Abstract
Evening chronotypes exhibit increased rates of affective dyregulation and sleep disturbances (e.g., insomnia and nightmares). Such symptoms are common to military veterans with posttraumatic stress disorder (PTSD); however, the influence of chronotype on this population remains unknown. We examined behavioral, psychological, and neural correlates of chronotype in 36 combat-exposed military veterans with varying degrees of posttraumatic stress symptomatology. We employed FDG-PET to assess neural activity across wakefulness and rapid eye movement (REM) sleep. We used polysomnography and diaries to monitor sleep, and a self-report survey to measure chronotype. Eveningness was associated with greater lifetime PTSD symptoms, more disturbed sleep, and more frequent and intense nightmares. Eveningness was also associated with greater brain activity in posterior cingulate/precuneus and brainstem regions across wakefulness and REM sleep, overlapping with regions related to arousal and REM sleep generation. Chronotype may be an important correlate of neural activity in REM sleep-generating and/or arousal regulatory regions among combat-exposed veterans with PTSD symptoms. Further investigations of the role of chronotype in PTSD are warranted.
Keywords: imaging, chronotype, sleep, PTSD, veterans, nightmares
INTRODUCTION
Individual differences in preferred sleep-wake timing, termed chronotype or morningness-eveningness, are associated with a variety of mental and physical health outcomes (e.g.,(Broms et al., 2011; Gau et al., 2007; Mongrain, Carrier, & Dumont, 2006; Negriff, Dorn, Pabst, & Susman, 2011; Randler, 2011; Urban, Magyarodi, & Rigo, 2011)). Specifically, the eveningness end of the chronotype spectrum is linked to affective and behavioral dysregulation, including depression and sleep disturbances such as insomnia and nightmares (e.g.,(Drennan, Klauber, Kripke, & Goyette, 1991; Gau, et al., 2007; Merikanto et al., 2012; Nielsen, 2010)). Chronotype may be relevant to combat-exposed military veterans, who often exhibit posttraumatic symptoms including affective disturbances, insomnia, and nightmares (Germain, in press; Germain, Buysse, & Nofzinger, 2008). However, the influence of chronotype on combat-exposed military veterans with posttraumatic symptoms remains unknown.
To date, eveningness has been most consistently associated with depression (unipolar and bipolar) and substance abuse (Adan, 1994; Broms, et al., 2011; Drennan, et al., 1991; Hasler, Allen, Sbarra, Bootzin, & Bernert, 2010; Randler, Stadler, Vollmer, & Diaz-Morales, 2012; Tonetti et al., 2010); suggesting dysregulated affect and motivation. We recently extended these findings and identified differences in affective circuitry between morning- and evening-types among a sample of adults with primary insomnia (Hasler et al., 2012). The evening-type insomniacs showed blunted daily patterns in positive affect and corresponding altered relative regional glucose metabolism within the medial prefrontal cortex (mPFC).
Recent evidence also links eveningness to more frequent and intense nightmares (Nielsen, 2010; Selvi et al., 2012). This link suggests that the affective dysregulation, which characterizes evening-types' waking state, may also extend to the rapid-eye movement (REM) sleep state during which nightmares primarily occur. The dorsolateral prefrontal cortex (dlPFC) is relatively deactivated during REM sleep, while medial prefrontal cortex and subcortical limbic areas show activation levels that are similar to, or even exceed, activation that is observed during wakefulness (Braun et al., 1997; Maquet et al., 1996; Nofzinger, Mintun, Wiseman, Kupfer, & Moore, 1997). Hypoactivity of the mPFC, which provides top-down modulation of the subcortical limbic regions including the amygdala, may facilitate the occurrence of nightmares (Germain, et al., 2008; Germain et al., 2013). Evening-types who experience diminished mPFC activity during REM sleep may thus be at particular risk of nightmares.
In the present analyses, we examined correlates of chronotype among military veterans with varying degrees of posttraumatic stress symptomatology. We used [18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to examine the relative regional cerebral metabolic rate of glucose (rCMRglc), as an index of brain activity, during wakefulness and REM sleep. We monitored sleep using both sleep diaries and polysomography (PSG), the gold standard for objective assessment of sleep continuity and sleep architecture. The first aim was to investigate the relationship between chronotype, psychological functioning, and sleep in military veterans with posttraumatic stress symptoms. We hypothesized that eveningness would be associated with greater depression, worse insomnia, and more nightmares. The second aim was to investigate the relationship between chronotype and brain activity during REM sleep. We hypothesized that eveningness would be associated with diminished rCMRglc in prefrontal regions during REM sleep, thus paralleling the hypothesized increased frequency of nightmares. Finally, the third aim was to evaluate the relationship between chronotype and PFC activity during wakefulness. We hypothesized that greater eveningness would be associated with lower rCMRglc in prefrontal regions during wakefulness, which may reflect reduced regulatory control that could account for psychological dysregulation.
METHODS
Participants
These data came from a study that was designed to examine the neurobiological correlates of posttraumatic stress disorder during sleep among combat-exposed military veterans. The study was approved by the University of Pittsburgh, Institutional Review Board, the Human Use Subcommittee of the Radioactive Drug Safety Committee, and the Human Research Protection Office of the Department of Defense. All participants provided written, informed consent.
Participants included thirty-six military returnees from Operation Iraqi Freedom and Operation Enduring Freedom (Table 1). Participants exhibited a range of posttraumatic stress symptomatology, from mild to severe, and 24 participants met criteria for Posttraumatic Stress Disorder (PTSD) as based on the Clinician-Administered PTSD Scale (CAPS;(Blake et al., 1995)), the gold standard for PTSD assessment (National Center for PTSD). Exclusion criteria were the presence of psychotic disorders, untreated, unstable psychiatric or medical disorders, bipolar disorder, and active substance abuse and/or dependence in the past 3 months. No participants carried current mood disorder diagnoses; four participants had histories of major depression. Furthermore, participants were asked to limit alcohol use to ≤ 2 drinks per day throughout the study, and breathalyzers and urinary drug screens were employed to confirm abstinence from alcohol and illicit drug use prior to each sleep/PET study. Participants were also excluded if they had an apnea-hypopnea index >15 or periodic limb movement arousal index >15 on the PSG screening night.
