Abstract
An earlier reported laboratory assay, performed in The Netherlands, to diagnose Schistosoma infections by detection of the parasite antigen CAA in serum was converted to a more user-friendly format with dry reagents. The improved assay requires less equipment and allows storage and worldwide shipping at ambient temperature. Evaluation of the new assay format was carried out by local staff at Ampath Laboratories, South Africa. The lateral flow (LF) based assay utilized fluorescent ultrasensitive up-converting phosphor (UCP) reporter particles, to be read by a portable reader (UPlink) that was also provided to the laboratory. Over a period of 18 months, about 2000 clinical samples were analyzed prospectively in parallel with a routinely carried out CAA-ELISA. LF test results and ELISA data correlated very well at CAA concentrations above 300 pg/mL serum. At lower concentrations the UCP-LF test indicates a better performance than the ELISA. The UCP-LF strips can be stored as a permanent record as the UCP label does not fade. At the end of the 18 months testing period, LF strips were shipped back to The Netherlands where scan results obtained in South Africa were validated with different UCP scanning equipment including a novel, custom developed, small lightweight UCP strip reader (UCP-Quant), well suited for testing in low resource settings.
Conclusion
The dry format UCP-LF assay was shown to provide a robust and easy to use format for rapid testing of CAA antigen in serum. It performed at least as good as the ELISA with respect to sensitivity and specificity, and was found to be superior with respect to speed and simplicity of use. Worldwide shipping at ambient temperature of the assay reagents, and the availability of small scanners to analyze the CAA UCP-LF strip, are two major steps towards point-of-care (POC) applications in remote and resource poor environments to accurately identify low (30 pg CAA/mL serum; equivalent to about 10 worm pairs) to heavy Schistosoma infections.
Keywords: Schistosomiasis, Circulating antigen, CAA, Lateral flow strip test, Up-converting phosphor, User-friendly, Dry reagent, Worldwide shipping
1. Introduction
Schistosomiasis control has recently gained increasing interest by the commitment of the 65th World Health Assembly (World Health Organization (WHO), 2012) to support interruption of transmission and even elimination is considered feasible in a number of countries. For these efforts, the availability of highly accurate diagnostics becomes a key issue and alternatives for the current standard of microscopical counting of parasite eggs in urine or stool to diagnose active schistosome infections are urgently needed. The detection of parasite-derived circulating antigens in various diagnostic assays with high sensitivity and specificity has been described extensively (Gabriel et al., 2012; Mendoza et al., 2009; Utzinger et al., 2011; van Lieshout et al., 2000; Wilson et al., 2006). Two well described circulating antigens for Schistosoma, both applied to diagnose active infection, are the Circulating Cathodic Antigen (CCA) and the Circulating Anodic Antigen (CAA).
The circulating cathodic antigen CCA can be detected in untreated urine of individuals with active S. mansoni infection. After initial development of monoclonal antibody based ELISA’s (de Jonge et al., 1990), a rapid point-of-care (POC) test for detection of CCA in urine has been described (van Dam et al., 2004). Several versions of this test were developed before eventually outsourcing it to Rapid Medical Diagnostics (Pretoria, South-Africa); the device has now been evaluated in various studies (Coulibaly et al., 2011; Legesse and Erko, 2007; Midzi et al., 2009; Shane et al., 2011; Standley et al., 2010). The CCA-POC test provides a rapid visual result based on a carbon or gold label but is developed for urine testing only. In the various evaluations it showed sufficiently high sensitivity and specificity to be taken up as an alternative to egg microscopy in mapping studies and field surveys. The test is particularly well-suited to accurately demonstrate moderate to heavy S. mansoni infections and can be considered as a useful method for S. mansoni diagnosis in peripheral health centers and schistosomiasis control programs (Coulibaly et al., 2011). Unfortunately, the accuracy of the CCA-POC test in S. haematobium infections is variable and needs to be further evaluated (Midzi et al., 2009; Obeng et al., 2008; Stothard et al., 2009).
Also for the second well-described schistosomal circulating antigen, circulating anodic antigen CAA, highly sensitive and specific monoclonal antibody based ELISA’s were developed and applied in numerous epidemiological and laboratory studies (Agnew et al., 1995; Deelder et al., 1989; Leutscher et al., 2008; van Dam et al., 1996a; van Lieshout et al., 1995). CAA is a genus-specific antigen with a unique carbohydrate structure (Bergwerff et al., 1994), present in serum and urine of hosts infected with various species of Schistosoma, including species infecting cattle (de Bont et al., 1996; Flowers et al., 2002; Gabriel et al., 2002). The test requires a trichloroacetic acid (TCA)-precipitation step after which CAA can be detected in the supernatant. Because of this sample pretreatment procedure and the fact that only an ELISA is available for testing, detection of CAA – despite showing a much larger potential for very sensitive detection of active schistosomiasis – for many years remained a laboratory-based assay. When the test is implemented in the daily routine, with several built-in controls and samples tested in duplicate, the ELISA may detect CAA in serum at levels as low as 40 pg/mL (Leutscher et al., 2008). When the assay is performed on a less regular basis and samples are tested only once, this sensitivity may not always be reached. ELISA standard series indicate an exponential increase in signal from around 300 to 10,000 pg/mL (Corstjens et al., 2008); a concentration of 300 pg/mL seems a “safe” level to prevent unacceptable high numbers of false positives when testing under non-optimal conditions.
Schistosoma worm pairs excrete a steady amount of CAA in the bloodstream upon feeding and the day-to-day variation of CAA in serum is fairly constant implying that the time of day is irrelevant for sample collection (Polman et al., 1998). Studies on in vitro incubated worms as well as studies with experimentally infected animals have indicated that a single worm pair would excrete a daily amount of CAA in the order of 40 ng, corresponding to 1–10 pg/mL blood (van Dam et al., 1996a; Wilson et al., 2006). In contrast to CCA which shares Lewis-X epitopes with various host components (van Dam et al., 1996b), the CAA carbohydrate structure (repeating GalNAC and GlcA disaccharides) is completely unique and no biological equivalent has so far been described. The use of CAA specific monoclonal antibodies in combination with ultra-sensitive detection platforms could thus be expected to result in further sensitivity improvements without compromising specificity.
In order to pursue the above, and also to further improve the robustness of the CAA assay and make it more applicable for future POC applications, we recently introduced a lateral flow based platform in combination with an ultrasensitive reporter technology. The resulting LF assay demonstrated an analytical sensitivity down to 1 pg/mL, about 10-fold better than the CAA-ELISA (Corstjens et al., 2008). However, the applied format was not yet optimal for distribution because of a limited batch size and due to the fact that some of the reagents needed refrigeration. Furthermore, the requirement of a sonication step added to the complexity of the assay. Here we describe a further advance towards a field applicable test through the introduction of dry reagents. The improved field-applicable assay was tested in a routine diagnostic setting in South Africa by local staff, with dry assay materials that were shipped at ambient temperature from the Netherlands. In parallel, a custom designed lightweight reader to analyze the UCP-LF strips was tested successfully.
