (Stelmack & Siddle, 1982). Finally, several studies have reported reliable habituation of the PDR across multiple presentations of task-irrelevant stimuli (Antikainen & Niemi, 1983; Maher & Furedy, 1979; Stelmack & Siddle, 1982).
The relationship between PDR amplitude and performance accuracy mirrors that observed for P3 amplitude and accuracy. That is, under data-limited conditions, larger pupil dilations are generally associated with better performance. Like for the P3, the main evidence comes from studies using the signal-detection paradigm. Hakerem and Sutton (1966) required subjects to detect near-threshold stimuli in a visual signal-detection task. These stimuli elicited a PDR only on trials in which the stimulus was correctly reported as seen. Beatty and Wagoner (1976, unpublished data; cited in Janisse, 1977) found similar results using an auditory signal-detection task: On signal-present trials, larger PDRs were associated with significantly more correct decisions and with higher confidence that the decisions made were correct.
Discussion
The above review reveals a striking resemblance between the conditions that evoke the P3, and those associated with two exemplary components of the orienting response, the SCR and the PDR. All three measures are preferentially sensitive to novelty, motivational significance (e.g., task relevance), and other salient stimulus characteristics that are potentially important for survival and goal-directed behavior. In addition, all three measures show clear evidence of habituation with repeated presentation of task-irrelevant stimuli. The occurrence of a P3, SCR or PDR is also clearly associated with better task performance. As described above, similar observations by other authors, mainly in the 1970s and 1980s, led to the question of how exactly the P3 might be related to autonomic components of the orienting response. In the next section, we discuss a recently proposed theory of the P3 that suggests a straightforward account of this relationship.
Before we turn to this theory, two issues are worth noting. First, the data reviewed above suggest that the SCR is more strongly correlated with P3 activity at frontocentral electrodes than with posterior P3 activity. The possible significance of this finding will be considered in the section ‘Summary and discussion’. Second, the few studies that have co-registered the P3 and autonomic nervous system measures have reported small or absent correlations between these phenomena. Although such findings may appear at odds with the remarkable similarities in the antecedents for these responses, it should be kept in mind that the between-subject correlations between the P3 and autonomic nervous system measures will be affected by their differential susceptibility to several types of variables (cf. Steinhauer & Hakerem, 1992). Such variables include, for example, exogenous influences like ambient lighting conditions and temperature, recent smoking behavior and time-of-last-meal, that may affect autonomic nervous system measures differently than the P3 (e.g., Polich & Kok, 1995). Studies that examined within-subject, cross-trial correlations between the P3 and autonomic nervous system responses have struggled with the vast differences in the time scales on which these response can be assessed. Attempts to overcome these methodological challenges are direly needed.
Link between the P3 and the locus coeruleus-norepinephrine (LC-NE) system
Recent research has suggested that the neuromodulatory brainstem nucleus locus coeruleus (LC) is critical for the regulation of cognitive performance (Aston-Jones & Cohen, 2005; Nieuwenhuis & Jepma, in press; Robbins, 1997; Sara, 2009; Yu & Dayan, 2005). The LC exhibits a strong phasic increase in activity during the processing of motivationally relevant stimuli, leading to the release of the neuromodulatory neurotransmitter norepinephrine (NE) in widespread cortical projection areas. This LC-mediated noradrenergic innervation increases the responsivity (or gain) of efferent target neurons (for a review, see Berridge & Waterhouse, 2003). It has been shown that when applied in a temporally strategic manner (e.g., when driven by the identification and evaluation of motivationally relevant stimuli), increases in gain produce an increase in the signal-to-noise ratio of subsequent processing and a concomitant improvement in the efficiency and reliability of behavioral responses (e.g., Servan-Schreiber, Printz, & Cohen, 1990). Accordingly, it has been found that LC phasic activation reliably precedes and is temporally linked to behavioral responses to attended stimuli (Aston-Jones, Rajkowski, Kubiak, Alexinsky, 1994; Bouret & Sara, 2004; Clayton, Rajkowski, Cohen, & Aston-Jones, 2004).
According to a recent theory, the scalp-recorded P3 is the electrophysiological correlate of LC-induced phasic enhancement of neural responsivity (gain) in the neocortex (Nieuwenhuis, Aston-Jones et al., 2005); this theory follows from earlier experimental work (Desmedt & Debecker, 1979 and Pineda, Foote, & Neville, 1989). Strong evidence for subcortical involvement in P3 generation has come from a study showing largely intact P3 components to unilaterally presented visual stimuli in the unstimulated hemisphere of a split-brain patient (Kutas, Hillyard, Volpe, & Gazzaniga, 1990). Given that in split-brain patients interhemispheric transfer of information is not possible at the cortical level, this finding indicates that critical input and/or output signals of the P3 process must have passed through one of the intact subcortical commissures.
Here, we briefly summarize the evidence for a specific role of LC phasic activity in P3 generation. (For an extensive review and a comparison with the context-updating hypothesis, see Nieuwenhuis, Aston-Jones et al., 2005.) First, the distribution and timing of intracranial and scalp-recorded P3 activity are consistent with the anatomical and physiological properties of the LC-NE system. For example, the diffuse P3 scalp distribution and observation of P3 activity in multiple intracranial structures (Soltani & Knight, 2000) are consistent with the widespread projections from the LC to cortical and subcortical areas. Furthermore, P3 onset latency in simple two-alternative forced choice tasks is consistent with the latency of LC phasic activity (~150–200 ms), if one takes into account the relatively slow conduction velocity of LC fibers (Aston-Jones, Foote, & Segal, 1985). Additionally, the relatively early timing of P3 activity in frontal (P3a) and subcortical areas (e.g., thalamus; Klostermann et al., 2006) is consistent with the trajectory of LC fibers, which first reach these areas and only then veer backwards to innervate posterior cortical areas (Morrison, Molliver, Grzanna, & Coyle, 1981), where the P3b is generated. Because the neuromodulatory effect of NE—presumed to be reflected in the P3—is to enhance processing in target areas, brain areas that are most engaged by a given task should show the greatest increases in activity. This may explain why the relative contribution of the P3a and P3b to the overall P3 scalp topography depends on the antecedent conditions. For example, the large P3a to novel stimuli, leading to a more anterior focus of the P3 scalp distribution, may reflect the greater contribution of prefrontal structures to novelty processing (Soltani & Knight, 2000), an effect that is enhanced by LC-NE engagement.
Second, the antecedent conditions for the P3, discussed above, are highly similar to those for the LC phasic response (for review, see Aston-Jones, Rajkowski, & Cohen, 2000). The LC phasic response is preferentially elicited by motivationally significant stimuli, including stimuli that are novel or unexpected, conditioned stimuli that require a response (e.g., in an oddball task), unconditioned auditory startle stimuli, appetive and aversive stimuli. The LC phasic response after task-irrelevant auditory stimuli varies directly with the intensity of those stimuli (Grant, Aston-Jones, & Redmond, 1988). Like the P3, the LC phasic response is relatively insensitive to the physical attributes of stimuli, and habituates as the salience of task-irrelevant stimuli decreases with repeated presentations (Sara, Vankov, & Herve, 1994). However, LC responses do not exhibit habituation for highly salient or task-relevant stimulus events (Aston-Jones & Bloom, 1981; Aston-Jones, Rajkowski et al., 1994). The parallel between the P3 and the LC phasic response is further supported by a study that simultaneously recorded the two phenomena, and found that their changes in amplitude in response to experimental manipulations followed a very similar time course (Aston-Jones, Chiang, & Alexinsky, 1991).