Table 1.
Demographics, clinical and sleep measures
| Mean ± SD | Range | |
|---|---|---|
| Age | 28.81 ± 5.62 | 22.18 – 45.84 |
| Sex (male/female) | 29/7 | |
| Composite Scale of Morningness | 35.86 ± 6.38 | 22 – 47 |
| Clinician-administered PTSD scale | ||
| Past month | 39.76 ± 22.74 | 3 – 82 |
| Life time | 59.97 ± 30.59 | 3 – 107 |
| Past month-nightmare item | 1.65 ± 2.23 | 0 – 6 |
| Life time-nightmare item | 3.31 ± 2.81 | 0 – 8 |
| Beck Depression Inventory | 5.38 ± 3.97 | 0 – 13 |
| Pittsburgh Sleep Quality Index | 6.37 ± 3.39 | 0 – 14 |
| PSQI - Addendum for PTSD | 2.53 ± 2.21 | 0 – 7 |
| Diary-based sleep parameters | ||
| Lights out | 0:04 ± 1:18 | 21:34 – 3:36 |
| Sleep onset | 0:27 ± 1.21 | 21:30 – 3:36 |
| Sleep offset | 7:43 ± 2:02 | 5:26 – 15:27 |
| Sleep onset latency (min.) | 23 ± 15 | 1.20 – 64.2 |
| Wake after sleep onset (min.) | 12 ± 13 | 0 – 49.20 |
| Total sleep time (hours) | 6.91 ± 1.15 | 4.93 – 9.76 |
| Sleep efficiency (%) | 92.04 ± 5.93 | 78.53 – 99.44 |
| PSG-based sleep parameters | ||
| Sleep onset latency (min.) | 23.37 ± 20.32 | 1 – 83 |
| Wake after sleep onset (min.) | 24.21 ± 15.15 | 7 – 76 |
| Total sleep time (hours) | 6.78 ± 0.76 | 4.81 – 8.18 |
| Sleep efficiency (%) | 89.46 ± .5.26 | 76.48 – 98.00 |
| REM latency (min.) | 80.48 ± 40.42 | 21 – 224 |
| REM fragmentation | 6.67 ± 4.01 | 1 – 16 |
| REM density (counts/min.) | 11.44 ± 7.14 | 0.77 – 26.40 |
Study design
Participants completed screening/baseline procedures that included a diagnostic interview, a physical examination, magnetic resonance imaging, and a laboratory-based sleep screen. The one-night sleep screen was completed at the University of Pittsburgh Neuroscience Clinical & Translational Research Center (N-CTRC), and served as an adaptation night. Participants completed a number of self-report measures (described below) during screening/baseline procedures, including completing a sleep diary for up to 10 consecutive nights. Eligible participants returned to the sleep laboratory for four consecutive PSG sleep study evaluations, one waking PET scan, one NREM sleep PET scan, and one REM sleep PET scan. Sleep schedules in the laboratory were based on habitual sleep schedules as measured by the previously obtained sleep diaries. Night 1 served as the baseline night. The next morning, 2 to 4 hours after awakening, participants underwent the waking PET scans. The NREM sleep PET scans occurred on the Night 2 (data not reported here). Night 3 was used as a recovery night from the mild sleep disruption induced by the scanning procedures conducted on Night 2. The REM sleep PET scans occurred on Night 4; [18F]-FDG was injected at the onset of the 2nd REM sleep period.
Measures
During the screening/baseline evaluation, participants completed the Composite Scale of Morningness (CSM;(Smith, Reilly, & Midkiff, 1989) to assess self-reported chronotype. The CSM includes 13 Likert-type items that are summed to obtain a total score ranging from 13 (extreme eveningness) to 55 (extreme morningness). The CSM has sound psychometric properties (Caci et al., 2005; Guthrie, Ash, & Bendapudi, 1995; Smith, et al., 1989) and CSM scores correlate with both diary- and actigrapy-derived sleep-wake timing (Randler, 2009; Thun et al., 2012). Participants also completed the Pittsburgh Sleep Quality Index (PSQI;(Buysse, Reynolds, Monk, Berman, & Kupfer, 1989)) to assess global sleep quality, and the Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A;(Germain, Hall, Krakow, Katherine Shear, & Buysse, 2005; Insana, Hall, Buysse, & Germain, in press)) to assess disruptive nocturnal behaviors (e.g., nightmares) related to PTSD. Participants completed a sleep diary, which included items on bedtime, lights out (the time participants closed their eyes with their intention to fall asleep), sleep onset, sleep offset, sleep onset latency (interval from lights out until sleep onset), and wakefulness after sleep onset (amount of wakefulness between sleep onset and sleep offset).
Participants' baseline levels of depression symptoms were assessed via the Beck Depression Inventory (BDI-1A;(Beck, Steer, Ball, & Ranieri, 1996)).
A trained Master's or doctoral level study personnel administered the CAPS to assess posttraumatic stress symptom severity over two timeframes—past month and lifetime. The CAPS includes an item about nightmares (frequency and intensity) that was analyzed separately for certain analyses discussed below.
Polysomnographic sleep assessment
The PSG montage included bilateral central and occipital electroencephalography (EEG) channels, electro-oculogram, submentalis electromyogram, and electrocardiogram. EEG signals were recorded at 20-second epochs, were digitized at 256 Hz, decimated to 128 Hz, and sleep stages were visually scored according to Rechtschaffen and Kales criteria (Brunner et al., 1996; Rechtschaffen & Kales, 1968; Vasko et al., 1997). Measures of interest included sleep onset latency, wake after sleep onset, and total sleep time. We also examined three REM-related variables: REM fragmentation (number of occurrences of consecutive epochs of wake and/or NREM within REM periods immediately preceded by and followed by an epoch of REM), REM latency (number of minutes from sleep onset to the first REM period), and REM density (REM counts/minute). We focused on PSG data from the baseline night (Night 1) for the present analyses. We also used REM density during the uptake period of the REM scans for the correlation analyses below.
