Abstract
Urinary tract infections (UTIs) are common and over half of women report having had at least one in their lifetime. Nearly a third of these women experience recurrent UTI episodes, but the mechanisms of these recurrences are not fully elucidated. Frequent use of antimicrobials for treatment and prevention of UTIs and other infections has contributed to the evolution of multidrug-resistant microorganisms globally. This is a looming worldwide crisis that has created an urgent need for novel strategies for the treatment and prevention of UTIs. Furthering our understanding of the mechanisms of recurrent UTIs, from both host and bacterial perspectives, will be paramount in developing targeted management strategies. In this review we discuss recent findings regarding recurrent UTIs in women, including progress in our understanding of the mechanisms of recurrence as well as emerging treatments.
Keywords: Recurrent Urinary Tract Infection, Antibiotic Resistance, Mannoside, Pilicide, FimH, Stress Response
Introduction
Urinary tract infections (UTIs) are the most common bacterial infections encountered in outpatient medicine with a strong predilection toward women. More than 50% of women experience at least one UTI during their lifetime, and each year approximately 11% of women report having had a UTI [1]. This high frequency leads to significant healthcare costs; in the United States alone, UTIs cost at least 2-3 billion dollars per annum [1-3].
Uncomplicated UTIs are infections of the lower urinary tract that are not associated with functional or anatomical abnormalities, diabetes, pregnancy, or urinary catheterization. The most common organism causing uncomplicated UTI is Escherichia coli, which causes about 85% of UTIs [2]. Using a mouse model, infection of the bladder by uropathogenic E. coli (UPEC) has been well characterized [4,5]. E. coli enter the bladder via the urethra and attach to the bladder epithelium. These host-pathogen interactions facilitate bacterial colonization and invasion, triggering apoptosis and exfoliation [6,7] and inducing elevated levels of cyclic AMP (cAMP) [8]. Upon internalization, UPEC can be exocytosed in a TLR-4 dependent process [8] however, bacteria can escape into the host cell cytoplasm, where they are able to subvert expulsion and innate defenses by replicating into biofilm-like intracellular bacterial communities (IBCs) [9]. Each IBC matures within hours into masses of approximately 104 cells from a single invaded bacterium. Bacteria then flux out of the host cell back into the lumen of the bladder, where they can invade naïve epithelial cells again. After an infection is cleared, latent bacterial cells, termed quiescent intracellular reservoirs (QIRs), can remain in underlying or superficial bladder epithelial tissue and are capable of causing recurrent UTIs [10]. Another potential outcome of these acute events is the establishment of long-lasting, chronic cystitis characterized by persistent bacteriuria (>104 colony forming units (CFU)/ml) and high titer bacterial bladder burdens accompanied by chronic inflammation and urothelial necrosis [11]. Selective pressure and bacterial population bottlenecks during colonization impact the ultimate fate of disease [12,13]. Numerous bacterial factors have been shown to be important in the ability of UPEC to colonize the bladder, including capsule, iron acquisition systems, toxins, a virulence plasmid, and pathogenicity islands [14,15].
Antimicrobials are the mainstay of treatment for UTI. Several options are recommended for uncomplicated cystitis, depending on the patient's allergy history and the prevalence of antibiotic resistance in their community. Nitrofurantoin (five-day treatment) and trimethoprim/sulfamethoxazole (TMP/SMX; three-day treatment) are recommended first-line drugs [16]. Fosfomycin trometamol (single dose) is another recommended first-line treatment option, though it has inferior efficacy and is not used widely in the United States. Fluoroquinolones (e.g. ciprofloxacin, levofloxacin) are highly effective for cystitis, but are best reserved for more serious infections given their propensity for causing adverse ecologic effects such as Clostridium difficile colitis [16]. Alarmingly, UPEC is becoming increasingly resistant to many of the antimicrobials used to treat UTI. Resistance to ciprofloxacin and other fluoroquinolones is rapidly rising worldwide, which is another reason to avoid using them to treat UTIs while they are still useful against other types of infections [17-19]. Additionally, resistance to TMP/SMX is high in many areas of the world [17,18], and the drug is not recommended for use in geographic areas with known or suspected prevalence of resistance 20% or higher [16].
Here, we discuss recent developments in our understanding of UTI in women. We focus on current issues relevant to recurrent UTIs, including pathogenesis and problems with antimicrobial management. We also discuss emerging treatments that hold promise for use in addition to or in replacement of antimicrobial therapy. Last, we highlight recent findings related to UPEC physiology and potentially future therapeutic targets.