Third, several studies have reported direct evidence for an LC generator of the P3. These include psychopharmacological studies, which have shown that P3 amplitude is modulated in a systematic fashion by noradrenergic agents such as clonidine (Swick, Pineda, & Foote, 1994), and entirely abolished following drug-induced norepinephrine depletion (Glover, Ghilardi, Bodis-Wollner, & Onofrj, 1988). Lesion studies have demonstrated a selective decrease in P3 amplitude in monkeys sustaining LC lesions (Pineda et al., 1989). Also, a recent study has found that individual differences in the noradrenergic gene that affects the activity of the alpha-2a receptor are a key determinant of P3 amplitude (Liu et al., 2009).
Finally, the tight link between the P3 and task performance (i.e., speed and accuracy) is consistent with the functional role ascribed to LC phasic activity, namely to facilitate post-decisional information processing and behavioral responding. Indeed, LC activity itself is closely related to behavioral performance: Larger LC responses are associated with higher performance accuracy, and the latency of LC phasic responses is positively correlated with the overt reaction times (Aston-Jones, Rajkowski et al., 1994; Aston-Jones et al., 2000; Clayton et al., 2004) a pattern similar to that for the P3. We note that the potentiating influence of the LC-NE system on behavioral responding is likely to be modest in typical laboratory tasks, which use simple stimuli and discrete button-press responses. These tasks are performed so quickly that the noradrenergic modulation of the relevant cortical areas (as reflected in the P3) may sometimes occur too late to facilitate the response. It is plausible that the facilitatory influence of the LC-NE system is more prominent in real-life situations, which are characterized by multimodal, crowded sensory environments and a range of potential, often time-consuming response options.
In sum, there is converging evidence from multiple research disciplines that indicates a crucial role for the LC-NE system in generating the P3. As we will discuss below, the tight relationship between the LC-NE system, the P3, and the orienting response is further supported by several findings that suggest a strong temporal correlation between LC-NE activity and SNS activity.
Correlation between LC-NE activity and SNS activity
The types of stimuli that are most effective for eliciting LC phasic responses are those that stop ongoing behavior and elicit a behavioral orienting response (Aston-Jones & Bloom, 1981; Aston-Jones, Valentino, Van Bockstaele, & Meyerson, 1994). This observation is supported by several studies that have measured correlations between autonomic nervous system changes and LC activity across various time scales. For example, Elam and colleagues found that noxious and non-noxious sensory stimuli produced parallel changes in LC-NE unit activity and peripheral sympathetic nerve discharge in rats (Elam, Svensson, & Thoren, 1986). Abercrombie and Jacobs (1987) found that changes in LC-NE activity induced by chronically presented stressful stimuli were closely correlated with changes in heart rate in cats. Studies examining LC activity after physiological manipulations that cause autonomic activation found that the two were frequently correlated, with higher LC activity for hypoglycemia, hypotension, hypervolemia, ambient heating and pyrogen-induced fever (Morilak, Fornal, & Jacobs, 1987a, 1987b, 1987c). Furthermore, Reiner (1986) reported parallel changes in activity of LC neurons and peripheral sympathetic tone across the stages of the sleep-wake cycle in behaving cats.
Finally, neurophysiological recordings in the monkey have indicated that tonic changes in pupil diameter closely track the time course of LC activity, and show the same relationship to behavioral performance as tonic LC activity (Aston-Jones & Cohen, 2005, Figure 7; Rajkowski, Kubiak, & Aston-Jones, 1993). Some of these findings have recently been corroborated in a series of human pupillometry experiments (Gilzenrat, Nieuwenhuis, Jepma, & Cohen, in press; Jepma & Nieuwenhuis, submitted). In these experiments, it was found that the magnitudes of baseline pupil diameter and task-evoked pupil dilations were inversely correlated, corresponding to the reciprocal relationship observed between LC tonic and phasic modes (Aston-Jones et al., 2000; Usher, Cohen, Servan-Schreiber, Rajkowski, & Aston-Jones, 1999). Furthermore, these measures of pupil diameter were sensitive to experimental manipulations of task utility, and predictive of behavioral indices of task (dis)engagement and exploratory behaviors in a manner consistent with predictions of the adaptive gain theory of LC function (Aston-Jones & Cohen, 2005).
The observed similarity in antecedent conditions and correlations between autonomic nervous system and LC-NE activity suggest that the LC-NE system in the brain is a central analogue of the peripheral SNS (cf. Amaral & Sinnamon, 1977; Aston-Jones et al., 1991), and that the two systems often operate in an integrated fashion. They also lend further credence to the notion that autonomic components of the orienting response and the P3 are intimately coupled. The critical question that motivates much of the remainder of this article is how the parallel activation of the SNS and the P3 (as a correlate of phasic LC-NE activity) can be understood in anatomical terms, given existing knowledge about the anatomy of the LC-NE system.
Anatomical link: Parallel activation of the LC-NE system and peripheral SNS by the rostral ventrolateral medulla
It is unlikely that the parallel activation of the LC-NE system and peripheral SNS reflects a direct influence of one on the other. Contrary to occasional claims in the literature (e.g., Szabadi & Bradshaw, 1996), there is no reliable evidence for a direct projection from the LC to the autonomic nuclei that regulate the pupil, sweat glands, heart, and other organs (Aston-Jones, 2004). Instead, some of these nuclei are innervated by lower medullary NE cell groups (e.g., Levitt & Moore, 1979).Although there is substantial evidence that autonomic (mainly cardiovascular) responses have an influence on LC activity (Berntson, Sarter, & Cacioppo, 1998; Morilak et al., 1987a, 1987b, 1987c; Svensson, 1987), this anatomical route is too slow to explain the rapid, phasic LC responses to motivationally significant stimuli and the resulting P3.
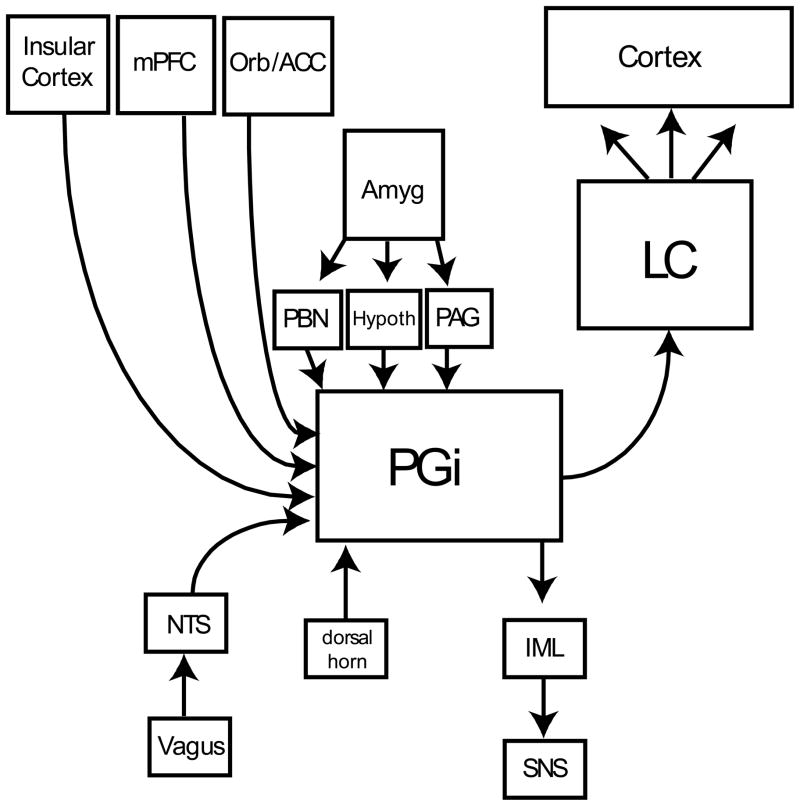
As an alternative explanation for the parallel activation of the LC and the SNS, we propose an anatomical model that introduces a third player: the nucleus paragigantocellularis (PGi), a highly integrative nucleus in the rostral ventrolateral medulla that plays a pivotal role in controlling both the LC and the SNS (see Figure 1). The PGi provides one of the major excitatory inputs to the LC (Aston-Jones, Ennis, Pieribone, Nickell, & Shipley, 1986). Furthermore, pharmacologic blockade of the PGi blocks LC responses to somatosensory stimulation (Chiang & Aston-Jones, 1993; Ennis & Aston-Jones, 1988), as does blockade of glutamate transmission in the LC, the major neurotransmitter in the PGi-to-LC pathway (Ennis & Aston-Jones, 1988). These and other studies have indicated that the PGi is a critical relay center for mediating the phasic LC responses evoked by at least certain sensory stimuli.