Magnetic resonance and PET study procedures
Prior to the PET procedures, all participants underwent a brain magnetic resonance (MR) scan on a Siemens 3T Trio scanner. The following axial series was oriented to the anterior commissure-posterior commissure line: fast spin-echo T2-weighted images (TE/TR=104/4660ms, FOV 18×24cm, 46 slices, 3.6mm slices), proton density-weighted images (TE/TR=23/4050ms, FOV 18×24cm, 46 slices, 3.6mm slices) and fast fluid-attenuated inversion recovery images (TE/TR/TI=90/9160/2500ms, FOV 21.2×25.6cm, 48 slices, 3mm slices). A volumetric MPRAGE sequence was acquired in the sagittal plane (TE/TR=2.98/2300ms, flip angle=9°, FOV 24×25.6cm, 160 slices, 1.2mm slices). MR data was registered with PET data using Automated Image Registration.
At the start of each PET study (morning wakefulness and REM sleep), two intravenous catheters were placed, one in each arm, with normal saline infused at the minimal rate to keep the vein open. The radioligand was injected through one catheter, and the other catheter was used to sample glucose and radioactivity. These PET procedures were originally described by Nofzinger and colleagues (Nofzinger et al., 1998).
For the wake PET scan (2–4 hours post-waking), participants lay supine with their eyes closed while their wakefulness was continuously monitored with PSG. For the REM sleep PET scan, [18F]-FDG was injected at the onset of the 2nd REM sleep period, consistent with previously-validated methods (Nofzinger, et al., 1997; Nofzinger et al., 1999). After 20 minutes of PSG-monitored wakefulness and at the beginning of the second REM sleep interval, an intravenous bolus of [18F]-FDG (M dose=6.52±1.66 mCi) was injected. Following the 20-minute uptake period during each respective state, participants were transported to the PET Center for a10–15 minute transmission scan and a 60-minute emission scan. PET studies were conducted on a Siemens/CTI ECAT HR+ PET scanner with a Neuro-insert (CTI PET Systems, Knoxville, TN) in 3D mode. PET images were reconstructed using standard commercial software as 63 2.4-mm transaxial slices. The estimated full-width half-maximum resolution of reconstructed images was 6 mm in the transverse plane.
Data Analysis
Analysis of clinical and sleep data
All analyses were conducted within SPSS 18.0. Non-normal sleep variables were transformed prior to statistical analysis as needed (Tables 2 and 3). In order to minimize Type II errors due to the small sample size, we report all results, highlighting which findings survive Bonferroni correction for multiple comparisons (requiring a p<0.0025; α=0.05/20 planned comparisons).
Table 2.
Correlations with diary-based sleep variables
| Variables | 1d | 2 | 3 |
|---|---|---|---|
| 1. Eveningness (CSM) | — | ||
| 2. rCMRglu - brainstem - WAKE | .58***,† | — | |
| 3. rCMRglu - brainstem - REM | .70***,† | .79***,† | — |
| 4. Sleep onset | .55**,† | .48* | .45* |
| 5. Sleep offset | .63***,† | .77***,† | .58**,† |
| 6. Sleep onset latencya | .39* | .41* | .45* |
| 7. Wake after sleep onsetb | −.00 | .03 | .04 |
| 8. Sleep efficiencyc | −.18 | −.18 | −.21 |
| 9. Total sleep time | .23 | .38 | .19 |
Ln-transformed;
Sqrt-transformed;
Reverse-scored and Ln-transformed
Note that the sign of correlations with the Composite Scale of Morningness are reversed to ease interpretation (positive values now indicate positive correlations with eveningness)
p < 0.001;
p < 0.01;
p < 0.05;
survived Bonferroni correction (p < 0.0025)
Table 3.
Correlations with polysomnography-based sleep variables
| Variables | 1c | 2 | 3 |
|---|---|---|---|
| 1. Eveningness (CSM)c | - | ||
| 2. rCMRglu - brainstem - WAKE | .59***,† | — | |
| 3. rCMRglu - brainstem - REM | .70***,† | .79***,† | — |
| 4. Sleep latencya | .43** | .51**,† | .47** |
| 5. Wake after sleep onsetb | −.14 | −.35* | −.32 |
| 6. Total sleep time | −.02 | −.18 | −.21 |
| 7. REM latency | −.02 | −.11 | −.11 |
| 8. REM fragmentation | −.36* | −.20 | −.12 |
| 9. REM density | .26 | .41* | .62**,† |
Ln-transformed;
Sqrt-transformed
Note that the sign of correlations with the Composite Scale of Morningness are reversed to ease interpretation (positive values now indicate positive correlations with eveningness)
p < 0.001;
p < 0.01;
p < 0.05;
survived Bonferroni correction (p < 0.0025)
Analysis of neuroimaging data
High-resolution structural MR images were aligned along the AC-PC line and medial longitudinal fissure using Statistical Parametric Mapping version 8 (SPM8; http://www.fil.ion/ucl/ac/uk/spm/software/spm8/), then normalized to the ICBM 152 template (Montreal Neurological Institute) via the unified segmentation technique (Ashburner & Friston, 2005). The FDG images were aligned and averaged over 60 to 90 minutes post-injection (six 5-minute frames) via established methods (Woods, Mazziotta, & Cherry, 1993). Summed FDG images were co-registered to their corresponding structural MR and the previously obtained transformation parameters applied. Normalized FDG images were smoothed using a 10 mm FWHM Gaussian filter.