Recurrent UTIs
A significant number of patients have recurrent UTIs following antimicrobial treatment. UTI recurs in 25% of young women with cystitis within 6 months after their first episode [20], and the recurrence rate increases with more than 1 prior UTI [20,21]. Recurrent UTIs are common in women at all ages, and some women are troubled with frequent recurrences over long periods of time. Although several mechanisms have been postulated to explain recurrent UTI, the syndrome is not well understood.
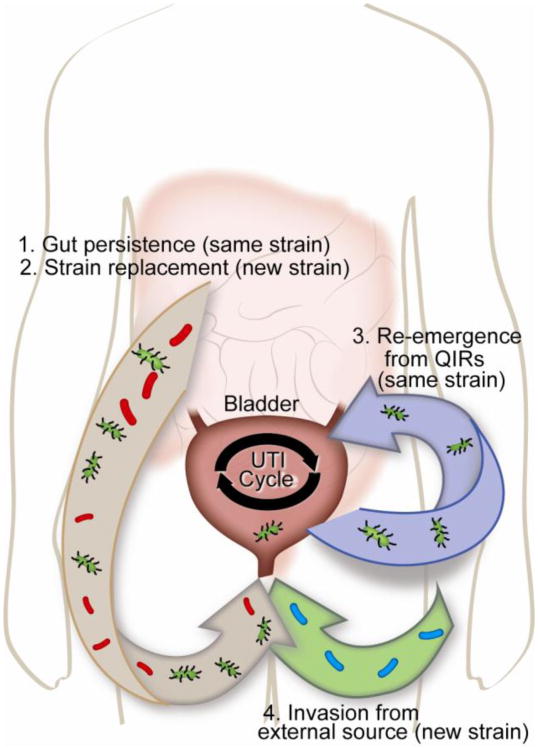
Identifying the strain(s) appearing in recurrent UTI episodes can provide insight into the mode of recurrence. Recurrent UTIs caused by the same UPEC strain can arise from reinfection from the gut or vagina with the same strain [22] or from QIRs that can persist in the bladder [10] (depicted in Figure 1). Recurrences with strains different than the first infection can only occur through the invasion of the bladder by another strain. When different-strain recurrent UTIs occur, the new strain causing the UTI may dwell in the patient's gut, as predicted by the gut-perineal-urethral hypothesis [23], or be introduced from another environmental source. The rates of same-strain versus different-strain recurrences have been investigated for nearly five decades, but estimates of the percentage of same-strain recurrences vary greatly between studies, as summarized in Table 1 [20,21,24-35]. This dramatic range of results may arise from differences in the demographics of the cohort studied, urine collection and bacterial culture methods, definition of symptoms, treatment regimens, duration of follow-up, and perhaps most importantly strain typing methods.
Figure 1.
Possible modes of UTI recurrence. Recurrent UTIs can be introduced from a number of sources. 1. The same UTI-causing strain (green bacteria) persists in the gut and repeatedly re-inoculates the bladder. 2. A different strain (red bacteria) is introduced first into the gut and then colonizes the bladder. 3. Quiescent bacteria reside in the bladder epithelium and re-emerge periodically to cause same-strain UTI (green bacteria). 4. A new strain of bacteria is introduced directly into the peritoneal area from the environment (blue bacteria).
Table 1. Studies of same-strain recurrences of UTI.
| Study | Typing Method | Cohort Age | Proportion with same-strain recurrences* |
|---|---|---|---|
| Pryles, 1965 | Serotype | Not described | 15% |
| McGeachie, 1966 | Serotype | Not described | 16% |
| Kunin, 1970 | Serotype | 6-8 yr | 20% |
| Karkkainen, 2000 | RAPD-PCR | 59 yr (median) | 25% |
| Ikaheimo, 1996 | Phenotype, RAPD-PCR | 51 yr (median) | 33% |
| Foxman, 2000 | Dot blot hybridization, PFGE | 18-39 yr | 34% |
| Luo, 2012 | PFGE | 68 yr (median) | 52% |
| Foxman, 1995 | Dot blot hybridization, PFGE | 21 yr (mean) | 58% |
| Skjot-Rasmussen, 2011 | PFGE | 60 yr (median) | 62% |
| Czaja, 2009 | PFGE | 22 yr (median) | 67% |
| Russo, 1995 | PFGE | 18-42 yr | 68% |
| Koljalg, 2009 | PFGE | 38 mo (median) | 78% |
| Ejmaes, 2006 | PFGE | 43 yr (median) | 82% |
Rate of same-strain recurrences is expressed as percent of total recurrent UTIs that were deemed due to the same strain as the first studied occurrence.