Figure 1.
Importantly, the PGi is also a key sympathoexcitatory brain region, with strong projections that directly innervate preganglionic sympathetic neurons of the intermediolateral cell column of the spinal cord (Guyenet, 1990; Loewy, Wallach, & McKellar, 1981). These preganglionic cells send axons to specific ganglia in the peripheral nervous system and synapse on postganglionic neurons, which in turn innervate various peripheral targets such as the dilator muscle of the pupil and the sweat glands. Stimulation of the PGi elicits electrodermal responses, pupil dilations, increases in blood pressure, and other sympathetic responses (Davison & Koss, 1975; Guyenet, 1990; Koss & Wang, 1972). Interestingly, stimuluation of PGi neurons can also increase cortical arousal, as indicated by changes in the EEG power spectrum (Wu, Stavarache, Pfaff, & Kow, 2007). The location of the PGi in the medullary reticular formation is consistent with early proposals regarding the origin of the orienting response in the reticular formation (Sokolov, 1963, 1975), and with classic experiments showing that stimulation of the reticular formation by implanted electrodes reproduces the autonomic, behavioral and EEG components of the orienting response (Moruzzi & Magoun, 1949; Scheibel, 1980). It is noteworthy, however, that evidence indicates that neurons projecting to sympathetic preganglionic areas and to the LC are often distinct but interdigitated cells in the ventrolateral medulla (Huangfu, Verberne, & Guyenet, 1992). Thus, the frequently observed parallel regulation of the LC and the SNS seems to involve similar inputs to parallel but distinct neurons innervating spinal sympathetic areas and LC.
Anatomical studies have revealed that the PGi itself receives inputs from a wide variety of brain areas involved in autonomic and visceral regulation, including the periaqueductal grey, the hypothalamus, and the insular cortex, and from multimodal association areas including the medial prefrontal cortex (Van Bockstaele, Aston-Jones, Ennis, Shipley, & Pieribone, 1991; Van Bockstaele, Pieribone, & Aston-Jones, 1989). The PGi is a critical relay center for the descending sympathoexcitatory pathways originating in the hypothalamus (Hilton & Smith, 1984). Furthermore, emotional signals from the amygdala may reach the PGi by way of the hypothalamus and periaqueductal grey. Thus, the PGi integrates several types of autonomic and sensory information, and provides potent parallel activation of the LC and the peripheral SNS (Aston-Jones, Valentino et al., 1994). These properties are consistent with the simultaneous occurrence of the P3 and OR in response to a wide array of motivationally significant events.
Alternative anatomical routes
Our model posits that the LC provides no direct efferent innervation of the nuclei subserving sympathetic tone, because the LC does not project substantially to preganglionic autonomic nuclei. However, experimental manipulation of the LC-NE system can result in changes in SNS activity. An early study found that electrical stimulation of the cat LC elicited an increase in heart rate and blood pressure (Gurtu, Pant, Sinha, & Bhargava, 1984). However, these effects must have been the result of activating other structures, either structures nearby the LC such as the periaqueductal grey, or antidromically activated afferents such as the PGi, because chemical stimulation of the LC decreases both blood pressure and heart rate (Murase, Takayama, & Nosaka, 1993; Sved & Felsten, 1987). These responses were eliminated by chemical destruction of noradrenergic LC neurons using local injection of 6-hydroxydopamine, a selective neurotoxin of catecholamine neurons. Lesions induced by 6-hydroxydopamine in the rat and cat dorsal noradrenergic bundle also resulted in the complete abolition of auditory-evoked SCRs (Yamamoto, Arai, & Nakayama, 1990; Yamamoto, Hoshino, Takahashi, Kaneko, & Ozawa, 1991).
In rats, the alpha-2 adrenoceptor agonist clonidine (which inhibits LC activity and decreases NE release) decreased the SCR amplitude, whereas the alpha-2 adrenoceptor antagonist yohimbine (which increases LC activity and NE release) substantially increased the amplitude of the SCR (Yamamoto, Ozawa, Shinba, & Hoshino, 1994). Similarly, Saiers and Campbell (1990) reported that a decrease of noradrenergic activity as a result of clonidine injections in rats disrupted the heart rate component of the orienting response to an auditory stimulus. In contrast, pharmacological modulations of the dopaminergic, cholinergic, and serotonergic systems did not affect heart rate responses. Administration of clonidine and yohimbine have also been found to change baseline pupil diameter (Koss, 1986; Phillips, Szabadi, & Bradshaw, 2000). However, it is hard to determine whether the effects of such noradrenergic agents on autonomic activity are mediated by adrenoceptors located on LC neurons or by other adrenoceptors, for example located on the lower medullary NE cell groups, which directly innervate autonomic nuclei, or even on autonomic nuclei themselves. Furthermore, some of the reported effects may be a consequence of a reduction in parasympathetic tone, instead of an increase in sympathetic tone (Koss, 1986).
As no direct anatomical connections have been documented between the LC and autonomic nuclei, there is no straightforward way to explain the effects of LC lesions/manipulations on SNS activity other than that they are produced via indirect pathways. Indeed, there are a number of possible indirect pathways by which LC manipulation could affect the SNS. In particular, the LC has dense ascending projections to various important higher brain centers involved in SNS control, which in turn project directly or indirectly to autonomic nuclei that regulate peripheral SNS responses (cf. Berntson et al., 1998). Anatomical and physiological studies suggest that these control centers include the anterior cingulate, the insula, amygda and hippocampus (Verberne & Owens, 1998). Electrical or chemical stimulation of these LC projection areas elicits a wide range of peripheral sympathetic responses, and lesions damaging these areas tend to reduce or abolish these peripheral responses (Critchley, 2002; Jordan, 1990; Knight, 1996; Verberne & Owens, 1998). Converging evidence from fMRI studies confirms that activity in the anterior cingulate and insula is modulated by stimulus frequency and novelty, consistent with a role for these regions in orienting to motivationally significant stimuli (Ranganath & Rainer, 2003). Finally, the hypothalamus is another major component of the descending pathways that regulate sympathetic and vagal neurons. However, LC projections to the hypothalamus are quite limited (Aston-Jones, 2004), indicating that other areas are the critical links in the circuit connecting the LC with the SNS.
Functional significance of the P3 and the orienting response: mobilization for action
Theoretical accounts of the function of the orienting response generally distinguish between two components: enhancing the perception of the eliciting event and facilitating action in response to the stimulus. The latter component refers to the energizing quality of the orienting response—the mobilization of somatic and autonomic systems for dealing with the immediate consequences of the triggering stimulus. As reviewed by Lynn (1966), somatic responses include the inhibition of ongoing activity, increases in general muscle tone that prepare the muscles for action, and changes in the skeletal muscles that direct the sense organs towards the source of the stimulus. This directional motor activity (e.g., ocular motion, pricking of the ears in animals) likely reflects the interaction of the spatially nonspecific orienting response with brain systems specialized in directing spatial attention. The multifaceted autonomic response, including cardiovascular, respiratory, hormonal, and pupillary changes, likewise seems to prepare the body for efficient action and increased energy expenditure (Lynn, 1966).