2. Materials and methods
2.1 Patient population and sample treatment
During a period of 18 months, 2,599 serum samples were routinely analyzed for schistosomiasis by CAA-ELISA using the standard operating procedures of the Department of Serology of Ampath Laboratories (ISO 15189 and GLP/GPC certified, under supervision of Dr. L.H. van Rooyen, Dr du Buisson, Kramer, Swart, Bouwer Inc., Centurion, South-Africa). These sera were additionally evaluated using the UCP-LF CAA strip, and after quality control, full data records of 1,979 samples were obtained. The samples were sent in by local physicians, mostly from schistosome endemic regions North and East of Pretoria, based on clinical complaints and suspicion of schistosomiasis. In this population, based on extensive testing in preceding years, about 8% was expected to have active schistosomiasis, predominantly caused by S. haematobium. Urine and serum samples were sent in, and as per request by the physicians, urine samples were subjected to the CCA-POC test (Rapid Medical Diagnostics, Pretoria, RSA), and serum samples to antibody detection (IgM, IgG, and/or IgE; Delta and ImmunoCAP, Thermo Fisher Scientific Inc.) and/or in-house CAA-ELISA. All samples that were tested with the CAA-ELISA were also evaluated by the dry-format CAA UCP-LF assay. In this study, samples were only tested once and results from the latter test were compared to the routinely used CAA-ELISA; other comparisons were not included. The serum samples tested by ELISA and UCP-LF obtained a TCA-treatment and were neutralized resulting in a 4-fold dilution compared to the original serum.
Negative sera were obtained from healthy lab volunteers and the status was confirmed by routine antibody serology.
2.2 CAA-specific UCP-LF strips
Large batches of lateral flow strips (up to 2000 LF strips) were prepared following protocols described earlier (Corstjens et al., 2001; Corstjens et al., 2008). The Test (T) line was comprised of 200 ng mouse monoclonal anti-CAA antibody 147-3G4 (MαCAA, dept. of Parasitology LUMC) and the Flow-Control (FC) line contained 100 ng goat anti-mouse (M8642, Sigma-Aldrich). Strips were stored dry in closed plastic containers with silica dry pellets and were shipped to South Africa at ambient temperature.
2.3 Dry UCPMαCAA reporter conjugate
Mouse monoclonal anti-CAA antibody 147-3G4 (MαCAA, dept. of Parasitology LUMC) was coupled to 400 nm UCP reporter particles (OraSure Technologies, Bethlehem, PA) as described previously (Corstjens et al., 2001; Corstjens et al., 2008). The resulting UCP-MαCAA reporter conjugate (1 mg UCP per mL) was diluted 100-fold in 5 mM borate buffer (pH 8.8) and sonicated at high power (2× 30 sec with 15 sec pause in a Bioruptor™ UCD-200 TO; Diagenode Inc.). The sonicated UCP solution was mixed with 1 volume of 20% w/v sucrose and 2 volumes of high salt lateral flow (HSLF) assay buffer. Aliquots of 40 μL were dried overnight at 37 °C in flat-bottom poly propylene tubes (Ratiolab® 0.65 mL micro-tubes for 96-well micro test plate; Ratiolab GmbH, Dreieich, Germany) or in medium binding polystyrene flat-bottom eight-wells strips (Sigma-Adrich; Greiner multiwell strip plates). Dried material was stored in aluminum foil bags (Lamigrip pouches; Overtoom International Nederland B.V., Den Dolder, Netherlands) with silica dry pellets.
2.4 Lyophilized HSLF assay buffer
HSLF assay buffer (100 mM HEPES pH 7.5, 270 mM NaCl, 0.5% v/v Tween-20, 1% w/v BSA) was freeze dried using a Christ (Alpha 2–4 LD Plus) lyophilizer. Generally, 1 mL frozen 10x HSLF was lyophilized in 15 mL centrifugation tubes. Tubes with lyophilized buffer were stored at room temperature in Lamigrip pouches with silica dry pellets.
2.5 UCP-LF CAA assay with dry reagents
The serum samples were pretreated with 1 volume of 4% (w/v) TCA to remove interfering proteins and to dissociate immune complexes, and supernatants were then mixed with 1 volume of CAA neutralization buffer (0.05 M NaOH, 0.1 M phosphate, 0.6% v/v Tween-20, 0.2% w/v BSA, 4 mM MgCl2, pH 11, Corstjens et al., 2008). This pretreatment procedure resulted in a 4-fold dilution compared to the original serum.
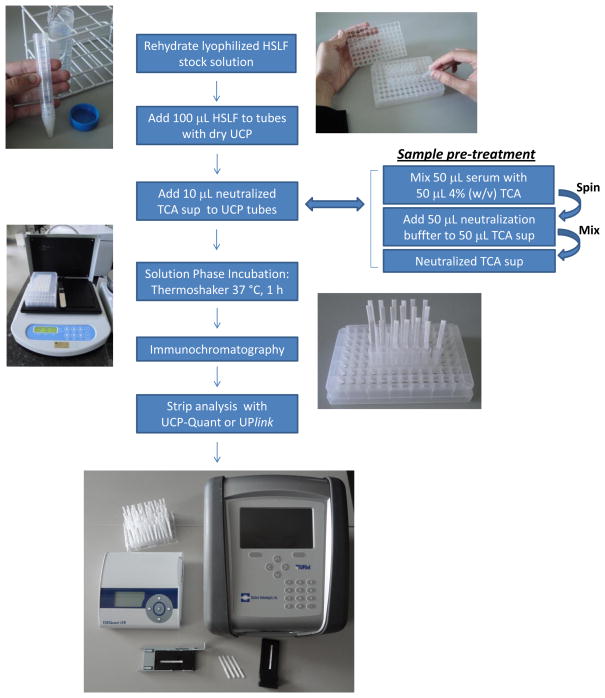
The assay employing dry reagents is schematically shown in Fig. 1. In detail, the dry reagent CAA UCP-LF assay consists of five steps: (i) addition of 100 μL rehydrated HSLF buffer to a flat bottom tube/well with 100 ng dry UCP reporter; (ii) addition of 10 μL pretreated serum sample to the rehydrated UCP reporter; (iii) incubation of the reporter-sample mixture in an orbital shaker (1,200 rpm) at 37 °C for 60 min; (iv) initiation of the lateral flow by placing a LF strip (sample pad down) in the tube/well with the reporter-sample mixture, allowing chromatography to continue until LF strips are dry; and (v) scanning of the strips using a UCP dedicated scanner (UPlink, UCP-Quant or a modified Packard Fluorocount reader). The total assay time for testing 20 samples is approximately 2 hours. This includes a chromatography step sustained for 30 min after which the strips are placed at elevated temperature for 10 min to dry before they are analyzed. Larger sample numbers would add about 2 minutes for each additional sample. The extra time is mainly required for scanning and analysis of individual LF strips when using single strip analyzers; it will be shorter when benchtop multi-strip scanners are available. The utilized 96-well micro test plate format allows an operator to work up 192 samples (including quality control standards) at a time.