Abbreviations: RAPD-PCR, random amplification of polymorphic DNA polymerase chain reaction; PFGE, pulsed field gel electrophoresis.
A number of findings regarding same-strain persistence of UPEC in the bladder have recently been described. A temporal pattern to same-strain recurrences has been identified, as recurrent UTIs that occur closer to the initial UTI are more likely to be caused by the same strain [21]. Work in ovariectomized mice suggested decreased levels of estrogen can lead to increased bacterial persistence in QIRs [36], possibly explaining the increased susceptibility of post-menopausal women to UTIs [37]. Additionally, strains with increased numbers of bacterial virulence factors, including adhesins, iron uptake systems, and toxins, were more likely to be associated with same-strain recurrent UTI episodes than strains with fewer virulence factors [30]. The function of particular virulence factors also appears to be important, as some virulence factors are associated with higher rates of recurrence than others [20,21,26,27,30,34].
The populations of UPEC strains in other body sites may also be an important factor in recurrent UTIs. A recent study found that the E. coli populations of the gut and the bladder may be correlated in recurrent UTI episodes [38]. In this research, fecal and urine isolates of E. coli from four female patients with several recurrent UTIs were analyzed. For each patient, the majority of E. coli strains isolated from the feces were identical to the urine isolate at the time of each UTI episode. In two patients, a recurrent UTI was caused by a different UPEC strain than the previous UTI, and this new strain found in the urine had also become the dominant member of the E. coli population in the gut while the previous urine strain was no longer found. Importantly, the new strain from one patient was more fit than the previous strain in both the urinary tract and the gut in a mouse model, demonstrating that there is not necessarily a fitness tradeoff between gut and urinary tract colonization. In addition, some of the same urovirulence factors, including adhesins and iron acquisition systems, have been shown to increase persistence of E. coli in the gut [39], indicating that there is some overlap in the factors that mediate fitness in the disparate gut and bladder environments. These findings suggest that the populations of E. coli in both the bladder and gut are linked, although it is not clear if the new strains were introduced first in the gut or in the urinary tract.
Taken together, these investigations show that recurrent UTIs, especially same-strain recurrences, result from complex interactions between the pathogen and host. Given the frequency of recurrent UTIs, additional research is needed to understand the mechanisms of recurrences in order to better predict, treat, and prevent recurrent UTI episodes.
Emerging Treatments for Recurrent UTIs
Increasing antimicrobial resistance is a particular problem for clinicians managing women with recurrent UTIs. One prevention strategy for women with recurrent UTIs is to use prophylactic antimicrobials. While highly effective, use of long-term antimicrobials adds selective pressure for increased antimicrobial resistance in both the gut and urinary tract. In one study, UTI prophylaxis with TMP/SMX led to 86% of fecal E. coli and 91% of bacteriuria E. coli isolates being resistant to TMP/SMX after one month of treatment [40]. Nitrofurantoin may be associated with a significantly lower risk of selecting for antimicrobial resistance in this population compared with TMP/SMX, but is comparatively less effective against many organisms other than E. coli[41,42]. Nitrofurantoin is not recommended for long-term use in the elderly and patients with decreased renal function, as the drug is not delivered to the bladder in sufficient concentrations and may also cause pulmonary complications [43]. Alternatively, if UPEC are dormant as reported in QIRs, they may be entirely recalcitrant to antimicrobials [44] and unaffected by all antimicrobial prophylaxis treatments. In light of the increasing rates of antimicrobial resistance and the adverse ecologic effects associated with antimicrobial prophylaxis used for prevention of recurrent UTIs, there is increasing interest in developing alternative strategies for treating and preventing this common syndrome.
The efficacy of cranberry products as preventive treatments for UTIs has been studied extensively but the data from these investigations are often conflicting. Cranberry studies are complicated by the fact that the mechanism of potential cranberry effect is unknown; it is possible that cranberries affect host responses, bacterial physiology, or both. At the bacterial level, cranberry juice compounds as well as urine from mice fed cranberry juice decreases the adhesion of UPEC to uroepithelial cells [45,46]; in vitro studies of human vaginal epithelial cells have likewise shown an anti-adhesive effect of cranberry [47].