Other theorists have emphasized the importance of the orienting response for enhancing sensory processing of the eliciting stimulus (Graham, 1979; Pavlov, 1927; Sokolov, 1963). The orienting response is associated with increased sensory receptor sensitivity, lowering perceptual thresholds (Lynn, 1966). However, aside from this, there is little empirical support for the notion that the orienting response enhances information uptake. For example, there is little or no evidence for the argument that a large pupil enhances perceptual sensitivity (cf. Lynn, 1966). Of course, information uptake will be enhanced by the orienting of body and sense organs, but these are motor changes, not changes in the perceptual system or SNS. Some psychophysiologists have also questioned the use of the orienting response for enhancing perceptual processing, given that the elicitation of the orienting response is contingent on a perceptual analysis of the stimulus for determining its motivational significance (e.g., Siddle & Spinks, 1979). This argument forces theorists to be explicit about their model of information processing and the corresponding aspects of perception that are enhanced by the orienting response. These limitations of the sensory-intake hypothesis, along with the apparent consensus that the distinction between the benefits for perception vs action afforded by the orienting response is largely irrelevant from a selection-for-action perspective (Allport, 1987), have led to the view that the primary role of the orienting response is in the mobilization for action (e.g, Donchin et al., 1984).
As discussed earlier, the theoretical integration of the OR and P3 literatures was challenged in the 1980s by the discrepancy between the action-oriented view of the orienting response and the reigning theory of the P3: the context-updating hypothesis (Donchin, 1981; Donchin & Coles, 1988). Donchin’s hypothesis was strongly inspired by Sokolov’s ideas about the antecedent conditions for the orienting response: the P3 was assumed to be elicited when there is a mismatch between a subject’s representation (“neuronal model”) of the environment (in its broadest sense) and actual experience. However, the context-updating hypothesis attributed a different function to the process manifested by the P3 process, namely the updating of the representation of the environment (context) to optimize decision making in response to future stimuli. Thus, according to this hypothesis the P3 reflects a strategic or memory function rather than the facilitation of responses.
Unlike the context-updating hypothesis, the recent theory that the P3 reflects phasic activity of the LC-NE system (Nieuwenhuis, Aston-Jones et al., 2005) suggests a shared functional interpretation of the P3 and the orienting response. For its assumptions about the functional significance of the P3, the theory draws on our current understanding of the function of LC phasic responses, which indicates that these LC responses facilitate behavioral responses to the outcome of task-specific decision processes (Aston-Jones & Cohen, 2005; Bouret & Sara, 2005). In addition, the broad projections of LC-NE neurons indicate that responses in these cells may also augment other processes important for decision execution besides motor activity, including sensory perception and memory (Hurley, Devilbiss, & Waterhouse, 2004; Sara et al., 1994). As reviewed above, the ensuing action-oriented view of the P3 is supported by the tight link between the latency and amplitude of the P3 and corresponding task performance. Taken together, this analysis suggests that the P3 and SNS components of the orienting response can be seen as manifestations of a global sympathetic system specialized in mobilization for action. The central nervous system limb of this system, the LC-NE system (manifested in the P3), facilitates the execution of cognitive decisions concerning proper behaviors in the face of urgent stimulus demand, while at the same time, the autonomic nervous system limb (manifested in the orienting response) facilitates physical execution of the chosen behaviors.
This theoretical integration of the orienting response and P3 suggests a highly efficient system for urgent responding: Stimuli are analyzed by cortical (and perhaps subcortical) areas capable of performing the precise computations that determine whether a stimulus is task-relevant or otherwise motivationally significant and what response should be elicited. The output of this analysis is passed down to lower brain areas, including the rostral ventrolateral medulla, which in turn projects to the LC and autonomic nuclei. In case a motivationally significant stimulus is detected and a response decision is reached, the LC is activated and produces a system-wide transient innervation of the brain (P3) that facilitates further processing of the eliciting stimulus and other stimuli related to the decision reached, speeding up the deployment of attention and the execution of a behavioral response. Simultaneously, the SNS is activated (orienting response) to facilitate motor action in response to the stimulus.
Summary and discussion
In this article, we have discussed the similarities between two psychophysiological phenomena: the orienting response, a collection of physiological responses in order to effectively cope with motivationally significant events; and the ubiquitous P3, the single most-studied component of the event-related potential. The orienting response and P3 generally co-occur; as we have reviewed, both are elicited by stimuli with learned or inherent motivational significance. This has raised the question whether the P3 should be seen as the central nervous system counterpart to the SNS components of the orienting response (Donchin et al., 1984; Friedman, 1978; Kimmel et al., 1979). The analysis of anatomy and function presented here suggests that the answer to this question is a cautious ‘yes’.
With regard to anatomy we have discussed that there is no direct connection between SNS nuclei and the LC,, the most probable initial generator of the P3 (Nieuwenhuis, Aston-Jones et al., 2005). Furthermore, the sensory feeback of actual autonomic responses to the LC is too slow to explain the rapid, phasic LC responses to motivationally significant stimuli. Instead, it is likely that the tight link between P3 and orienting response reflects common afferent projections to the LC and sympathetic preganglionic neurons: the major source of input to the LC is a key sympathoexcitatory region of the rostral ventrolateral medulla—in particular the PGi. This highly integrative medullary area could be responsible for the observed parallel activation of the LC-NE system and peripheral SNS in response to various types of motivationally significant stimuli. The LC and its ascending projections thus carry efferent copies of the medulla’s command signals to the peripheral SNS. This suggests that the LC-NE system may implement one of Damasio’s (1999) ‘as-if’ loops—the notion that somatic markers can reflect not only states of the body (e.g., in the peripheral SNS) but also representations (i.e., copies) of body states in, for example, brainstem neuromodulatory systems. In other words, feedback from the body is short-circuited by direct signals from brainstem areas to regions representing body state.
With regard to function, a comparison of the functional significance of the LC-NE system and SNS suggests that the P3 and orienting response reflect complementary contributions to the mobilization for action following motivationally significant stimuli. Phasic LC responses (giving rise to the P3) may optimize information processing following decisions regarding appropriate behavioral responses at the same time that the peripheral sympathetic system prepares the subject physically to execute these responses. The wide range of antecedent conditions for the P3 and orienting response is consistent with our anatomical model, given the integrative properties of the rostral ventrolateral medulla. There has been a lot of debate about the common denominator of these antecendent conditions (e.g., Bernstein, 1979; Maltzman, 1979; O’Gorman, 1979). We doubt whether this is a useful debate; the brain has evolved to favour the processing of salient, significant, unexpected and novel stimuli (Corbetta, Patel, & Shulman, 2008; Desimone & Duncan, 1995; Ranganath & Rainer, 2003) and the antecedent conditions for the P3 and orienting response merely reflect this preference.