Whole-brain voxel-wise regression models ran in SPM8 were calculated to test whether there was an association between chronotype (CSM scores) and rCMRglc. Although the prior literature suggests that candidate regions (e.g., prefrontal areas) may be related to chronotype, given the novelty of our sample and methodology (i.e., scan during REM sleep), we focused on whole-brain voxel-wise approaches rather than region-of-interest analyses. We included age, sex, and the CAPS-lifetime score as covariates in all analyses; the latter covariate was included in order to account for the cumulative effect of posttraumatic stress symptoms. We applied a height threshold of p<0.01, minimum extent of 50 contiguous voxels, and corrected cluster-level threshold of p<0.05 (familywise error rate) to these analyses. After identifying clusters of interest, we extracted their mean rCMRglc value using the MATLAB toolbox rex (web.mit.edu/swg/rex/rex.pdf) for correlation analyses within SPSS 18.0. Given potential time-of-day effects on brain activity, we also explored correlations between rCMRglc and scan time during wakefulness and REM sleep.
RESULTS
Sample characteristics (Table 1)
Participants spanned the continuum of chronotype (CSM), falling on average into the intermediate range between extreme evening- and morning-types (13–26=evening-type; 27–41=intermediate-type; 42–55=morning-type; (Natale & Alzani, 2001)). On average, the sample exhibited subclinical levels of depression symptoms (BDI <10) and significant symptoms of posttraumatic stress according to the CAPS, particularly when considering the lifetime timeframe. Based on the CAPS nightmare item, the sample reported more frequent and intense nightmares over the lifetime timeframe than over the past month. The sample reported poor overall sleep quality (mean PSQI >5). On average, the sample reported mild levels of PTSD-related disruptive nocturnal behaviors according to the PSQI-A (mean PSQI-A < 4).
Diary-based sleep (Table 1)
Participants completed the diary for a mean (± SD) of 6.50±1.06 days. Based on mean diary data, the sample did not report clinically significant symptoms of insomnia (both sleep onset latency and wake after sleep onset were <30 minutes, sleep efficiency >85%).
Polysomnography (PSG; Table 1)
PSG- and diary-based sleep parameters were similar, suggesting that the diary data was an accurate reflection of participants' sleep outside the laboratory. The mean REM latency was 80.48±40.42 minutes, the mean REM fragmentation was 6.67±4.01, and the mean REM density was 8.07±4.30 counts/minute.
We used PSG to confirm that participants were predominantly in REM sleep or wakefulness during the respective FDG-PET uptake periods. Participants spent 94.64±9.16% of the first 15 minutes post-injection in REM sleep, and 95.52±3.48% of the first 15 minutes post-injection awake, during the REM sleep and waking FDG-PET uptake periods, respectively.
Correlations between chronotype, clinical, and sleep measures
Clinical measures
Greater eveningness correlated with higher levels of ifetime posttraumatic stress symptoms (CAPS-lifetime; r=−0.418, p=0.015).1 This correlation was not driven by greater sleep disturbance among evening-types, as it remained unchanged after removing the insomnia item (r=−0.415, p=0.016). Chronotype did not significantly correlate with past month's posttraumatic stress symptoms (CAPS-past month; r=−0.23, p=0.20). Contrary to our expectations, chronotype was not significantly associated with depression (BDI; r=−0.26, p=0.15), nor was it significantly associated with age (r=0.28, p=0.10).
Greater eveningness was unrelated to overall sleep quality over the past month (PSQI; r=−0.04, p=0.81), but was associated with the extent of PTSD-specific nocturnal disturbances at a trend-level (PSQI-A; r=−0.29, p=0.083). Greater eveningness was also associated with more frequent and intense nightmares, as measured by the nightmare item from the CAPS (lifetime: r=−0.41, p=0.019; past month: r=−0.38, p=0.036).
None of the correlations between chronotype and posttraumatic stress symptoms, depression, or sleep quality survived the strict Bonferroni correction for multiple comparisions (p < 0.0025).
Diary-based sleep measures (Table 2)
Greater eveningness correlated with later sleep timing (onset and offset) and longer sleep onset latency based on diary measures, but was not correlated with wake after sleep onset, sleep efficiency, or total sleep time.
Polysomnography-based sleep measures (Table 3)
Similar to the diary findings, greater eveningness correlated with longer sleep onset latency based on PSG. Greater eveningness was also significantly associated with less REM fragmentation, but was not correlated with REM density or REM latency.
Chronotype and brain activity during wakefulness
Eveningness was positively associated with rCMRglc during wakefulness in three clusters (Figure 1A), after accounting for age, sex, and CAPS-lifetime score2. The first cluster was composed of 2,163 voxels (peak voxel [6,−28,−16], t=5.14, p<0.001, cluster-pFWE=0.02) and extended into the brainstem, midbrain, and thalamus. The second cluster was composed of 1,785 voxels (peak voxel [−16,−42,36], t=4.63, p<0.001, cluster-pFWE=0.04) and extended into the left precuneus and dorsal portion of the posterior cingulate cortex. The third cluster was composed of 1,761 voxels (peak voxel [32,−34,28], t=4.15, p<0.001, cluster-pFWE=0.04) that extended into the right precentral gyrus and inferior parietal lobe. There were no clusters in which rCMRglc during wakefulness were significantly associated with greater morningness.
Figure 1.
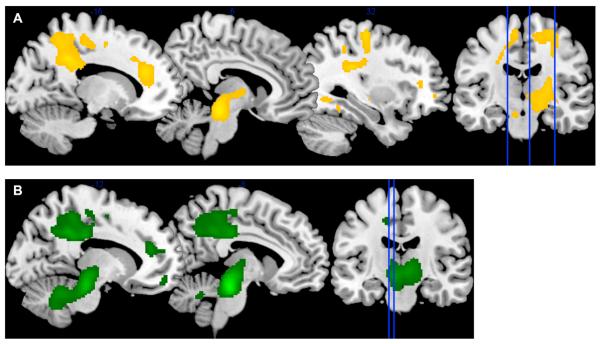
Relative cerebral glucose metabolism (rCMRglc) associated with eveningness during wakefulness (A) and REM sleep (B) in combat-exposed military veterans with posttraumatic stress symptoms. Sagittal sections correspond to the x-coordinates of the peak voxel for each cluster of interest. Clusters during wakefulness were centered in the precuneus/posterior cingulate, brainstem, and precentral gyrus/inferior parietal lobe (left to right). Clusters during REM sleep were centered in the precuneus/posterior cingulated and brainstem.