A recent study of college-age women showed that drinking cranberry juice did not reduce the incidence of recurrent UTI compared with placebo [48]. As only a small number of the patients in either group experienced recurrent UTI, studies like this are often underpowered to detect statistical differences. Another recent trial found no difference in recurrence in younger patients drinking cranberry juice versus a placebo, but did find that cranberry juice decreased recurrent UTI frequency in women 50 or more years old [49], raising the possibility that the efficacy of cranberry changes with age. Two recent meta-analyses of numerous cranberry trials determined that cranberry products only have a slight protective effect, if any, against UTI in most populations [50,51]. Overall, whether cranberry products can effectively treat or prevent UTIs requires further study to determine conclusively.
A recent pilot study compared the effect of treatment with ciprofloxacin versus ibuprofen for uncomplicated cystitis [52]. Surprisingly, both treatments were equally effective at symptom resolution and were not significantly different in bacterial clearance from the bladder. The pilot study was small and underpowered, but these initial results are intriguing and suggest that the anti-inflammatory effect of ibuprofen helps clear bladder infections. This is consistent with the finding that severe inflammation of bladder epithelial cells predisposes mice to chronic and recurrent UTIs [11]. Alternatively, ibuprofen could have other unknown anti-infection properties.
As cAMP levels in superficial bladder epithelial cells are raised during UPEC infection as part of the TLR4-based immune response, increasing cAMP induction is a potential therapeutic strategy. When mice were treated with forskolin, a compound that increases levels of intracellular cAMP, their bladder epithelia were significantly more resistant to invasion by UPEC [8]. Thus, cAMP inducers could represent a new class of promising therapeutics, particularly for patients with defects in TLR4-mediated signaling.
There are other, more specific, therapies being developed that are based on new knowledge about the pathophysiology of UTI targeting adhesive pili. Adhesive pili assembled by the chaperone/usher pathway (CUP), such as type 1 pili, contain adhesins at their tips that are thought to play an important role in host-pathogen interactions (reviewed in [53]). Each sequenced UPEC strain has been found to encode a multitude of CUP operons [54]. Some CUP adhesins are known to recognize specific receptors with stereochemical specificity [55]. For example, FimH, the type 1 pilus tip adhesin, has been shown to bind mannosylated glycoproteins, as well as N-linked oligosaccharides on β1 and α3 integrins and the pattern recognition receptor TLR4, which are expressed throughout the luminal surface of human and murine bladders [56,57]. Certain mannose analogs, mannosides, can prevent the binding of FimH to host cells by binding in the adhesin pocket with greater affinity than mannose [58]. These mannosides have been effective in vitro and in mouse models in decreasing adhesion of E. coli to bladder epithelium and subsequently decreasing titers of bacteria that are found in the bladder during experimental infections [59-64]. Lead candidate mannoside molecules have both treated existing UTI and prevented UTI when used prophylactically in mice [61,63]. Additionally, pharmacokinetic studies identified an optimized candidate with high oral bioavailability [61]. These mannosides are continuing to be refined and will be tested against human UTIs in the near future.
Another strategy to prevent bacteria from adhering to the bladder epithelium is to prevent cells from generating CUP pili. Small molecules that prevent pilus assembly have been identified and are termed pilicides [65,66]. The mechanism of action of one pilicide was shown to be in part due to binding to the chaperone to prevent formation of chaperone-subunit-usher ternary interactions [65]. Pilicides have also been shown to prevent expression of the genes encoding type 1 pili as well as other adhesive pili [67] by mechanisms yet to be elucidated. Though pilicides have been mostly studied in vitro thus far, these compounds are potentially able to thwart infection by preventing pilus biosynthesis and thus prevent adhesion to epithelial cells. Pre-treatment of UPEC with pilicides has been shown to decrease virulence in a mouse model, supporting the predicted in vivo efficacy of these compounds [68].
Perhaps the most intriguing potential treatment strategy is a combination of antimicrobials and these new anti-adhesion molecules. It was recently shown that the combination of mannosides and TMP/SMX had a synergistic effect and was more effective in mice than either treatment alone and able to circumvent antibiotic resistance [61]. Combinations of mannosides, other antimicrobials, pilicides, or cranberry extract could potentially become optimal treatments for recurrent UTI.