A challenge for our anatomical model, suggested by our review of the similarities between the orienting response and P3, is that the SCR seems more strongly correlated with P3 activity at frontocentral electrodes (P3a) than with posterior P3 activity (P3b). A speculative explanation for this finding is that posterior cortical areas, which are not directly connected to the LC, synapse on frontal neurons that are directly connected to the LC (Arnsten & Goldman-Rakic, 1984; Aston-Jones & Cohen, 2005; Lee, Kim, & Waterhouse, 2005), whereas other frontal neurons connect to the LC via the rostral ventrolateral medulla (Van Bockstaele et al., 1989). As we have discussed, there are also a number of possible indirect pathways by which the LC could affect the SNS. At the level of the cortex these pathways include frontal areas (e.g., anterior cingulate cortex) and not posterior areas, which may also partly account for the pattern of correlation between P3a, P3b and orienting response. Future research should address these possibilities. Our theory of the P3 also suggests another, more general explanation for dissociations between the orienting response and subcomponents of the P3. The theory claims that the P3 reflects the neuromodulatory effect of NE in cortical target areas. Therefore, cortical areas that are most engaged by a given stimulus or task should show the greatest increases in activity, and cortical areas that are not involved should show little or no noradrenergic modulation. The implication of this conjecture is that a putative correlation between the orienting response and a P3 subcomponent may be confounded by variance in the involvement of the cortical areas that directly generate the P3 subcomponent.
However, it is implausible that these explanations can account for all of the observed dissociations between the orienting response and P3 subcomponents. Indeed, although our anatomical model offers an attractive account of the similarities between these phenomena, it is unlikely to provide a complete account of their relationship. Both the locus coeruleus and sympathetic preganglionic neurons receive projections from a wide range of areas other than the rostral ventrolateral medulla, including forebrain, hypothalamic and other brainstem areas (Aston-Jones 2004; Berridge & Waterhouse 2003; Dampney et al 2003; Sved et al 2001).
Our analysis has several other limitations. First, although our hypothesis about the relationship between the orienting response and P3 is based on a multitude of data from psychophysiologal and neurophysiological literatures that have not previously been connected in this context, the exact value of the hypothesis awaits new empirical tests. For example, future animal studies could simultaneously record cell activity in the PGi, the scalp P3, and components of the orienting response, and test the prediction that the corresponding measures should be highly correlated. Second, our analysis is too focused to do justice to some of the complexities of the orienting response literature, for example the subtle but crucial difference between the orienting response and the defense reflex (Graham, 1979). A third limitation is that our analysis is fully focused on the role of the LC-NE system in generating the P3, even though other neurochemical systems almost certainly influence the P3 as well (Polich & Criado, 2006). A better understanding of the complex interactions between neuromodulatory systems such as the cholinergic, dopaminergic, and noradrenergic systems, would almost certaintly further enhance our understanding of the current topic (Briand, Gritton, Howe, Young, & Sarter, 2007).
These limitations notwithstanding, we believe that our analysis is a valuable step towards establishing the precise relationship between the P3 and the orienting response. More broadly, the present research illustrates the value of integrating the psychophysiological and neurophysiological literature. Knowledge of the LC-NE system played a crucial role in developing a novel hypothesis regarding the relationship between the P3 and peripheral manifestations of the orienting response—that they reflect the co-activation of the LC-NE system and the peripheral SNS by a common medullary pathway that has evolved to afford rapid action in response to motivationally significant stimuli.
Acknowledgments
This research was supported by the Netherlands Organization for Scientific Research.
Footnotes
By motivationally significant stimuli, we mean stimuli that are either relevant to the current task or that have the potential to be associated with some form of utility (positive or negative).
References
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. Journal of Neuroscience. 1987;7:2844–2848. doi: 10.1523/JNEUROSCI.07-09-02844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport DA. Selection for action: Some behavioral and neurophysiological considerations of attention and action. In: Heuer H, Sanders AF, editors. Perspectives on perception and action. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1987. pp. 395–419. [Google Scholar]
- Amaral DG, Sinnamon HM. The locus coeruleus: neurobiology of a central noradrenergic nucleus. Progress in Neurobiology. 1977;9:147–196. doi: 10.1016/0301-0082(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Antikainen J, Niemi P. Neuroticism and the pupillary response to a brief exposure to noise. Biological Psychology. 1983;17:131–135. doi: 10.1016/0301-0511(83)90013-3. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Research. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. Locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. 3. Elsevier Academic Press; San Diego: 2004. pp. 259–294. [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. Journal of Neuroscience. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Progress in Brain Research. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, Segal M. Impulse conduction properties of noradrenergic locus coeruleus axons projecting to monkey cerebrocortex. Neuroscience. 1985;15:765–777. doi: 10.1016/0306-4522(85)90077-6. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen JD. Locus coeruleus and regulation of behavioral flexibility and attention. Progress in Brain Research. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in the monkey are selectively activated by attended stimuli in a vigilance task. Journal of Neuroscience. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Valentino RJ, Van Bockstaele EJ, Meyerson AT. Locus coeruleus, stress, and PTSD: Neurobiological and clinical parallels. In: Murburg MM, editor. Cathecholamine Function in Post-Traumatic Stress Disorder: Emerging Concepts. 1. Washington, DC: American Psychiatric Press, Inc; 1994. pp. 17–62. [Google Scholar]
- Bahramali H, Gordon E, Lim CL, Li W, Lagopoulos J, Leslie J, Rennie C, Meares RA. Evoked related potentials associated with and without an orienting reflex. Neuroreport. 1997;18:2665–2669. doi: 10.1097/00001756-199708180-00006. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Low-intensity auditory stimulation and the GSR orienting response. Physiological Psychology. 1975;3:98–100. [Google Scholar]
- Barry RJ. A factor-analytic examination of the unitary OR concept. Biological Psychology. 1979;8:161–178. doi: 10.1016/0301-0511(79)90045-0. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Habituation of the orienting reflex and the development of Preliminary Process Theory. Neurobiology of Learning and Memory. 2009;92:235–242. doi: 10.1016/j.nlm.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Feldman S, Gordon E, Cocker KI, Rennie C. Elicitation and habituation of the electrodermal orienting response in a short interstimulus interval paradigm. International Journal of Psychophysiology. 1993;15:247–253. doi: 10.1016/0167-8760(93)90008-d. [DOI] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91:276–292. [PubMed] [Google Scholar]
- Beatty J, Lucero-Wagoner B. The pupillary system. In: Caccioppo J, Tassinary LG, Berntson G, editors. The Handbook of Psychophysiology. Hillsdale, NJ: Cambridge University Press; 2000. [Google Scholar]
- Bernstein AS. The orienting response as novelty and significance detector: reply to O’Gorman. Psychophysiology. 1979;16:263–273. doi: 10.1111/j.1469-8986.1979.tb02989.x. [DOI] [PubMed] [Google Scholar]
- Bernstein AS, Taylor KW. The interaction of stimulus information with potential stimulus significance in eliciting the skin conductance orienting response. In: Kimmel H, Van Olst E, Orlebeke J, editors. The orienting reflex in humans. Hillsdale, NJ: Lawrence Erlbaum; 1979. pp. 499–519. [Google Scholar]