Chronotype and brain activity during REM sleep
We observed a similar pattern of associations between chronotype and rCMRglc during REM sleep. Greater eveningness was significantly associated with greater rCMRglc in two clusters (Figure 1B), after accounting for age, sex, and CAPS-lifetime score2. The first cluster was composed of 3,378 voxels (peak voxel [−8,−26,−20], t=7.04, p<0.001, cluster-pFWE=0.001) and extended into the brainstem, midbrain, and thalamus. The second cluster was composed of 2,197 voxels (peak voxel [−12,−44,36], t=4.35, p<0.001, cluster-pFWE=0.001) and extended into the left precuneus and dorsal posterior cingulate cortex.
Brainstem cluster and REM density analyses
The eveningness-associated brainstem cluster that we previously identified during REM sleep appeared to coincide anatomically with the known mesopontine REM-generating brainstem regions (i.e., the pedunculopontine [PPT] and laterodorsal tegmental nuclei [LDT]). Lacking the necessary measures (e.g., radioligands for acetylcholine) to confirm this anatomical colocalization, we instead sought supporting evidence by performing additional analyses to explore whether this eveningness-associated brainstem cluster might also be associated with REM density, which served as an index of REM-generating activity.
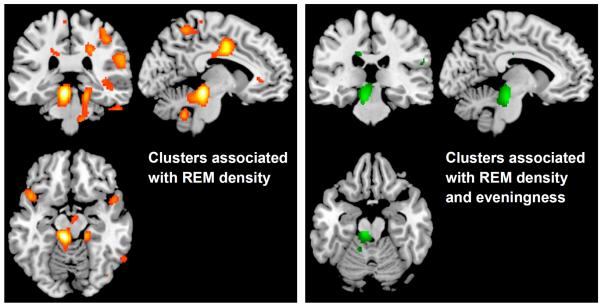
First, a REM density brain model was calculated. We ran a whole-brain voxel-wise regression model testing whether REM sleep rCMRglc was associated with PSG-measured REM density. We included age and sex as covariates. As shown in Figure 2A, REM density was significantly associated with greater rCMRglc in a 4,010 voxel cluster (peak voxel [−8,−32,−14], t=4.27, p<0.001, cluster-puncorr=0.01) and a 2,880 voxel cluster (peak voxel [54,14,−4], t=3.85, p<0.001, cluster-puncorr=0.03). The first cluster included portions of the brainstem, midbrain, and cerebellum. The second cluster was restricted to the right hemisphere, and included portions of the inferior frontal gyrus, superior temporal gyrus, and insula. Second, using the results of the REM density model as a mask in the subsequent analysis, we investigated the overlap between the REM density model and brain areas associated with chronotype. We ran a new SPM model testing whether chronotype was associated with REM sleep rCMRglc in any clusters within this mask from the REM density model. Age, sex, and CAPS-lifetime score were included as covariates. Greater eveningness was associated with an 833 voxel cluster (peak voxel [−8,−26,−20], t=7.04, p<0.001, cluster-puncorr=0.02) encompassing portions of the brainstem and midbrain (Figure 2B). These preliminary data support our post-hoc hypothesis that greater eveningness would be associated with higher rCMRglc in brainstem regions implicated in REM sleep generation.
Figure 2.
Relative cerebral glucose metabolism (rCMRglc) associated with REM activity (REM counts/minute) during REM sleep. Figure 2A shows rCMRglc associations with REM activity in a whole-brain voxel-wise analysis. Figure 2B shows a brainstem cluster in which rCMRglc was associated with eveningness after masking using the regions associated with REM activity from Figure 2A in a region of interest analysis.
Brain activity correlations with chronotype, clinical, and sleep measures
For the following correlation analyses, we extracted mean rCMRglc values from the brainstem and precuneus/PCC clusters that were associated with eveningness during wakefulness and REM sleep, yielding four variables (i.e., both clusters within both states, as described in the preceding section). In order to focus on the brainstem regions most relevant to REM-generating mechanisms in the REM sleep scan, we used rCMRglc values from the cluster masked by the REM density model–rather than from the initial unmasked cluster. The mean rCMRglc in this cluster (Figure 2B) was more strongly correlated with eveningness (r=− 0.70, p<0.001) than the broader unmasked brainstem cluster (r=−0.59, p<0.001; Figure 1B). We did not similarly mask the eveningness-associated brainstem cluster from the waking scan (Figure 1A) because we did not have any theoretical bases to focus on REM-generating mechanisms during wakefulness. Higher mean brainstem rCMRglc during wakefulness was moderately correlated with greater eveningness (r=−0.58, p<0.001).
The extracted mean CMRglc values from the precuneus/PCC clusters (Figures 1A/B) showed strong correlations with chronotype (i.e., higher CMRglc-greater eveningness) during wakefulness (r=0.73, p<0.001) and REM (r=0.69, p<0.001).
During both wakefulness and REM sleep, brainstem and precuneus/PCC rCMRglc generally correlated with the time of the respective scan, as based on the FDG injection time (see Supplement). Given the high degree of covariation between chronotype and scan time, we elected to not include scan time as a covariate in subsequent analyses.
Clinical measures
Mean brainstem rCMRglc (waking and REM) did not significantly correlate with measures of depression (BDI) or posttraumatic symptoms (CAPS). Mean brainstem rCMRglc (waking and REM) was also unrelated to disruptive nocturnal behaviors (PSQI-A), and overall sleep quality (PSQI). Mean precuneus rCMRglc (waking and REM) did not correlate with any clinical measures.
Diary-based sleep measures (Table 2)
Higher brainstem rCMRglc during wakefulness and REM sleep both correlated with later sleep timing and longer sleep onset latency based on diary measures. However, they were not correlated with wake after sleep onset, sleep efficiency, or total sleep time. Mean precuneus rCMRglc (waking and REM) did not correlate with any diary-based sleep measures.