There have been a number of efforts to create a vaccine against UTI, particularly to be used in populations with recurrent infections (reviewed in [69]). Vaccine strategies using whole cell lysates of UPEC and other uropathogens have been tried in human subjects with limited success. However, vaccines developed against iron uptake proteins have been developed and shown to be effective in mice [70,71]. Additionally, a vaccine against the FimH adhesin elicited protection in mice [72,73], and protected cynomolgus monkeys from colonization and infection with UPEC [74]. Recently, mice have been protected from bladder and kidney colonization with a vaccine that included both FimH and a flagellum component, FliC [75]. Vaccines against other UPEC adhesins, FdeC and PapG, have also elicited protection against kidney infection in a mouse model [76] and a cynomolgus monkey model [77], respectively. Like the emerging chemotherapeutics strategies in other conditions, perhaps a vaccine that targeted multiple adhesins or physiological structures at the same time could be the most effective in preventing either cystitis or pyelonephritis in susceptible populations.
Recent Developments in UPEC Physiology Identify New Therapeutic Targets
In addition to the role of type 1 pili, new research continues to identify aspects of bacterial physiology that are important in urinary tract pathogenesis. Recent findings suggest that UPEC are able to grow in urine using amino acids as nutrient sources, and mutants that are unable to catabolize amino acids are reduced in virulence [78,79]. UPEC also require iron siderophores to scavenge iron from the low-iron environment in the bladder [80-82], and as described above it has been suggested that this physiology could be a target for vaccine development [70,83,84]. A recent study uncovered a surprising new function of the yersiniabactin siderophore: the ability to scavenge copper from urine, in addition to iron [85]. Uncovering physiological details such as the role of amino acids or siderophores not only helps us understand how UPEC is able to survive in the urine and bladder, but will also facilitate the development of smart strategies to target UPEC with minimal impact on the patient flora and help prevent the rapid spread of antimicrobial resistance across the globe.
Recently, two-component regulatory systems have been found to be important in UPEC pathogenesis. Two-component signaling systems are critical for bacterial pathogens to adapt quickly to environment changes, and these systems generally work via a sensor protein, often a histidine kinase, and a regulatory protein which gets phosphorylated by the sensor protein and subsequently alters gene expression [86]. The two-component regulatory system QseBC was found to be involved in E. coli UTI [87]. The QseBC system is involved in regulation of type 1 pili and flagella expression, and other factors required for virulence [88]. In the absence of QseC (the sensor kinase), a number of metabolic genes important for bladder infection are misregulated thus attenuating virulence, demonstrating a potential novel target for therapy [89].
The Cpx two-component signaling system, consisting of histidine kinase CpxA and response regulator CpxR, was also found to contribute to urinary tract pathogenesis [90]. The Cpx system responds to envelope stress, suggesting that the bacterial cell envelope integrity is compromised when UPEC invades the bladder. The Cpx system also regulates expression of another UPEC attachment fiber, the P pilus [91,92]. Though P pili are not absolutely required for cystitis, they provide a competitive advantage in the bladder [93] and are an important virulence factor for E. coli strains that cause pyelonephritis (reviewed in [94]). A third two-component system, OmpR-EnvZ, is possibly involved in virulence, likely by influencing the expression of type 1 pili genes, particularly in acidic and high-osmolarity environments [95,96]. These two-component regulatory systems could provide novel drug targets. In addition to the anti-adhesion therapies currently in development, two-component systems would be good targets because they are exposed to or sensitive to the external environment. Interrupting any of these signaling pathways would not immediately lead to cell death but could disrupt virulence gene expression and decrease both invasion and persistence of the bacteria in the bladder.
Other types of stress response systems are also involved in UPEC pathogenesis in the bladder. The canonical oxidative stress response is induced in the presence of reactive oxygen species (ROS) such as peroxide and superoxide. These ROS can damage enzyme cofactors, DNA, and proteins, ultimately leading to cell death if the ROS are not managed [97]. ROS damage is a significant antimicrobial mechanism used by phagocytic cells during infection. To counteract oxidative stress, UPEC RpoS is induced during bladder infection to increase expression of proteins that can eliminate the ROS produced by invading phagocytes [79,98]. Nitrosative stress caused by high levels of nitric oxide is another environmental stress faced by UPEC in the bladder [99,100], and UPEC uses polyamines to help quench such reactive nitrogen species in vivo [101]. Any of these stress response systems could be effective targets for therapeutics, as eliminating such responses would make it harder for bacteria to survive in the bladder as well as lead to more efficient clearance by the immune system.