- Bernstein AS, Taylor KW, Weinstein E. The phasic electrodermal response as a differentiated complex reflecting stimulus significance. Psychophysiology. 1975;12:158–169. doi: 10.1111/j.1469-8986.1975.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behavioral Brain Research. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. European Journal of Neuroscience. 2003;18:2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: Modulation of behavioral state and statedependent cognitive processes. Brain Research Reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus–medial frontal cortex interplay during learning. European Journal of Neuroscience. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Progress in Neurobiology. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G. Response of locus coeruleus neurons to footshock stimulation is mediated by neurons in the rostral ventral medulla. Neuroscience. 1993;53:705–715. doi: 10.1016/0306-4522(93)90618-p. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. Journal of Neuroscience. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Covington JW, Polich J. P300, stimulus intensity, and modality. Electroencephalography and Clinical Neurophysiology. 1996;100:579–584. doi: 10.1016/s0168-5597(96)96013-x. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Electrodermal responses: what happens in the brain. Neuroscientist. 2002;8:132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Davison MA, Koss MC. Brainstem loci for activation of electrodermal response in the cat. American Journal of Physiology. 1975;229:930–934. doi: 10.1152/ajplegacy.1975.229.4.930. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, editors. Handbook of Psychophysiology. 2. Cambridge University Press; Cambridge, U.K: 2000. pp. 200–223. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Debecker J. Wave form and neural mechanism of the decision P350 elicited without pre-stimulus CNV or readiness potential in random sequences of near-threshold auditory clicks and finger stimuli. Electroencephalography and Clinical Neurophysiology. 1979;47:648–70. doi: 10.1016/0013-4694(79)90293-1. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!...Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Donchin E, Heffley E, Hillyard SA, Loveless N, Maltzman I, Ohman A, Rosler F, Ruchkin D, Siddle D. Cognition and event-related potentials. II. The Orienting Reflex and P300. Annals of the New York Academy of Sciences. 1984;425:39–57. doi: 10.1111/j.1749-6632.1984.tb23522.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: The variation of event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Edelberg R. Electrodermal recovery rate, goal-orientation, and aversion. Psychophysiology. 1972;9:512–520. doi: 10.1111/j.1469-8986.1972.tb01805.x. [DOI] [PubMed] [Google Scholar]
- Elam M, Svensson TH, Thoren P. Locus coeruleus neurons and sympathetic nerves: activation by cutaneous sensory afferents. Brain Research. 1986;366:254–261. doi: 10.1016/0006-8993(86)91302-8. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. Journal of Neuroscience. 1988;8:3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roth WT, Kopell BS. Auditory evoked potentials to unpredictable shifts in pitch. Psychophysiology. 1976;13:32–39. doi: 10.1111/j.1469-8986.1976.tb03333.x. [DOI] [PubMed] [Google Scholar]
- Friedman D. The late positive component and orienting behavior. In: Otto D, editor. Multidisciplinary perspectives in event-related brain potential research. Washington, D.C: U.S. EPA; 1978. pp. 178–180. [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience & Biobehavioral Reviews. 2001;5:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Hakerem G, Sutton S, Fleiss JL. Effect of stimulus uncertainty on the pupillary dilation response and the vertex evoked potential. Electroencephalography and Clinical Neurophysiology. 1973;34:475–484. doi: 10.1016/0013-4694(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognitive, Affective, & Behavioral Neuroscience. doi: 10.3758/CABN.10.2.252. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover A, Ghilardi MF, Bodis-Wollner I, Onofrj M. Alterations in event-related potentials (ERPs) of MPTP-treated monkeys. Electroencephalography and Clinical Neurophysiology. 1988;71:461–468. doi: 10.1016/0168-5597(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Gonsalvez CL, Polich J. P300 amplitude is determined by target-to-target interval. Psychophysiology. 2002;39:388–396. doi: 10.1017/s0048577201393137. [DOI] [PubMed] [Google Scholar]
- Graham FK. Distinguishing among orienting, defense and startle reflexes. In: Kimmel HD, Olst EH, van Orlebeke JF, editors. The orienting reflex in humans. Lawrence Erlbaum Associates; New York: 1979. pp. 137–168. [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychological Bulletin. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Grant S, Aston-Jones G, Redmond DE. Responses of primate locus coeruleus neurons to simple and complex stimuli. Brain Research Bulletin. 1988;21:401–410. doi: 10.1016/0361-9230(88)90152-9. [DOI] [PubMed] [Google Scholar]
- Gurtu S, Pant KK, Sinha JN, Bhargava KP. An investigation into the mechanism of cardiovascular responses elicited by electrical stimulation of locus coeruleus and subcoeruleus in the cat. Brain Research. 1984;301:59–64. doi: 10.1016/0006-8993(84)90402-5. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Role of the ventral medulla oblongata in blood pressure regulation. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. pp. 145–167. [Google Scholar]
- Hakerem G, Sutton S. October 29) Pupillary response at visual threshold. Nature. 1966;212:485–486. doi: 10.1038/212485a0. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K. Neurophysiological networks integrating human emotions. In: Gazzaniga M, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. pp. 1137–1151. [Google Scholar]
- Hillyard SA, Squires KC, Bauer JW, Lindsay PH. Evoked potential correlates of auditory signal detection. Science. 1971 Jun 25;172:1357–1360. doi: 10.1126/science.172.3990.1357. [DOI] [PubMed] [Google Scholar]
- Hilton SM, Smith PR. Ventral medullary neurones excited from the hypothalamic and mid-brain defence areas. Journal of the Autonomic Nervous System. 1984;11:35–42. doi: 10.1016/0165-1838(84)90006-7. [DOI] [PubMed] [Google Scholar]
- Holm A, Ranta-aho PO, Sallinen M, Karjalainen PA, Müller K. Relationship of P300 single-trial responses with reaction time and preceding stimulus sequence. International Journal of Psychophysiology. 2006;61:244–252. doi: 10.1016/j.ijpsycho.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Verberne AJ, Guyenet PG. Rostral ventrolateral medullary neurons projecting to locus coeruleus have cardiorespiratory inputs. Brain Research. 1992;598:67–75. doi: 10.1016/0006-8993(92)90169-a. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Current Opinion in Neurobiology. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jackson JC. Amplitude and habituation of the orienting reflex as a function of stimulus intensity. Psychophysiology. 1974;11:647–659. doi: 10.1111/j.1469-8986.1974.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Janisse M-P. Pupillometry: The Psychology of the Pupillary Response. Washington, D.C: Hemisphere Publishing Co; 1977. [Google Scholar]
- Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: Evidence for the adaptive gain theory of locus coeruleus function. doi: 10.1162/jocn.2010.21548. submitted. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30:90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Johnston VS, Miller DR, Burleson MH. Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology. 1986;23:684–694. doi: 10.1111/j.1469-8986.1986.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Jordan D. Autonomic changes in affective behavior. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. pp. 349–366. [Google Scholar]
- Kahneman D. Attention and effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Karis D, Fabiani M, Donchin E. “P300” and memory: Individual differences in the von Restorff effect. Cognitive Psychology. 1984;16:177–216. [Google Scholar]
- Kimmel H, Van Olst EH, Orlebeke JF. The orienting reflex in humans. Hillsdale, NJ: Erlbaum; 1979. [Google Scholar]
- Kleinsmith LJ, Kaplan S. Interaction of arousal and recall interval in nonsense syllable paired-associate learning. Journal of Experimental Psychology. 1964;67:124–126. doi: 10.1037/h0045203. [DOI] [PubMed] [Google Scholar]