Figure 1.
The CAA UCP-LF dry assay format and portable strip readers.
Various steps in the procedure are indicated starting with the rehydration of the lyophilized LF assay buffer. The UCP dry reagent is provided as a dry pellet in individual 0.65 mL flat bottom tubes (VWR; Ratiolab 0.65 mL Micro-Tube-System) or alternatively (not shown) in plastic strips comprised of 8 microtiter plate wells (Sigma Aldrich; Greiner multiwell strip plates). The two portable readers used in this study to analyze the UCP-LF strips are the UPlink (Mokkapati et al., 2007) and UCP Quant. The UPlink scanner (with matching tray for a single LF strip) with a low-power 1.0 W infrared laser and read head with 1-dimensional movement was used in South Africa. The UCP Quant scanner is a customized commercially available small lightweight ESEQuant LFS reader (weight, 620 g; dimensions HxWxD, 46×178×165 mm) adapted with infrared laser diode to analyze UCP-LF strips and drawer with a custom designed tray fitting a single LF strip. The system is provided with dedicated software (LateralFlowStudio) and can be used with internal/external RFID or barcode reader to identify specific assays and sample ID.
Test line signals were normalized to control signals (FC) of the individual strips and results are expressed as the resulting ratio value (T/FC). Serial dilutions of the TCA-soluble fraction of Schistosoma adult worm antigen (AWA-TCA) (Deelder et al., 1976) were assayed with each set of clinical samples. The cut-off threshold T/FC ratio was determined based on 86 laboratory negatives, which were confirmed antibody negative in all the routine antibody tests. A high specificity cut-off threshold was defined as the highest T/FC ratio of this series plus 2 standard deviations (SD), while a low specificity cut-off was defined as average T/FC ratio + 2 SD’s (Corstjens et al., 2008).
2.6 CAA-ELISA
CAA concentrations were determined in serum as described previously (Polman et al., 2000). The applied standard operating protocol implied a 6 h time-span to perform the various ELISA steps plus an overnight staining at 4 °C before reading the OD415 result. Serial dilutions of AWA-TCA and several reference samples were assayed simultaneously on each ELISA plate for quality control and to determine the cut-off threshold. The clinical samples were analyzed in singular and the cut-off threshold optical density value (OD415) routinely used by Ampath-Serology (based on the average of 4 buffer controls + 3 SD’s) was defined as plate cut-off or – for this study – low specificity cut-off threshold. This low threshold may lead to some false positives, but was chosen by the pathologist because infection levels were generally low and prevention of missing potentially positive patients was given priority. For a final indication of the infection status, the ELISA result by itself was not conclusive but was always combined with other diagnostic observations/tests and anamnesis. For the current study, a high specificity cutoff was chosen, viz. the OD value that is 50% above the plate cut-off. This is similar to the OD value for 30 pg CAA/ml or the average of all plate cut-off plus 3 SD’s.
2.7 Statistical analysis
For statistical analysis, SPSS software v18 was used. All analyses were performed on raw OD 415 values (CAA-ELISA) and T/FC ratio values (CAA-UCP), except for the correlation of the sample results for the CAA-ELISA with the CAA UCP-LF assay, where CAA-ELISA values were normalized by calculating them as percentages relative to the (plate) cut-off threshold (Table 3). For these correlations, samples were categorized in 4 groups based on CAA-UCP LF values: negative (T/FC ratio < high specificity cut-off), low positive (cut-off to 30 pg CAA/ml), positive (30–300 pg CAA/ml) and high positive (>300 pg CAA/ml). Statistical differences in mean ELISA and UCP values for the QC standards were calculated using an independent samples T-test. Correlations were calculated using both Spearman’s rho or Pearson’s r coefficients.
Table 3.
Correlation of the ELISA and UCP-LF CAA concentration values when grouping the clinical samples according to four infection levels.
| Clinical Samplesa | Spearmanb | Pearsonb | |||
|---|---|---|---|---|---|
| Infection levelc | Nd | value | significance | value | significance |
| > 300 pg/mL | 52 | 0.34 | 0.015 | 0.59 | < 0.001 |
| 30–300 pg/mL | 61 | 0.25 | 0.057 | 0.28 | 0.028 |
| cut-off to < 30 pg/mL | 56 | 0.038 | 0.782 | 0.061 | 0.66 |
| < cut-off | 1810 | −0.069 | 0.003 | −0.039 | 0.101 |
1,979 clinical samples were used in the analysis.
The Spearman and Pearson values represent the correlation of the clinical samples with the outcome of the CAA-ELISA. ELISA OD values were normalized for day-to-day variance for the different batches by dividing them by the plate cut-off and then calculating as percentage ((ODsample/ODplate cut-off− 1) × 100%). CAA-UCP-LF T/FC values did not vary significantly from day to day and were not normalized.
Infection level expressed as the concentration of CAA measured in serum.
N is the number of samples with respective CAA concentrations as determined by UCP –LF CAA.
3. Results
3.1 Development of a robust and user friendly platform
Fig. 1 shows an overview of the dry reagent UCP-LF assay format. Drying of UCP reporter materials into a specific reporter release pad was described by Niedbala et al. (Niedbala et al., 2001). The same protocol with minor modifications (e.g. sucrose instead of trehalose) was used to dry the reporter in flat-bottom polypropylene tubes or polystyrene 8-wells strips. The reporter solution dried homogenously, sticking as a ring to the wall at the bottom of the tube/well; rehydration occurred gradually upon addition of assay buffer and during initial incubation of the solution phase. The polypropylene tubes and polystyrene 8-well strips fit into microtiter plate format racks, allowing convenient arrangement of 96 tubes/wells per rack. The micro-racks provide a frame to store the tubes with dry UCP reagent, and they can be used during the solution phase incubation and are stable enough to hold the tubes with LF strip. This provides a suitable template to perform testing with larger numbers of LF strips. High throughput of LF strips benefit from a reader capable of scanning multiple strips, similar to the two-dimensional UCP reader as first described by Niedbala et al. (Niedbala et al., 2000).
The availability of dry UCP reagents allowed omission of the sonication step, replacing it with a straightforward dissolving step which considerably reduced the complexity of the assay. In addition, the dry reagents demonstrated prolonged shelf life (>2 years) and allowed convenient worldwide shipping at ambient temperature as well. Assay materials that were kept in South Africa for more than a year were sent back to the Netherlands where they were stored for another year without relevant loss in performance (results not shown); materials were always kept at ambient temperature in aluminum Lamigrip pouches with silica dry packs. Local staff in the Serology Department of Ampath Laboratories received a few hours training after which the practical use and performance of the dry assay format was successfully evaluated and compared to the use of the CAA-ELISA.