Toxin-antitoxin (TA) systems assist in oxidative and nitrosative stress resistance as well as persister cell formation [102]. A TA system works by generating both a toxin and cognate immunity protein (the antitoxin); if the antitoxin is lost, the cells are killed by the toxin. One TA pair, PasT and PasI, was recently shown to provide a benefit to UPEC strains in murine kidney colonization [103]. The presence of PasT/PasI also promoted persister cell formation, which leads to more cells surviving antimicrobial treatment. Interfering with this PasT/PasI system would be a unique antimicrobial strategy, as inhibiting only the antitoxin PasI would induce PasT toxin-mediated killing. Thus, TA systems, in addition to other factors that promote persister cells, could be important targets for therapeutics specifically targeting recurrent UTIs that are caused by recrudescence or QIRs.
Conclusions
Urinary tract infections afflict the majority of women today, with many experiencing recurrent UTIs. Recent work has begun to unravel host and pathogen factors that mediate recurrent UTI, suggesting that management of the disease will likely require consideration of bacterial reservoirs in other body sites in addition to the urinary tract. Current antimicrobial treatments are rapidly selecting for resistant bacteria, both in the gut and in the bladder, and this practice is unsustainable. Emerging therapeutics are being developed to target UPEC without causing antimicrobial resistance and ideally not disrupting the healthy microbiota in other body sites. These therapeutics are based on our knowledge of UPEC physiology, highlighting the importance of continued investigation of basic microbiology and translational research. Advancing our understanding of recurrent UTIs will require continued investigations at the nexus of genomics, bacteriology, and the human microbiome in order to develop appropriate and responsible treatment strategies.
Acknowledgments
Dr. Scott J. Hultgren was supported by grants from NIAID R01 AI029549, NIAID R01 AI048689, ORWH NIDDK P50 DK064540, NIDDK R01 DK051406, NIAID U01 AI095542, and NIAID R01 AI099099. Dr. Hultgren also has a pending grant from NIH/NIAID (U01 AI095776). Dr. Hultgren has a board membership, has received consultancy from, and owns stock in Fimbrion Therapeutics, Inc.
Footnotes
Compliance with Ethics Guidelines: Conflict of Interest: Dr. Jennifer A. Silverman and Dr. Henry L. Schreiber IV reported no potential conflicts of interest relevant to this article.
Dr. Thomas M. Hooton serves on the board and owns stock in the company of Fimbrion Therapeutics, Inc.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of outstanding importance
- 1.Foxman B, Barlow R, D'Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 2.Griebling TL. Urinary Tract Infection in Women. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. US DHHS, PHS, NIH, NIDDKD; Washington DC: 2007. pp. 587–620. US GPO. [Google Scholar]
- 3.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 4.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannan TJ, Totsika M, Mansfield KJ, et al. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev. 2012;36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thumbikat P, Berry RE, Schaeffer AJ, Klumpp DJ. Differentiation-induced uroplakin III expression promotes urothelial cell death in response to uropathogenic E. coli. Microbes Infect. 2009;11:57–65. doi: 10.1016/j.micinf.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thumbikat P, Berry RE, Zhou G, et al. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 2009;5:e1000415. doi: 10.1371/journal.ppat.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J, Bishop BL, Li G, et al. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–298. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson GG, Palermo JJ, Schilling JD, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 10.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Hannan TJ, Mysorekar IU, Hung CS, et al. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. This important work identified a connection between inflammation and UTI outcome in mice. More severe inflammatory responses correlated with a predisposition for chronic UTI, suggesting the immune response to an initial UTI affects the likelihood of experiencing future UTIs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun. 2011;79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walters MS, Lane MC, Vigil PD, et al. Kinetics of uropathogenic Escherichia coli metapopulation movement during urinary tract infection. MBio. 2012;3 doi: 10.1128/mBio.00303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203–221. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 15.Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7:430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 16•.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–120. doi: 10.1093/cid/ciq257. This reference discusses the emerging worldwide problem of antimicrobial resistance and current empiric treatment strategies for uncomplicated cystitis and pyelonephritis. [DOI] [PubMed] [Google Scholar]