- Klostermann F, Wahl M, Marzinzik F, Schneider GH, Kupsch A, Curio G. Mental chronometry of target detection: human thalamus leads cortex. Brain. 2006;129:923–931. doi: 10.1093/brain/awl014. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Koss MC. Pupillary dilation as an index of central nervous system alpha 2-adrenoceptor activation. Journal of Pharmacological Methods. 1986;15:1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- Koss MC, Wang SC. Brainstem loci for sympathetic activation of the nictitating membrane and pupil in the cat. American Journal of Physiology. 1972;222:900–905. doi: 10.1152/ajplegacy.1972.222.4.900. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA, Volpe BT, Gazzaniga MS. Late positive event-related potentials after commissural section in humans. Journal of Cognitive Neuroscience. 1990;2:258–271. doi: 10.1162/jocn.1990.2.3.258. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Waterhouse BD. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Journal of Comparative Neurology. 2005;481:179–193. doi: 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- Levitt P, Moore RY. Origin and organization of brainstem catecholamine innervation in the rat. Journal of Comparative Neurology. 1979;186:505–528. doi: 10.1002/cne.901860402. [DOI] [PubMed] [Google Scholar]
- Li R, Keil A, Principe JC. Single-trial P300 estimation with a spatiotemporal filtering method. Journal of Neuroscience Methods. 2009;177:488–496. doi: 10.1016/j.jneumeth.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Liu J, Kiehl KA, Pearlson G, Perrone-Bizzozero NI, Eichele T, Calhoun VD. Genetic determinants of target and novelty-related event-related potentials in the auditory oddball response. Neuroimage. 2009;46:809–816. doi: 10.1016/j.neuroimage.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD, Wallach JH, McKellar S. Efferent connections of the ventral medulla oblongata in the rat. Brain Research. 1981;228:63–80. doi: 10.1016/0165-0173(81)90012-6. [DOI] [PubMed] [Google Scholar]
- Lynn R. Attention, arousal, and the orientation reaction. Oxford: Pergamon Press; 1966. [Google Scholar]
- Lyytinen H, Blomberg AP, Näätänen R. Event-related potentials and autonomic responses to a change in unattended auditory stimuli. Psychophysiology. 1992;29:523–534. doi: 10.1111/j.1469-8986.1992.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Maher TF, Furedy JJ. A comparison of the pupillary and electrodermal components of the orienting reflex in sensitivity fo initial stimulus presentation, repetition and change. In: Kimmel HD, Olst EH, van Orlebeke JF, editors. The orienting reflex in humans. Lawrence Erlbaum Associates; New York: 1979. pp. 381–391. [Google Scholar]
- Makeig S, Delorme A, Westerfield M, Jung TP, Townsend J, Courchesne E, Sejnowski TJ. Electroencephalographic brain dynamics following manually responded visual targets. PloS Biology. 2004;2:747–762. doi: 10.1371/journal.pbio.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman I. Orienting reflexes and classical conditioning in humans. In: Kimmel HD, Olst EH, van Orlebeke JF, editors. The orienting reflex in humans. Lawrence Erlbaum Associates; New York: 1979a. pp. 323–351. [Google Scholar]
- Maltzman I. Orienting reflexes and significance: a reply to O’Gorman. Psychophysiology. 1979b;16:274–283. doi: 10.1111/j.1469-8986.1979.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Maltzman I, Kantor W, Langdon B. Immediate and delayed retention, arousal, and the orienting and defensive reflexes. Psychonomic Science. 1966;6:445–446. [Google Scholar]
- Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. I. Thermoregulatory challenge. Brain Research. 1987a;422:17–23. doi: 10.1016/0006-8993(87)90535-x. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. II. Cardiovascular challenge. Brain Research. 1987b;422:24–31. doi: 10.1016/0006-8993(87)90536-1. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. III. Glucoregulatory challenge. Brain Research. 1987c;422:32–39. doi: 10.1016/0006-8993(87)90537-3. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Molliver ME, Grzanna R, Coyle JT. The intracortical trajectory of the coeruleo-cortical projection in the rat: a tangentially organized cortical afferent. Neuroscience. 1981;6:139–158. doi: 10.1016/0306-4522(81)90051-8. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr aphy and Clinical Neurophysiology. 1949;1:455–473. [PubMed] [Google Scholar]
- Murase S, Takayama M, Nosaka S. Chemical stimulation of the nucleus locus coeruleus: cardiovascular responses and baroreflex modification. Neuroscience Letters. 1993;153:1–4. doi: 10.1016/0304-3940(93)90062-p. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Gilzenrat MS, Holmes BD, Cohen JD. The role of the locus coeruleus in mediating the attentional blink: A neurocomputational theory. Journal of Experimental Psychology: General. 2005;134:291–307. doi: 10.1037/0096-3445.134.3.291. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Jepma M. Investigating the role of the noradrenergic system in human cognition. In: Robbins T, Delgado M, Phelps E, editors. Decision making. Attention & Performance. XXIII. Oxford: Oxford University Press; in press. [Google Scholar]
- O’Gorman J. The orienting reflex: Novelty or significance detector? Psychophysiology. 1979;16:253–262. doi: 10.1111/j.1469-8986.1979.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Öhman A, Bjorkstrand PA, Ellstrom PE. Effect of explicit trial-by-trial information about shock probability in long interstimulus interval GSR conditioning. Journal of Experimental Psychology. 1973;98:145–151. doi: 10.1037/h0034313. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford: Clarendon Press; 1927. [Google Scholar]
- Peavler WS. Pupil size, information overload, and performance differences. Psychophysiology. 1974;11:559–566. doi: 10.1111/j.1469-8986.1974.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Roth WT, Kopell BS. Age differences in P3-reaction time associations. Electroencephalography and Clinical Neurophysiology. 1980;49:257–265. doi: 10.1016/0013-4694(80)90220-5. [DOI] [PubMed] [Google Scholar]
- Phillips MA, Szabadi E, Bradshaw CM. Comparison of the effects of clonidine and yohimbine on pupillary diameter at different illumination levels. British Journal of Clinical Pharmacology. 2000;50:65–68. doi: 10.1046/j.1365-2125.2000.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JA, Foote SL, Neville HJ. Effects of locus coeruleus lesions on auditory, long-latency, event-related potentials in monkey. Journal of Neuroscience. 1989;9:81–93. doi: 10.1523/JNEUROSCI.09-01-00081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biological Psychology. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychological Bulletin. 1981;89:506–540. [PubMed] [Google Scholar]
- Qiyuan J, Richer F, Wagoner BL, Beatty J. The pupil and stimulus probability. Psychophysiology. 1985;22:530–534. doi: 10.1111/j.1469-8986.1985.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Correlations between locus coeruleus (LC) neural activity, pupil diameter and behavior in monkey support a role of LC in attention [Abstract] Society for Neuroscience Abstracts. 1993;19:974. [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nature Reviews Neuroscience. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Raskin DC, Kotses H, Bever J. Autonomic indicators of orienting and defensive reflexes. Journal of Experimental Psychology. 1969;80:423–433. doi: 10.1037/h0027491. [DOI] [PubMed] [Google Scholar]
- Reiner PB. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Brain Research. 1986;378:86–96. doi: 10.1016/0006-8993(86)90288-x. [DOI] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HG., Jr Association cortex potentials and reaction time in auditory discrimination. Electroencephalography and Clinical Neurophysiology. 1972;33:547–555. doi: 10.1016/0013-4694(72)90245-3. [DOI] [PubMed] [Google Scholar]
- Ritter W, Vaughan HG, Jr, Costa LD. Orienting and habituation to auditory stimuli: A study of short term changes in average evoked responses. Electroencephalography and Clinical Neurophysiology. 1968;25:550–556. doi: 10.1016/0013-4694(68)90234-4. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Roth WT. Auditory evoked responses to unpredictable stimuli. Psychophysiology. 1973;10:125–138. doi: 10.1111/j.1469-8986.1973.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Roth WT. A comparison of P300 and the skin conductance response. In: Gaillard AWK, Ritter W, editors. Tutorials in ERP research - Endogenous Components. Amsterdam: North-Holland Publishing Co; 1983. pp. 177–199. [Google Scholar]