3.2 Availability of scanners applicable for the UCP reporter technology
In several of the previous studies (e.g. (Corstjens et al., 2001; Corstjens et al., 2008; Niedbala et al., 2001)), UCP-based scanning of LF strips was performed with a modified Packard FluoroCount fluorescence microtiter plate reader. The reader was equipped with an external 980 nm IR laser for excitation and specific band-pass filters for appropriate emission wavelength. Up to 20 LF strips can be loaded in this device, equipped with a microstepper motor for accurate control of x- and y-axis movement. The modified reader is an excellent tool for research and development and for testing large numbers of samples e.g. for epidemiological studies. However, it is not a low-cost portable device and it is not commercially available. Other optical scanners and equipment for various immunoassays with UCP labels have been described (Corstjens et al., 2005; Hampl et al., 2001; Hong et al., 2010; Li et al., 2008; van de Rijke et al., 2001; Zijlmans et al., 1999), as well as two portable readers for UCP-LF strips: a prototype UCP-based biosensor (Yan et al., 2006) and the UPlink reader (Mokkapati et al., 2007). The latter was used in the current evaluation in South Africa (Fig. 1). An obstacle for the general introduction of UCP-LF assays is that both readers are not readily available. Therefore, in this study we also evaluated the commercially available reader ESEQuant LFS that was adapted for the detection of the UCP label (referred to as UCP-Quant, Fig. 1). This is a lightweight reader and can be used for single strip scanning with the possibility to use it as a standalone battery operated device. For routine use, it is connected to a computer (or laptop) allowing quantitative data analysis. The performance of the UCP-Quant reader with the UCP-LF CAA dry reagent assay could be evaluated successfully by comparison to the scan results obtained in South Africa with the UPlink reader.
3.3 Quality control on the performance of ELISA and UCP-LF assays in South Africa
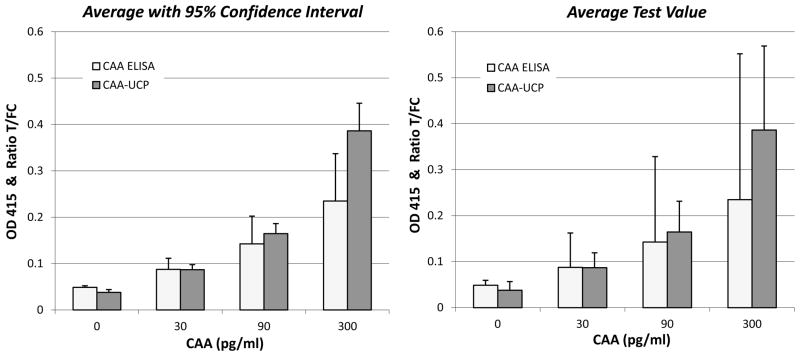
The performance of the CAA-ELISA and the dry format CAA UCP-LF assay was monitored during the 18 months period in which samples were collected and tested in one or two batches per week. Each batch included a freshly prepared quality control (QC) standard dilution series of AWA-TCA, representing 0, 30, 90 and 300 pg CAA/ml. Analysis of 38 standard series showed that both assay formats allow detection of 30 pg CAA per mL, but the UCP-LF showed significantly less variation (as indicated by the error bars in Fig. 2). Statistical analysis (T-test) further indicated that in the CAA-ELISA assay OD-values obtained with the standard sample with 90 pg CAA/ml cannot be significantly discriminated from those obtained with the 30 pg and the 300 pg samples. The CAA UCP-LF test on the other hand shows highly significant differences between all QC standards (Table 1). These results indicate that similar to the earlier reported laboratory assay with wet reagents (Corstjens et al., 2008), the new dry reagent UCP-LF assay format may improve the discrimination of low-positives from negatives when testing clinical samples.
Figure 2.
Comparison between CAA-ELISA and CAA-UCP-LF values for the CAA QC standard dilution series in serum.
Left panel showing the average OD and T/FC values of 38 QC standard series presented in histograms with 1x standard deviation error bars. Right panel showing the same histogram with bars indicating the 95% confidence interval.
Table 1.
Statistical differences between ELISA and UCP-LF values of QC samples.
| CAA-ELISA | CAA UCP-LF | |||
|---|---|---|---|---|
| QC samplesa | t-valueb | significance | t-valueb | Significance |
| 30 pg versus0 pg | 3.1 | 0.004 | 7.9 | <0.001 |
| 90 pg versus 0 pg | 3.1 | 0.004 | 11.1 | <0.001 |
| 90 pg versus 30 pg | 1.7 | 0.10 | 6.4 | <0.001 |
| 300 pg versus 0 pg | 3.6 | 0.001 | 11.6 | <0.001 |
| 300 pg versus 30 pg | 2.8 | 0.009 | 9.8 | <0.001 |
| 300 pg versus 90 pg | 1.5 | 0.13 | 6.9 | <0.001 |
QC samples with different CAA concentrations (0, 30, 90 and 300 pg CAA/ml) from 38 different QC standard series.
Independent samples T-test with 2-tailed significance levels.
3.4 Evaluation of UCP-LF assay using clinical samples, and ELISA correlation
The 1,979 samples with full data records were used to compare the ELISA and UCP-LF results. Serum samples, including positive and negative reference samples, were pre-treated for the CAA-ELISA according to standard operating procedures at Ampath-Serology; an aliquot of the pre-treated sample was used for the CAA UCP-LF. Because samples were tested only once, both a high and low specificity cut-off threshold was applied to evaluate the results. Thresholds were determined from a set of confirmed negative controls similar to the method described for the wet reagent UCP-LF assay. In Table 2, the number of individuals with a CAA positive test result are presented. The CAA UCP-LF assay detected a larger number of positive cases, thereby reflecting the expected higher sensitivity. Applying the high specificity cut-off, the number of positives identified with the UCP-LF assay approached the predicted experience-based 8% positives in this patient group more closely than the CAA-ELISA. When using the low specificity cut-off threshold the number of positives was found to be relatively high. In the earlier wet assay format (Corstjens et al., 2008), samples indicating CAA levels between the low and high cut-off threshold were labeled as indecisive and would require re-testing in triplicate or with another, more sensitive test. Applying the high specificity cut-off for the ELISA, only 3% positives were identified, whereas the low specificity cut-off threshold indicated 14% positives. The samples that in the ELISA indicated concentrations over 300 pg CAA/ml correlated well with UCP-LF test (Table 3). At lower CAA concentrations the correlation between both assays became less, which is in line with the noted decrease in the statistical significance of the CAA-ELISA test when discriminating low positives and negatives (Table 1).
Table 2.