- 17.Zhanel GG, Hisanaga TL, Laing NM, et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA) Int J Antimicrob Agents. 2005;26:380–388. doi: 10.1016/j.ijantimicag.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Schito GC, Naber KG, Botto H, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34:407–413. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Bonkat G, Muller G, Braissant O, et al. Increasing prevalence of ciprofloxacin resistance in extended-spectrum-beta-lactamase-producing Escherichia coli urinary isolates. World J Urol. 2013 doi: 10.1007/s00345-013-1031-5. [DOI] [PubMed] [Google Scholar]
- 20.Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 21.Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 22.Hooton TM. Clinical practice Uncomplicated urinary tract infection. New Engl J Med. 2012;366:1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Tsukamoto T, Terai A, et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol. 1997;157:1127–1129. [PubMed] [Google Scholar]
- 24.Czaja CA, Stamm WE, Stapleton AE, et al. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract Infection in women. J Infect Dis. 2009;200:528–536. doi: 10.1086/600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejrnaes K, Sandvang D, Lundgren B, et al. Pulsed-field gel electrophoresis typing of Escherichia coli strains from samples collected before and after pivmecillinam or placebo treatment of uncomplicated community-acquired urinary tract infection in women. J Clin Microbiol. 2006;44:1776–1781. doi: 10.1128/JCM.44.5.1776-1781.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foxman B, Zhang L, Tallman P, et al. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 27.Kärkkäinen U, Ikaheimo R, Katila ML, Siitonen A. Recurrence of urinary tract infections in adult patients with community-acquired pyelonephritis caused by E. coli: a 1-year follow-up. Scand J Infect Dis. 2000;32:495–499. doi: 10.1080/003655400458767. [DOI] [PubMed] [Google Scholar]
- 28.Kõljalg S, Truusalu K, Vainumäe I, et al. Persistence of Escherichia coli clones and phenotypic and genotypic antibiotic resistance in recurrent urinary tract infections in childhood. J Clin Microbiol. 2009;47:99–105. doi: 10.1128/JCM.01419-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunin CM. A ten-year study of bacteriuria in schoolgirls: final report of bacteriologic, urologic, and epidemiologic findings. J Infect Dis. 1970;122:382–393. doi: 10.1093/infdis/122.5.382. [DOI] [PubMed] [Google Scholar]
- 30.Luo Y, Ma Y, Zhao Q, et al. Similarity and divergence of phylogenies, antimicrobial susceptibilities, and virulence factor profiles of Escherichia coli isolates causing recurrent urinary tract infections that persist or result from reinfection. J Clin Microbiol. 2012;50:4002–4007. doi: 10.1128/JCM.02086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGeachie J. Recurrent infection of the urinary tract: reinfection or recrudescence? Brit Med J. 1966;1:952–954. doi: 10.1136/bmj.1.5493.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pryles CV, Glagovsky A. Serological characterization of Escherichia coli study in acute and recurrent urinary tract infections in infants and children. Pediatrics. 1965;36:219–224. [PubMed] [Google Scholar]
- 33.Russo TA, Stapleton A, Wenderoth S, et al. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–445. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 34.Skjøt-Rasmussen L, Hammerum AM, Jakobsen L, et al. Persisting clones of Escherichia coli isolates from recurrent urinary tract infection in men and women. J Med Microbiol. 2011;60:550–554. doi: 10.1099/jmm.0.026963-0. [DOI] [PubMed] [Google Scholar]
- 35.Winberg J, Andersen HJ, Bergström T, et al. Epidemiology of symptomatic urinary tract infection in childhood. Acta Paediatrica. 1974;63:1–20. doi: 10.1111/j.1651-2227.1974.tb05718.x. [DOI] [PubMed] [Google Scholar]
- 36•.Wang C, Symington JW, Ma E, et al. Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect Immun. 2013;81:733–739. doi: 10.1128/IAI.01234-12. This paper establishes an ovariectomized mouse model to mimic menopause, finding that more QIRs are present in ovariectomized mice and observing a protective effect of estrogen therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foxman B. Urinary Tract Infection in Postmenopausal Women. Curr Infect Dis Rep. 1999;1:367–370. doi: 10.1007/s11908-999-0043-1. [DOI] [PubMed] [Google Scholar]