Polysomnography-based sleep measures (Table 3)
Higher brainstem rCMRglc during wakefulness and REM sleep both correlated with longer sleep onset latency based on PSG. Higher brainstem rCMRglc during wakefulness and REM sleep, respectively, showed significant and trend-level associations with less wake after sleep onset. Finally, higher brainstem rCMRglc during wakefulness and REM sleep both showed significant associations with greater REM density. Mean precuneus rCMRglc (waking and REM) did not correlate with any PSG-based measures.
As reported above, both chronotype and brainstem rCMRglc (waking and REM) were consistently associated with diary- and PSG-based sleep onset latency. Therefore, we speculated that greater activity within the PPT/LDT, and thus greater arousal during wakefulness and REM sleep, might contribute to the longer sleep onset latencies. Exploratory mediation analyses (see Supplement) were generally consistent with this notion.
DISCUSSION
The present analyses are the first to examine correlates of chronotype in military veterans with posttraumatic stress symptoms. Consistent with previous studies, eveningness was associated with greater affective and sleep disturbances, including subjective (diary) and objective (PSG) sleep measures. However, other symptoms associated with eveningness were novel, particularly an association with greater lifetime symptoms of posttraumatic stress. The FDG-PET scans during wakefulness and REM sleep revealed associations between eveningness and objective measures of neural activity in multiple brain regions, although not within the prefrontal regions that we predicted. Notably, one eveningness-associated cluster encompassed brainstem areas associated with REM sleep generation and arousal. Taken together, these findings suggest that chronotype may be an important correlate of neural activity in REM-generating and/or arousal regulatory regions among combat-exposed military veterans with PTSD symptoms and/or nightmares.
Our findings complement other recent evidence that veterans with PTSD experience elevated neural activity that persists throughout wakefulness and REM sleep and extends throughout the brain, including the REM-generating and arousal regulatory brainstem regions highlighted in the current findings, as well as limbic regions implicated in fear responses, and striatal areas linked to reward processing (Germain, et al., 2013). Although these studies are limited by cross-sectional designs, as a whole, the data suggest that trauma exposure may be associated with changes throughout sleep, arousal, fear, and reward regulatory systems, and these changes may contribute to PTSD symptoms during both wakefulness (e.g., hypervigilance) and sleep (e.g., nightmares).
Our novel finding that greater eveningness correlated with higher brainstem rCMRglc across wakefulness and REM sleep suggests that eveningness in combat-exposed veterans is associated with chronic elevated arousal across sleep and wake states. Consistent with this interpretation, greater eveningness was also associated with longer sleep onset latency (perhaps due to higher arousal at bedtime interfering with sleep), worse lifetime PTSD symptoms, and nightmares. Both eveningness and brainstem rCMRglc also correlated with later sleep timing, underscoring the apparent importance of the sleep timing aspects of chronotype. Furthermore, our exploratory mediation analyses revealed a possible pathway by which elevated brainstem activity during wakefulness may account for the association between eveningness and longer sleep onset latencies. However, the cross-sectional nature of our data precludes any causal interpretations.
Indeed, our findings raise questions about the extent to which self-reported chronotype reflects trait-like endogenous circadian timing, rather than current sleep-wake behavior. Although chronotype measures tend to correlate with physiological measures of circadian phase (e.g., Duffy et al, 2009), substantial variance remains unaccounted for in these correlations, and other factors (e.g., individual differences in homeostatic sleep drive (Mongrain 2006)) may influence one's preference for sleep-wake timing. A plausible alternative model is that posttraumatic stress symptoms, including hyperarousal (per the greater brainstem rCMRglc) and pre-sleep concern about safety and/or nightmares, lead to difficulty initiating sleep and later sleep/wake timing, which are then misinterpreted as eveningness. Disentangling these models will require inclusion of endogenous measures of internal circadian timing, obviating any concern about whether chronotype reflects a stable trait or the individual's biased interpretation of his or her own current behavior. These measures would also be useful in determining whether the observed correlations between brain activity and scan timing were driven by circadian phase differences (see Supplement).
Consistent with previous studies, eveningness in this sample was associated with more frequent and intense nightmares. In contrast to our initial hypotheses, this association does not appear to be explained by altered PFC function, which could permit greater affective dysregulation during REM sleep and manifest as nightmares. Instead, our data suggest that eveningness is associated with less REM sleep fragmentation and more robust neural activity in REM sleep-generating brainstem regions, which are in turn associated with higher REM density. Accordingly, higher REM density has been previously linked to chronic PTSD in some (Mellman, Nolan, Hebding, Kulick-Bell, & Dominguez, 1997; Ross et al., 1994) but not all studies (e.g., (Engdahl, Eberly, Hurwitz, Mahowald, & Blake, 2000; Germain & Nielsen, 2003). Chronobiological factors may also contribute to increased nightmare generation among more evening-type individuals, such as circadian modulation of REM sleep (Dijk & Czeisler, 1995; Nielsen, 2010), memory consolidation (Nielsen, 2010; Walker & Stickgold, 2006), and mood (Murray et al., 2009). Altered alignment between sleep-wake timing and internal circadian phase among evening-types may contribute to dysregulation in these processes (Duffy, 1999; Nielsen, 2010; Wittmann, Dinich, Merrow, & Roenneberg, 2006).
The unanticipated association between eveningness and greater activity in a posterior cingulate cortex/precuneus cluster may also be relevant to increased nightmares and lifetime posttraumatic stress symptoms. Recent evidence suggests that the posterior cingulate and/or precuenus may be implicated in dreaming (Desseilles, Dang-Vu, Sterpenich, & Schwartz, 2011; Domhoff, 2011). Although the posterior cingulate cortex and precuneus are typically hypoactive during REM sleep relative to wakefulness (Braun, et al., 1997; Maquet, et al., 1996; Nofzinger, et al., 1997), as noted above, new evidence indicates that waking hypermetabolism in these regions persists into REM sleep in veterans with PTSD (Germain, et al., 2013). Likewise, our findings of increased activity associated with eveningness during both REM sleep and wake states could indicate that more symptomatic evening-types are experiencing chronic activation of these regions. The posterior cingulate cortex and precuneus also appear to be relevant to PTSD (Germain, et al., 2013); however, prior reviews and meta-analyses have diverged on whether these regions are consistently hypo- or hyperactive in PTSD (Bremner, 2002; Hayes, Hayes, & Mikedis, 2012; Patel, Spreng, Shin, & Girard, 2012). Other recent reports have specifically linked the precuneus to dissociative responses (Lanius et al., 2002) and dysregulated memory processes in PTSD (Geuze, Vermetten, de Kloet, & Westenberg, 2008; Hayes et al., 2011).