Number of positives found by CAA-ELISA and CAA UCP-LF assay with respect to defined specificity cut-off thresholds.
| number of CAA positives | ||
|---|---|---|
| Total number of samples: N=1979 | high specificity cut-offa | low specificity cut-offb |
| ELISA | 51 (3%) | 282 (14%) |
| UCP-LF | 170 (9%) | 536 (27%) |
The high specificity cut-off threshold OD for the CAA-ELISA is 1,5 x OD-value of plate cutoff; plate cut-off is defined as the average OD of 4 buffers + 3 SD’s. The high specificity cut-off threshold T/FC ratio for the CAA UCP-LF assay is the maximum ratio of 86 negative samples + 2 SD’s.
The low specificity cut-off OD for the CAA-ELISA is the plate cuf-off. The low specificity T/FC ratio CAA UCP-LF assay is the average ratio + 3 SD’s).
3.5 Comparison of the UPlink and the lightweight UCP-Quant reader
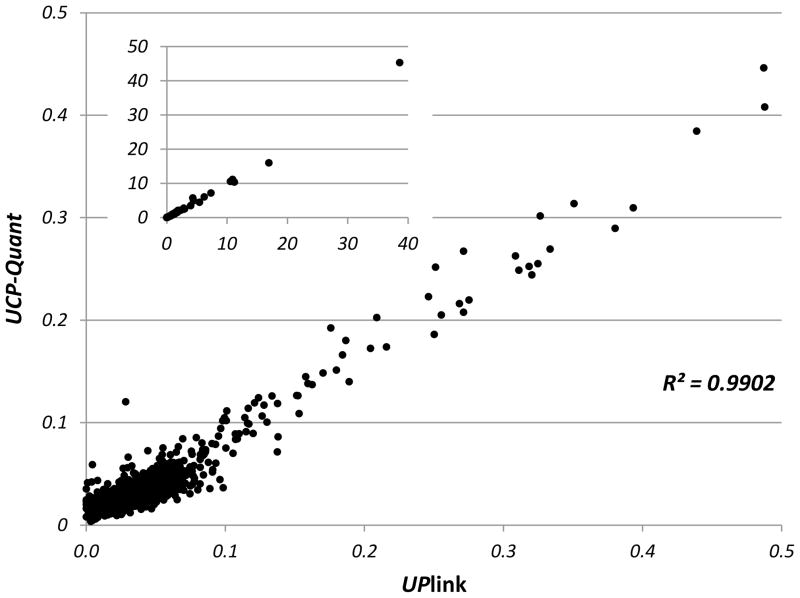
An UPlink reader was used to scan and analyze individual UCP-LF strips in South Africa. Strips were then sent to LUMC in The Netherlands where they were re-analyzed with the multi-strip reader, a modified Fluorocount Packard, to validate the UPlink scan results. In the analysis of the LF strips, peak area calculations of Test and Flow-Control line were performed with the same software (OTI-Connect, OraSure Technologies Inc.). The scan results were highly correlated and did not indicate relevant discrepancies (data not shown). LF strips were also analyzed with a commercial scanner that became available recently, the lightweight portable UCP-Quant reader. The reader was operated utilizing different software (LateralFlowStudio, QIAGEN Lake Constance GmbH) with a potentially different peak area calculation algorithm. Fig. 3 shows the correlation of UPlink and UCP-Quant scan results; again, relevant discrepancies were not observed. Although the UPlink reader with adjustable photomultiplier is at least an order of magnitude more sensitive in detecting the UCP emission (results not shown), this extra sensitivity is not required for the UCP-LF CAA assay described here.
Figure 3.
Correlation of T/FC ratio’s determined with UCP-Quant and UPlink reader.
A total of 2304 LF strips (clinical samples and quality controls) analyzed in South Africa with the UPlink reader were shipped to the Netherlands and scanned with a custom designed UCP-Quant reader. Ratio values were calculated from T and FC area values as determined with software provided with the respective readers (OTI Connect and LateralFlowStudio).
3.6 Relevance of the high and low specificity cut-off threshold
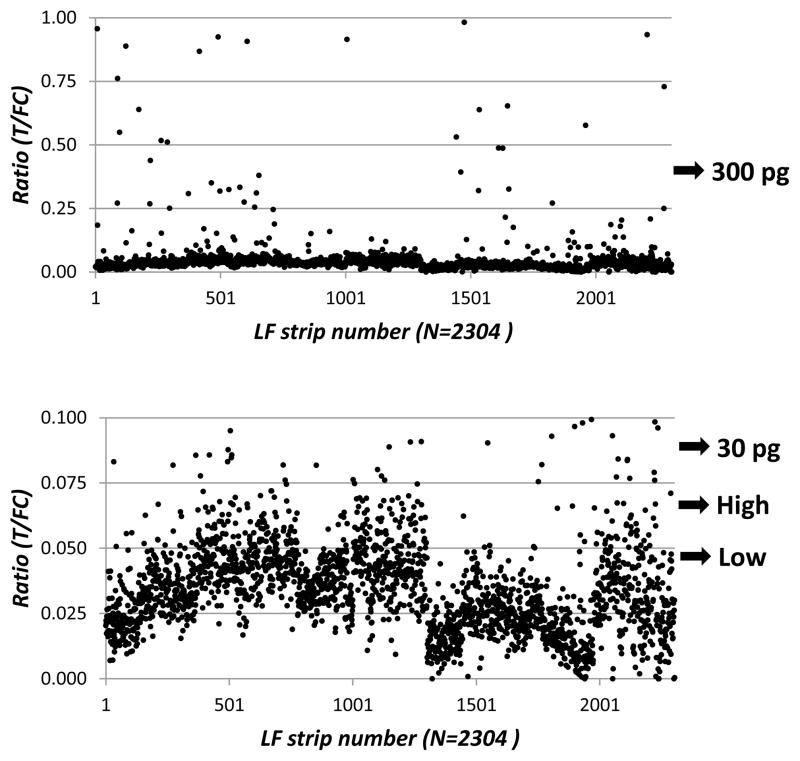
In analogy to the approach described earlier (Corstjens et al., 2008), the high and low specificity cut-off threshold implies the definition of a group of “potentially positives”. Fig. 4 shows a scatter plot of the T/FC ratio values of the patient samples (the same data points as presented in Fig. 3, but only with a T/FC lower than 1) by the UPlink reader. Looking more closely at the low signals the resolution between CAA “potentially positives” and CAA negatives expectedly becomes less pronounced. A similar pattern was observed for the ELISA result. Note that the identification of positive samples above CAA levels of 30 pg/mL (medium low) with the UCP-LF assay was very clear and that the high specificity cut-off threshold was still well above the average value of the alleged negatives. To further improve the resolution between low positives and negatives, assay formats with higher analytical sensitivities involving larger sample input are being developed.
Figure 4.
UPlink results focusing on the low and high specificity cut-off thresholds.
The two panels show the UCP-LF ratio’s from Fig. 3 for the UPlink reader at the lower ratio values. The low and high specificity cut-off thresholds and the 30 and 300 pg CAA/mL are indicated.