- Roth WT, Blowers GH, Doyle CM, Kopell BS. Auditory stimulus intensity effects on components of the late positive complex. Electroencephalography and Clinical Neurophysiology. 1982;54:132–146. doi: 10.1016/0013-4694(82)90155-9. [DOI] [PubMed] [Google Scholar]
- Roth WT, Dorato KH, Kopell BS. Intensity and task effects on evoked physiological responses to noise bursts. Psychophysiology. 1984;21:466–481. doi: 10.1111/j.1469-8986.1984.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Rushby JA, Barry RJ. Event-related potential correlates of phasic and tonic measures of the orienting reflex. Biological Psychology. 2007;75:248–259. doi: 10.1016/j.biopsycho.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Rushby JA, Barry RJ. Single-trial event-related potentials to significant stimuli. International Journal of Psychophysiology. 2009;74:120–131. doi: 10.1016/j.ijpsycho.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Rust J. Habituation and the orienting response in the auditory cortical evoked potential. Psychophysiology. 1977;14:123–126. doi: 10.1111/j.1469-8986.1977.tb03361.x. [DOI] [PubMed] [Google Scholar]
- Saiers JA, Campbell BA. Disruption of noradrenergic, but not serotonergic or opiate, functioning blocks both cardiac and behavioral components of the orienting response in preweanling rats. Behavioral and Neural Biology. 1990;54:254–270. doi: 10.1016/0163-1047(90)90628-j. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews in Neuroscience. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Research Bulletin. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Scheibel AB. Anatomical and physiological substrate of arousal: a view from the bridge. In: Hobson JA, Brazier MAB, editors. The Reticular Formation Revisited. Raven Press; New York: 1980. pp. 55–66. [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: Gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Siddle AT, Heron PA. Effects of length of training and amount of tone frequency change on amplitude of autonomic components of the orienting response. Psychophysiology. 1976;13:281–287. doi: 10.1111/j.1469-8986.1976.tb03076.x. [DOI] [PubMed] [Google Scholar]
- Siddle DA, O’Gorman JG, Wood L. Effects of electrodermal lability and stimulus significance on electrodermal response amplitude to stimulus change. Psychophysiology. 1979;16:520–527. doi: 10.1111/j.1469-8986.1979.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Siddle DA, Remington B, Churchill M. Effects of stimulus change on the electrodermal orienting response. Biological Psychology. 1984;18:33–39. doi: 10.1016/0301-0511(84)90024-3. [DOI] [PubMed] [Google Scholar]
- Siddle DA, Spinks JA. Orienting response and information-processing: Some theoretical and empirical problems. In: Kimmel HD, Olst EH, van Orlebeke JF, editors. The orienting reflex in humans. Lawrence Erlbaum Associates; New York: 1979. pp. 473–497. [Google Scholar]
- Simons RF. Event-related slow brain potentials: a perspective from ANS psychophysiology. Advances in Psychophysiology. 1988;3:223–267. [Google Scholar]
- Simons RF, Graham FK, Miles MA, Chen X. On the relationship of P3a and the Novelty-P3. Biological Psychology. 2001;56:207–218. doi: 10.1016/s0301-0511(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Simons RF, Rocksroh B, Elbert T, Fiorito E, Lutzenberger W, Birbaumer N. Evocation and habituation or autonomic and event-related potential responses in a nonsignal environment. Journal of Psychophysiology. 1987;1:45–59. [Google Scholar]
- Sokolov EN. Perception and the Conditioned Reflex. Oxford: Pergamon Press; 1963. [Google Scholar]
- Sokolov EN. The neuronal mechanisms of the orienting reflex. In: Sokolov EN, Vinogradova OS, editors. Neuronal mechanisms of the orienting reflex. Erlbaum; Hillsdale, NJ: 1975. pp. 217–235. [Google Scholar]
- Sokolov EN, Spinks JA, Näätänen R, Lyytinen H. The orienting Response in Information Processing. London: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Critical Reviews in Neurobiology. 2000;14:199–224. [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Steinhauer SR, Hakerem G. The pupillary response in cognitive psychophysiology and schizophrenia. Psychophysiology and experimental psychopathology: A tribute to Samuel Sutton. In: Friedman D, Bruder G, editors. Annals of the New York Academy of Sciences. Vol. 658. 1992. pp. 182–204. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Zubin J. Vulnerability to schizophrenia: Information processing in the pupil and event-related potential. In: Usdin E, Hanin I, editors. Biological markers in psychiatry and neurology. Oxford: Pergamon Press; 1982. pp. 371–385. [Google Scholar]
- Stelmack RM, Siddle DA. Pupillary dilation as an index of the orienting reflex. Psychophysiology. 1982;19:706–708. doi: 10.1111/j.1469-8986.1982.tb02529.x. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965 Nov 26;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Sutton S, Tueting P, Zubin J, John ER. Information delivery and the sensory evoked potential. Science. 1967 Mar 17;155:1436–1439. doi: 10.1126/science.155.3768.1436. [DOI] [PubMed] [Google Scholar]
- Suwazono S, Shibasaki H, Nishida S, Nakamura M, Honda M, Nagamine T, Ikeda A, Ito J, Kimura J. Automatic detection of P300 in single sweep records of auditory event-related potential. Journal of Clinical Neurophysiology. 1994;11:448–460. doi: 10.1097/00004691-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Sved AF, Felsten G. Stimulation of the locus coeruleus decreases arterial pressure. Brain Research. 1987;414:119–132. doi: 10.1016/0006-8993(87)91332-1. [DOI] [PubMed] [Google Scholar]
- Svensson TH. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications for psychiatry and psychopharmacology. Psychopharmacology. 1987;92:1–7. doi: 10.1007/BF00215471. [DOI] [PubMed] [Google Scholar]
- Swick D, Pineda JA, Foote SL. Effects of systemic clonidine on auditory event-related potentials in squirrel monkeys. Brain Research Bulletin. 1994;33:79–86. doi: 10.1016/0361-9230(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Szabadi E, Bradshaw CM. Autonomic pharmacology of α2-adrenoceptors. Journal of Psychopharmacology. 1996;10:6–18. [Google Scholar]
- Turpin G, Siddle DA. Effects of stimulus intensity on electrodermal activity. Psychophysiology. 1979;16:582–591. doi: 10.1111/j.1469-8986.1979.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G, Ennis M, Shipley MT, Pieribone VA. Subregions of the periaqueductal gray topographically innervate the rostral ventrolateral medulla in the rat. Journal of Comparative Neurology. 1991;309:305–327. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pieribone VA, Aston-Jones G. Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. Journal of Comparative Neurology. 1989;290:561–584. doi: 10.1002/cne.902900410. [DOI] [PubMed] [Google Scholar]
- Van Olst EH, Heemstra ML, Ten Kortenaar T. Stimulus signicance and the orienting reaction. In: Kimmel HD, Olst EH, van Orlebeke JF, editors. The orienting reflex in humans. Lawrence Erlbaum Associates; New York: 1979. pp. 521–547. [Google Scholar]
- Verbaten MN. The influence of information on habituation of cortical, autonomic and behavioral components of the orienting response. In: Gailard AWK, Ritter W, editors. Tutorials in ERP Research: Endogenous Components. Amsterdam: Elsevier; 1983. pp. 201–216. [Google Scholar]
- Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Progress in Neurobiology. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997;34:131–156. doi: 10.1111/j.1469-8986.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Wu HB, Stavarache M, Pfaff DW, Kow LM. Arousal of cerebral cortex electroencephalogram consequent to high-frequency stimulation of ventral medullary reticular formation. Proceedings of the National Academy of Sciences. 2007;104:18292–18296. doi: 10.1073/pnas.0708620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Arai H, Nakayama S. Skin conductance response after 6-hydroxydopamine lesion of central noradrenaline system in cats. Biological Psychiatry. 1990;28:151–160. doi: 10.1016/0006-3223(90)90632-c. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hoshino T, Takahashi Y, Kaneko H, Ozawa N. Skin conductance activity after intraventricular administration of 6-hydroxydopa in rats. Biological Psychiatry. 1991;29:365–375. doi: 10.1016/0006-3223(91)90222-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ozawa N, Shinba T, Hoshino T. Functional influence of the central noradrenergic system on the skin conductance activity in rats. Schizophrenia Research. 1994;13:145–150. doi: 10.1016/0920-9964(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]