In contrast to past studies, eveningness was not associated with depression or brain activity in prefrontal regions implicated in affect regulation, but was more clearly associated with measures of posttraumatic stress symptoms, and both subjective and objective elevations in sleep onset latency. Combined with our neuroimaging findings, these data suggest that chronotype may be operating differently among military veterans with posttraumatic stress symptomatology than among previously-studied samples of community-based adolescents and adults. The relationship between stress and chronotype is just starting to gain attention, including a recent paper reporting that sleep quality mediated the association between chronotype and response to a an arithmetic stress-induction task (Roeser, Meule, Schwerdtle, Kubler, & Schlarb, 2012). Furthermore, previous studies have generally presumed that chronotype is a stable, trait-like construct that reflects endogenous internal timing, and consequently have therefore conceptualized eveningness as a precipitating or perpetuating factor for affective and behavioral dysregulation. Morningness, in contrast, has been proposed as a protective factor (e.g., (Gelbmann et al., 2012). As previously noted, our current findings suggest that self-reported eveningness could also be a consequence of such dysregulation, particularly PTSD symptoms, which may delay sleep timing.
Limitations of our study include the cross-sectional design and reliance on self-reported chronotype. Longitudinal studies beginning prior to trauma exposure would best articulate the direction of the relationships between chronotype, sleep, PTSD symptoms, and brain activity. Perhaps a more pragmatic next step would be to retrospectively assess chronotype and sleep timing prior to trauma exposure. Another viable next step would be to assess these relationships during the course of treatment for sleep disturbances such as sleep onset insomnia and nightmares; thus, determining whether chronotype shifted towards morningness with resolution of other symptoms. Finally, our small sample size may limit the generalizability of these findings and increase the risk for Type II error. In particular, the non-significant correlation between chronotype and depression may be partly a function of limited statistical power; the magnitude of both the chronotype-depression and chronotype-age correlations in our sample were consistent with those observed in other studies (e.g., (Drennan, et al., 1991; Hasler, et al., 2010; Randler, et al., 2012) and might prove statistically significant with a larger sample size.
This study is the first to report that chronotype is associated with lifetime posttraumatic stress symptoms, sleep, and brain activity among combat-exposed military veterans. As seen in other populations, eveningness was generally associated with worse functioning and altered brain metabolism. However, the specific pattern of eveningness correlates in this sample was unique; particularly, the elevated glucose metabolism in brainstem and precuenus/posterior cingulate regions across both wakefulness and REM sleep. These associations may provide insight into the hyperarousal symptoms characteristic of PTSD. Our findings suggest that further investigations into the role of chronotype in PTSD, including whether it might be a novel target of sleep-based treatment, would be well justified.
Supplementary Material
Highlights
Chronotype may be relevant to combat-exposed military veterans with PTSD
We examined the correlates of chronotype in veterans using FDG-PET and PSG
Chronotype was associated with lifetime PTSD, sleep disturbance, and nightmares
Chronotype was associated with brainstem rCMRglc across wakefulness and REM sleep
ACKNOWLEDGEMENTS
The authors would like to thank the veterans who participated in this study. The authors would also like to thank the staff of the University of Pittsburgh PET and MR Center for their invaluable technical assistance. This work was supported by a grant from the Department of Defense, W81XWH-08-1-0637-PT073961 (Germain), and by grants from the National Institutes of Health, including R21MH083035 (Germain), T32HL082610 (Buysse), and UL1-RR024153.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
Drs. Hasler, Insana, and Germain, as well as Mr. James, report no biomedical financial interests or potential conflicts of interest.
Note that higher CSM scores indicated greater morningness, or less eveningness, and thus positive correlations between eveningness and clinical measures are expressed as negative r-values.
We also ran the same models using the CAPS-past month total score as a covariate; results were unchanged.