4. Discussion
The need for accurate diagnosis of active schistosome infections has recently been emphasized in several review papers (Bergquist et al., 2009; Ekpo et al., 2012; Utzinger et al., 2011). Routine diagnosis in field settings is still based on stool and urine microscopy with obvious limitations and shortcomings as low sensitivity, high variability, laborious and unhygienic procedures. Particularly, infants and preschool-aged children are difficult to diagnose by stool- and urine-based parasitological diagnosis and therefore infections in these groups go easily unnoticed (Ekpo et al., 2012; Stothard et al., 2011). In addition, in the diagnostic setting in South-Africa, many low grade active infections go undetected in routine microscopy while antibody detection does not provide information about present or past infections. In addition, due to migrating populations, patients may be infected with different species of Schistosoma making the diagnosis of an active infection by microscopy even more difficult. Therefore, an alternative diagnostic like detection of the schistosome excretory antigens CAA and CCA is highly needed, in particular if this can be supplied in a point-of-care or other user-friendly format.
Currently, the only available rapid POC test for detection of active schistosomiasis is based on the demonstration of CCA in urine of patients and this test is mainly suited for detection of S. mansoni infections, as only S. mansoni infections result in high enough concentrations of CCA in urine. The CCA-POC test performs excellently in medium to heavy S. mansoni infections (as is the case in highly endemic countries/regions) but lacks sensitivity when detecting lower infection levels (as in most situations in South-Africa). This test is currently extensively evaluated in SCORE-sponsored projects in various countries with the conclusions that the test is an excellent large-scale screening and mapping tool with equal or improved sensitivity as compared to (multiple) Kato-Katz slides (Colley et al., 2013) (Coulibaly et al., 2011; Tchuem Tchuente et al., 2012). Earlier studies by other groups in various countries came to the same conclusion (Shane et al., 2011; Standley et al., 2010; Stothard et al., 2006).
The previously described UCP-LF test (Corstjens et al., 2008) can be applied to detect schistosome-derived CAA in serum and urine samples. Like the earlier described CAA-ELISA, the assay is applicable for the diagnosis of all human and veterinary Schistosoma species. The introduction of the UCP-LF assay format improved robustness and analytical sensitivity of CAA detection, but the first generation of the test was not ready for implementation in external laboratories. In the second generation of the UCP-LF assay, presented here, a critical sonication step could be removed through implementation of a fully dry assay-reagent design. The two components that previously were provided as wet reagents, the UCP reporter particles and the HSLF assay buffer, are now available as dry reagents. The HSLF assay buffer is a simple lyophilized, easy to hydrate material, and the UCP reporter particles are provided as dry pellets in individual polypropylene tubes. Overall, the dry assay is much more user friendly, can be supplied as a kit with extended shelf life and allows shipping of reagents to external laboratories. The evaluation of the current dry assay format in the Department of Serology of Ampath Laboratories (Centurion, South Africa) demonstrated a performance at least as good as the ELISA. The correlation between ELISA and UCP-LF was good in the medium to heavy infection levels (above 300 pg CAA/ml), while low grade infections would appear to be more easily identified with the UCP-LF assay. The LOD of the here presented dry assay format allows detection well below a CAA concentration of 30 pg/mL. The use of a high and low cut-off threshold (as applied in the current analysis) when testing samples in singlet is preferred to improve clinical sensitivity and specificity. Avoiding classification of false positives will be achieved using the high cut-off threshold, whereas the use of the low cut-off threshold is helpful to reduce the chance of missing the light infections. Further validation of the UCP-LF assay is required including a thorough determination of the cut-off threshold with an extended set of unambiguous negatives, but the current evaluation by Ampath Laboratories local staff demonstrated the robustness of the new dry assay format. Moreover, the possibility of worldwide shipping without the need for a cold chain and the availability of lightweight UCP dedicated readers will allow integration/validation of the test in various schistosomiasis endemic regions (e.g. (Downs et al., 2012)).
A third version of the CAA UCP-LF assay is already in development. It will build on the current dry assay format but includes a concentration step, allowing a further 10- to 100-fold lower detection level. This should allow unequivocal detection of very light infection levels (a few worms) and, it might eliminate the need to use low and high cut-off thresholds; the assay thus could become the “golden standard” to determine active schistosome infections. The integration of this concentration step eventually may allow omission of the solution phase incubation (UCP reporter with the TCA treated clinical sample), and will thus further reduce complexity which is important when targeting POC applications. For those situations fully integrated robust microfluidic chips operated by simple and inexpensive but dedicated spring loaded devices (Liu et al., 2009) would appear to be well suited for POC applications in remote areas. The development of fully automated higher complexity microfluidic chips with ultimate sensitivity may however require a more sophisticated technology (Zhou et al., 2010).
In conclusion
The second version of the UCP-LF assay for CAA detection in serum samples involves only dried reagents and therefore allows storage and worldwide shipping of the assay at ambient temperature. The omission of a sonication step reduced complexity of the assay thereby allowing the assay to be performed by third parties after minimal training. The sensitivity of the dry assay format has been shown to be at least as good as that of the more complex and lab-based CAA-ELISA, but the variability in performance in routine settings with single sample testing was found to be significantly lower resulting in a much more reliable result and discrimination between positive and negative samples. The availability of lightweight (stand-alone) UCP dedicated readers will allow POC applications of this test even in remote and low-resource regions.
Highlights.
Improved lateral flow strip assay to diagnose active Schistosoma infection.
Assay materials can be stored and shipped worldwide, both at ambient temperature.
Assay materials shipped from the Netherlands, evaluated in Africa by local staff.
The lateral flow strip assay performed as least as good as the ELISA.
Validation of lightweight reader for analysis of the test strips.
Acknowledgments
Ms. D. Kornelis at LUMC and Mrs. Gloria Molaba at Ampath Laboratories are acknowledged for performing the UCP-LF assays, strip analysis and data processing. The UPlink™ reader and UCP particles were previously provided by OraSure Technologies Inc. Part of this development work was supported with financial support from US National Institutes of Health Grant UODE017855 and the University of Georgia Research Foundation, Inc. (SCORE project).
Abbreviations
- AWA
adult worm antigen
- CAA
circulating anodic antigen
- CCA
circulating cathodic antigen
- FC
flow control
- HSLF
high salt lateral flow
- LF
lateral flow
- MαCAA
mouse anti-CAA
- POC
point-of-care
- QC
quality control
- SD
standard deviation
- T
test
- TCA
trichloroacetic acid
- UCP
up-converting phosphor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnew A, Fulford AJ, de Jonge N, Krijger FW, Rodriguez-Chacon M, Gutsmann V, Deelder AM. The relationship between worm burden and levels of a circulating antigen (CAA) of five species of Schistosoma in mice. Parasitology. 1995;111(Pt 1):67–76. doi: 10.1017/s0031182000064611. [DOI] [PubMed] [Google Scholar]
- Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol. 2009;25:151–156. doi: 10.1016/j.pt.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Bergwerff AA, van Dam GJ, Rotmans JP, Deelder AM, Kamerling JP, Vliegenthart JF. The immunologically reactive part of immunopurified circulating anodic antigen from Schistosoma mansoni is a threonine-linked polysaccharide consisting of --> 6)-(beta-D-GlcpA-(1 --> 3))-beta-D-GalpNAc-(1 --> repeating units. J Biol Chem. 1994;269:31510–31517. [PubMed] [Google Scholar]
- Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuente LA, N’Goran EK, Erko B, Karanja DM, Kabatereine NB, van Lieshout L, Rathbun S. A Five-Country Evaluation of a Point-of-Care Circulating Cathodic Antigen Urine Assay for the Prevalence of Schistosoma mansoni. Am J Trop Med Hyg. 2013 doi: 10.4269/ajtmh.12-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corstjens P, Zuiderwijk M, Brink A, Li S, Feindt H, Niedbala RS, Tanke H. Use of up-converting phosphor reporters in lateral-flow assays to detect specific nucleic acid sequences: a rapid, sensitive DNA test to identify human papillomavirus type 16 infection. ClinChem. 2001;47:1885–1893. [PubMed] [Google Scholar]
- Corstjens PL, Li S, Zuiderwijk M, Kardos K, Abrams WR, Niedbala RS, Tanke HJ. Infrared up-converting phosphors for bioassays. IEE Proc Nanobiotechnol. 2005;152:64–72. doi: 10.1049/ip-nbt:20045014. [DOI] [PubMed] [Google Scholar]
- Corstjens PL, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, van Dam GJ. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol. 2008;46:171–176. doi: 10.1128/JCM.00877-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly JT, Knopp S, N’Guessan NA, Silue KD, Furst T, Lohourignon LK, Brou JK, N’Gbesso YK, Vounatsou P, N’Goran EK, Utzinger J. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Cote d’Ivoire. PLoS Negl Trop Dis. 2011;5:e1384. doi: 10.1371/journal.pntd.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge N, Kremsner PG, Krijger FW, Schommer G, Fillie YE, Kornelis D, van Zeyl RJ, van Dam GJ, Feldmeier H, Deelder AM. Detection of the schistosome circulating cathodic antigen by enzyme immunoassay using biotinylated monoclonal antibodies. Trans R Soc Trop Med Hyg. 1990;84:815–818. doi: 10.1016/0035-9203(90)90094-u. [DOI] [PubMed] [Google Scholar]
- de Bont J, van Lieshout L, Deelder AM, Ysebaert MT, Vercruysse J. Circulating antigen levels in serum of cattle naturally infected with Schistosoma mattheei. Parasitology. 1996;113(Pt 5):465–471. doi: 10.1017/s0031182000081531. [DOI] [PubMed] [Google Scholar]
- Deelder AM, de Jonge N, Boerman OC, Fillie YE, Hilberath GW, Rotmans JP, Gerritse MJ, Schut DW. Sensitive determination of circulating anodic antigen in Schistosoma mansoni infected individuals by an enzyme-linked immunosorbent assay using monoclonal antibodies. Am J Trop Med Hyg. 1989;40:268–272. doi: 10.4269/ajtmh.1989.40.268. [DOI] [PubMed] [Google Scholar]
- Deelder AM, Klappe HT, van den Aardweg GJ, van Meerbeke EH. Schistosoma mansoni: demonstration of two circulating antigens in infected hamsters. ExpParasitol. 1976;40:189–197. doi: 10.1016/0014-4894(76)90081-3. [DOI] [PubMed] [Google Scholar]
- Downs JA, van Dam GJ, Changalucha JM, Corstjens PL, Peck RN, de Dood CJ, Bang H, Andreasen A, Kalluvya SE, van Lieshout L, Johnson WD, Jr, Fitzgerald DW. Association of Schistosomiasis and HIV Infection in Tanzania. Am J Trop Med Hyg. 2012 doi: 10.4269/ajtmh.2012.12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpo UF, Oluwole AS, Abe EM, Etta HE, Olamiju F, Mafiana CF. Schistosomiasis in infants and pre-school-aged children in sub-Saharan Africa: implication for control. Parasitology. 2012;139:835–841. doi: 10.1017/S0031182012000029. [DOI] [PubMed] [Google Scholar]
- Flowers JR, Hammerberg B, Wood SL, Malarkey DE, van Dam GJ, Levy MG, McLawhorn LD. Heterobilharzia americana infection in a dog. Journal of the American Veterinary Medical Association. 2002;220:193–196. doi: 10.2460/javma.2002.220.193. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Blocher J, Dorny P, Abatih EN, Schmutzhard E, Ombay M, Mathias B, Winkler AS. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis. 2012;6:e1851. doi: 10.1371/journal.pntd.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S, De Bont J, Phiri IK, Masuku M, Riveau G, Schacht AM, Deelder AM, van Dam GJ, Vercruysse J. Transplacental transfer of schistosomal circulating anodic antigens in cows. Parasite Immunology. 2002;24:521–525. doi: 10.1046/j.1365-3024.2002.00494.x. [DOI] [PubMed] [Google Scholar]
- Hampl J, Hall M, Mufti NA, Yao YM, MacQueen DB, Wright WH, Cooper DE. Upconverting phosphor reporters in immunochromatographic assays. AnalBiochem. 2001;288:176–187. doi: 10.1006/abio.2000.4902. [DOI] [PubMed] [Google Scholar]
- Hong W, Huang L, Wang H, Qu J, Guo Z, Xie C, Zhu Z, Zhang Y, Du Z, Yan Y, Zheng Y, Huang H, Yang R, Zhou L. Development of an up-converting phosphor technology-based 10-channel lateral flow assay for profiling antibodies against Yersinia pestis. J Microbiol Methods. 2010;83:133–140. doi: 10.1016/j.mimet.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Legesse M, Erko B. Field-based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy. Trans R Soc Trop Med Hyg. 2007;101:668–673. doi: 10.1016/j.trstmh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Leutscher PD, van Dam GT, Reimert CM, Ramarakoto CE, Deelder AM, Ornbjerg N. Eosinophil cationic protein, soluble egg antigen, circulating anodic antigen, and egg excretion in male urogenital schistosomiasis. Am J Trop Med Hyg. 2008;79:422–426. [PubMed] [Google Scholar]
- Li JJ, Ouellette AL, Giovangrandi L, Cooper DE, Ricco AJ, Kovacs GT. Optical scanner for immunoassays with up-converting phosphorescent labels. IEEE Trans Biomed Eng. 2008;55:1560–1571. doi: 10.1109/TBME.2007.914674. [DOI] [PubMed] [Google Scholar]