REFERENCES
- Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89(4):455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain : a journal of neurology. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Current Psychiatry Reports. 2002;4(4):254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- Broms U, Kaprio J, Hublin C, Partinen M, Madden PA, Koskenvuo M. Evening types are more often current smokers and nicotine-dependent-a study of Finnish adult twins. Addiction. 2011;106(1):170–177. doi: 10.1111/j.1360-0443.2010.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner DP, Vasko RC, Detka CS, Monahan JP, Reynolds CF, 3rd, Kupfer DJ. Muscle artifacts in the sleep EEG: automated detection and effect on all-night EEG power spectra. Journal of Sleep Research. 1996;5(3):155–164. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caci H, Adan A, Bohle P, Natale V, Pornpitakpan C, Tilley A. Transcultural properties of the composite scale of morningness: the relevance of the “morning affect” factor. Chronobiology international. 2005;22(3):523–540. doi: 10.1081/CBI-200062401. [DOI] [PubMed] [Google Scholar]
- Desseilles M, Dang-Vu TT, Sterpenich V, Schwartz S. Cognitive and emotional processes during dreaming: a neuroimaging view. Consciousness and cognition. 2011;20(4):998–1008. doi: 10.1016/j.concog.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. Journal of Neuroscience. 1995;15(5 Pt 1):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domhoff GW. The neural substrate for dreaming: Is it a subsystem of the default network? Consciousness and Cognition. 2011;20:1163–1174. doi: 10.1016/j.concog.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Drennan M, Klauber M, Kripke D, Goyette L. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of Affective Disorders. 1991;23(2):93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Duffy J, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. Journal of Investigative Medicine. 1999;47(3):141–150. [PMC free article] [PubMed] [Google Scholar]
- Engdahl BE, Eberly RE, Hurwitz TD, Mahowald MW, Blake J. Sleep in a community sample of elderly war veterans with and without posttraumatic stress disorder. Biological Psychiatry. 2000;47(6):520–525. doi: 10.1016/s0006-3223(99)00201-2. [DOI] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22(3):268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Gelbmann G, Kuhn-Natriashvili S, Pazhedath TJ, Ardeljan M, Wöber C, Wöber-Bingöl Ç. Morningness: Protective factor for sleep-related and emotional problems in childhood and adolescence? Chronobiology International. 2012;29(7):898–910. doi: 10.3109/07420528.2012.686946. [DOI] [PubMed] [Google Scholar]
- Germain A. Sleep disturbances as the hallmark of PTSD: Where are we now? American Journal of Psychiatry. doi: 10.1176/appi.ajp.2012.12040432. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Medicine Reviews. 2008;12(3):185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Hall M, Krakow B, Katherine Shear M, Buysse DJ. A brief sleep scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. Journal of anxiety disorders. 2005;19(2):233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Germain A, James J, Insana S, Herringa RJ, Mammen O, Price J, Nofzinger E. A window into the invisible wound of war: Functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Research: Neuroimaging. 2013;211(2):176–179. doi: 10.1016/j.pscychresns.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Nielsen TA. Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biol Psychiatry. 2003;54(10):1092–1098. doi: 10.1016/s0006-3223(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, de Kloet CS, Westenberg HG. Precuneal activity during encoding in veterans with posttraumatic stress disorder. Progress in Brain Research. 2008;167:293–297. doi: 10.1016/S0079-6123(07)67026-5. [DOI] [PubMed] [Google Scholar]
- Guthrie JP, Ash RA, Bendapudi V. Additional validity evidence for a measure of morningness. Journal of Applied Psychology. 1995;80:186–190. [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of BAS and positive affect. Psychiatry Research. 2010;176(2–3):166–173. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, Buysse DJ. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. Journal of Sleep Research. 2012 doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders. 2012;2(1):9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, LaBar KS, McCarthy G, Selgrade E, Nasser J, Dolcos F, Morey RA. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. Journal of Psychiatric Research. 2011;45(5):660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insana SP, Hall M, Buysse DJ, Germain A. Validation of the Pittsburgh Sleep Quality Index Addendum for Posttraumatic Stress Disorder (PSQI-A) in male military veterans. Journal of Traumatic Stress. doi: 10.1002/jts.21793. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2002;52(4):305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383(6596):163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20(1):46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Lahti T, Partonen T. Relation of chronotype to sleep complaints in the general Finnish population. Chronobiology International. 2012;29(3):311–317. doi: 10.3109/07420528.2012.655870. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. Journal of Sleep Research. 2006;15(2):162–166. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9(5):705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Natale V, Alzani A. Additional validity evidence for the composite scale of morningness. Personality and Individual Differences. 2001;30(2):293–301. [Google Scholar]
- Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research. 2011;185(3):408–413. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T. Nightmares associated with the eveningness chronotype. Journal of biological rhythms. 2010;25(1):53–62. doi: 10.1177/0748730409351677. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Price J, Meltzer CC, Townsend D, Buysse DJ, Moore RY. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain research. Brain research protocols. 1998;2(3):191–198. doi: 10.1016/s1385-299x(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain research. 1997;770(1–2):192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Nichols TE, Meltzer CC, Price J, Steppe DA, Miewald JM, Moore RY. Changes in forebrain function from waking to REM sleep in depression: preliminary analyses of [18F]FDG PET studies. Psychiatry Research. 1999;91(2):59–78. doi: 10.1016/s0925-4927(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Randler C. Validation of the full and reduced Composite Scale of Morningness. Biological Rhythm Research. 2009;40(5):413–423. [Google Scholar]
- Randler C. Association between morningness-eveningness and mental and physical health in adolescents. Psychology, Health & Medicine. 2011;16(1):29–38. doi: 10.1080/13548506.2010.521564. [DOI] [PubMed] [Google Scholar]
- Randler C, Stadler L, Vollmer C, Diaz-Morales JF. Relationship between depressive symptoms and sleep duration/chronotype in women. Journal of Individual Differences. 2012;33(3):186–191. [Google Scholar]
- Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. National Institutes of Health; 1968. [DOI] [PubMed] [Google Scholar]
- Roeser K, Meule A, Schwerdtle B, Kubler A, Schlarb AA. Subjective sleep quality exclusively mediates the relationship between morningness-eveningness preference and self-perceived stress response. Chronobiology International. 2012;29(7):955–960. doi: 10.3109/07420528.2012.699124. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biological Psychiatry. 1994;35(3):195–202. doi: 10.1016/0006-3223(94)91152-5. [DOI] [PubMed] [Google Scholar]
- Selvi Y, Aydin A, Gulec M, Boysan M, Besiroglu L, Ozdemir PG, Kilic S. Comparison of dream anxiety and subjective sleep quality between chronotypes. Sleep and Biological Rhythms. 2012;10(1):14–22. [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74(5):728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Thun E, Bjorvatn B, Osland T, Steen VM, Sivertsen B, Johansen T, Pallesen S. An actigraphic validation study of seven morningness-eveningness inventories. European Psychologist. 2012;17(3):222–230. [Google Scholar]
- Tonetti L, Adan A, Caci H, De Pascalis V, Fabbri M, Natale V. Morningnes-seveningness preference and sensation seeking. European Psychiatry. 2010;25(2):111–115. doi: 10.1016/j.eurpsy.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Urban R, Magyarodi T, Rigo A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiology International. 2011;28(3):238–247. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko RC, Jr., Brunner DP, Monahan JP, Doman J, Boston JR, el-Jaroudi A, Kupfer DJ. Power spectral analysis of EEG in a multiple-bedroom, multiple-polygraph sleep laboratory. International journal of medical informatics. 1997;46(3):175–184. doi: 10.1016/s1386-5056(97)00064-6. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annual review of psychology. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23(1&2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17(4):536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.