- Liu C, Qiu X, Ongagna S, Chen D, Chen Z, Abrams WR, Malamud D, Corstjens PL, Bau HH. A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab Chip. 2009;9:768–776. doi: 10.1039/b814322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza N, Li A, Gill A, Tyring S. Filariasis: diagnosis and treatment. DermatolTher. 2009;22:475–490. doi: 10.1111/j.1529-8019.2009.01271.x. [DOI] [PubMed] [Google Scholar]
- Midzi N, Butterworth AE, Mduluza T, Munyati S, Deelder AM, van Dam GJ. Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis. Trans R Soc Trop Med Hyg. 2009;103:45–51. doi: 10.1016/j.trstmh.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Mokkapati VK, Sam NR, Kardos K, Perez RJ, Guo M, Tanke HJ, Corstjens PL. Evaluation of UPlink-RSV: prototype rapid antigen test for detection of respiratory syncytial virus infection. Ann NY Acad Sci. 2007;1098:476–485. doi: 10.1196/annals.1384.021. [DOI] [PubMed] [Google Scholar]
- Niedbala RS, Feindt H, Kardos K, Vail T, Burton J, Bielska B, Li S, Milunic D, Bourdelle P, Vallejo R. Detection of analytes by immunoassay using up-converting phosphor technology. AnalBiochem. 2001;293:22–30. doi: 10.1006/abio.2001.5105. [DOI] [PubMed] [Google Scholar]
- Niedbala RS, Vail TL, Feindt H, Li S, Burton JL. Multiphoton up-converting phosphors for use in rapid immunoassays. In: Cohn GE, editor. In-vitro diagnostic instrumentation. 2000. pp. 193–203. [Google Scholar]
- Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, van Dam GJ, van Lieshout L. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625–633. doi: 10.1179/136485908X337490. [DOI] [PubMed] [Google Scholar]
- Polman K, Diakhate MM, Engels D, Nahimana S, van Dam GJ, Ferreira STMF, Deelder AM, Gryseels B. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Tropical Medicine & International Health. 2000;5:534–537. doi: 10.1046/j.1365-3156.2000.00600.x. [DOI] [PubMed] [Google Scholar]
- Polman K, Engels D, Fathers L, Deelder AM, Gryseels B. Day-to-day fluctuation of schistosome circulating antigen levels in serum and urine of humans infected with Schistosoma mansoni in Burundi. Am J Trop Med Hyg. 1998;59:150–154. doi: 10.4269/ajtmh.1998.59.150. [DOI] [PubMed] [Google Scholar]
- Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PN, Butler SE, Karanja DM, Secor WE. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS Negl Trop Dis. 2011;5:e951. doi: 10.1371/journal.pntd.0000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley C, Lwambo N, Lange C, Kariuki H, Adriko M, Stothard J. Performance of circulating cathodic antigen (CCA) urine-dipsticks for rapid detection of intestinal schistosomiasis in schoolchildren from shoreline communities of Lake Victoria. Parasit Vectors. 2010;3:7. doi: 10.1186/1756-3305-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, Webster JP, Fenwick A. Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Trop. 2006;97:219–228. doi: 10.1016/j.actatropica.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Sousa-Figueiredo JC, Standley C, van Dam GJ, Knopp S, Utzinger J, Ameri H, Khamis AN, Khamis IS, Deelder AM, Mohammed KA, Rollinson D. An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta Trop. 2009;111:64–70. doi: 10.1016/j.actatropica.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Sousa-Figuereido JC, Betson M, Adriko M, Arinaitwe M, Rowell C, Besiyge F, Kabatereine NB. Schistosoma mansoni Infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable? PLoS. Negl Trop Dis. 2011;5:e938. doi: 10.1371/journal.pntd.0000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuem Tchuente LA, Kuete Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo NC, Kenfack CM, Gipwe NF, Nana ED, Stothard JR, Rollinson D. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl Trop Dis. 2012;6:e1758. doi: 10.1371/journal.pntd.0001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzinger J, N’Goran EK, Caffrey CR, Keiser J. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120(Suppl 1):S121–S137. doi: 10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- van Dam GJ, Bogitsh BJ, van Zeyl RJ, Rotmans JP, Deelder AM. Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. JParasitol. 1996a;82:557–564. [PubMed] [Google Scholar]
- van Dam GJ, Claas FH, Yazdanbakhsh M, Kruize YC, van Keulen AC, Ferreira ST, Rotmans JP, Deelder AM. Schistosoma mansoni excretory circulating cathodic antigen shares Lewis-x epitopes with a human granulocyte surface antigen and evokes host antibodies mediating complement-dependent lysis of granulocytes. Blood. 1996b;88:4246–4251. [PubMed] [Google Scholar]
- van Dam GJ, Wichers JH, Ferreira TM, Ghati D, van Amerongen A, Deelder AM. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol. 2004;42:5458–5461. doi: 10.1128/JCM.42.12.5458-5461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijke F, Zijlmans H, Li S, Vail T, Raap AK, Niedbala RS, Tanke HJ. Up-converting phosphor reporters for nucleic acid microarrays. Nat Biotechnol. 2001;19:273–276. doi: 10.1038/85734. [DOI] [PubMed] [Google Scholar]
- van Lieshout L, Polderman AM, De Vlas SJ, de Caluwe P, Krijger FW, Gryseels B, Deelder AM. Analysis of worm burden variation in human Schistosoma mansoni infections by determination of serum levels of circulating anodic antigen and circulating cathodic antigen. J Infect Dis. 1995;172:1336–1342. doi: 10.1093/infdis/172.5.1336. [DOI] [PubMed] [Google Scholar]
- van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 2000;77:69–80. doi: 10.1016/s0001-706x(00)00115-7. [DOI] [PubMed] [Google Scholar]
- Wilson RA, van Dam GJ, Kariuki TM, Farah IO, Deelder AM, Coulson PS. The detection limits for estimates of infection intensity in schistosomiasis mansoni established by a study in non-human primates. International Journal for Parasitology. 2006;36:1241–1244. doi: 10.1016/j.ijpara.2006.07.002. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Elimination of schistosomiasis. 65th World Health Assembly, resolution WHA65.21 agenda item 13.11; 2012; Geneva. 2012. [Google Scholar]
- Yan ZQ, Zhou L, Zhao YK, Wang J, Huang LH, Hu KX, Liu HH, Wang H, Guo ZB, Song YJ, Huang HJ, Yang RF. Rapid quantitative detection of Yersinia pestis by lateral-flow immunoassay and up-converting phosphor technology-based biosensor. Sensors and Actuators B-Chemical. 2006;119:656–663. doi: 10.1016/j.snb.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Young L, Chen Z. Weak solvent based chip lamination and characterization of on-chip valve and pump. Biomed Microdevices. 2010;12:821–832. doi: 10.1007/s10544-010-9436-z. [DOI] [PubMed] [Google Scholar]
- Zijlmans HJ, Bonnet J, Burton J, Kardos K, Vail T, Niedbala RS, Tanke HJ. Detection of cell and tissue surface antigens using up-converting phosphors: a new reporter technology. Anal Biochem. 1999;267:30–36. doi: 10.1006/abio.1998.2965. [DOI] [PubMed] [Google Scholar]