- 38••.Chen SL, Wu M, Henderson JP, et al. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci Transl Med. 2013;5:184r. doi: 10.1126/scitranslmed.3005497. a160. This work describes a seminal finding: E. coli isolates found in the urinary tract are not necessarily less fit than those found in the intestine, and UTI recurrences could be due to the introduction of more fit strains of E. coli than the ones that caused previous episodes. This paper calls attention to the need to investigate the gut as a reservoir for UPEC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowrouzian F, Hesselmar B, Saalman R, et al. Escherichia coli in infants' intestinal microflora: colonization rate, strain turnover, and virulence gene carriage. Pediatr Res. 2003;54:8–14. doi: 10.1203/01.PDR.0000069843.20655.EE. [DOI] [PubMed] [Google Scholar]
- 40.Beerepoot MA, ter Riet G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections: a randomized double-blind noninferiority trial in premenopausal women. Arch Intern Med. 2011;171:1270–1278. doi: 10.1001/archinternmed.2011.306. [DOI] [PubMed] [Google Scholar]
- 41.Cunha BA. Prophylaxis for recurrent urinary tract infections: nitrofurantoin, not trimethoprim-sulfamethoxazole or cranberry juice. Arch Intern Med. 2012;172:82. doi: 10.1001/archinternmed.2011.613. author reply 82-83. [DOI] [PubMed] [Google Scholar]
- 42.McOsker CC, Fitzpatrick PM. Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother. 1994;33(Suppl A):23–30. doi: 10.1093/jac/33.suppl_a.23. [DOI] [PubMed] [Google Scholar]
- 43.American Geriatrics Society 2012 Beers Criteria Update Expert Panel: American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blango MG, Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother. 2010;54:1855–1863. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CS, Ho DR, Chang PJ, et al. Urine post equivalent daily cranberry juice consumption may opsonize uropathogenicity of Escherichia coli. J Infect Chemother. 2013 doi: 10.1007/s10156-013-0565-1. [DOI] [PubMed] [Google Scholar]
- 46.Sobota AE. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J Urol. 1984;131:1013–1016. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- 47.Gupta K, Chou MY, Howell A, et al. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol. 2007;177:2357–2360. doi: 10.1016/j.juro.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbosa-Cesnik C, Brown MB, Buxton M, et al. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52:23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi S, Hamasuna R, Yasuda M, et al. A randomized clinical trial to evaluate the preventive effect of cranberry juice (UR65) for patients with recurrent urinary tract infection. J Infect Chemother. 2013;19:112–117. doi: 10.1007/s10156-012-0467-7. [DOI] [PubMed] [Google Scholar]
- 50.Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:988–996. doi: 10.1001/archinternmed.2012.3004. [DOI] [PubMed] [Google Scholar]
- 51.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Bleidorn J, Gagyor I, Kochen MM, et al. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?--results of a randomized controlled pilot trial. BMC Med. 2010;8:30. doi: 10.1186/1741-7015-8-30. This is a small pilot trial that raises the intriguing possibility that anti-inflammatories may have a role in the treatment of acute cystitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz DJ, Hultgren SJ. Uropathogenic Escherichia coli Virulence and Gene Regulation. In: Darwin AJ, Vasil ML, editors. Regulation of Bacterial Virulence. Washington DC: ASM Press; 2012. pp. 135–155. [Google Scholar]
- 54.Spurbeck RR, Stapleton AE, Johnson JR, et al. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect Immun. 2011;79:4753–4763. doi: 10.1128/IAI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hung CS, Bouckaert J, Hung D, et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol Microbiol. 2002;44:903–915. doi: 10.1046/j.1365-2958.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- 56.Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 57.Wu XR, Sun TT, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci U S A. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouckaert J, Berglund J, Schembri M, et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol Microbiol. 2005;55:441–455. doi: 10.1111/j.1365-2958.2004.04415.x. [DOI] [PubMed] [Google Scholar]
- 59.Han Z, Pinkner JS, Ford B, et al. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J Med Chem. 2010;53:4779–4792. doi: 10.1021/jm100438s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Z, Pinkner JS, Ford B, et al. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J Med Chem. 2012;55:3945–3959. doi: 10.1021/jm300165m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Cusumano CK, Pinkner JS, Han Z, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med. 2011;3:109ra115. doi: 10.1126/scitranslmed.3003021. This work describes optimization and murine bioavailability studies of small anti-adhesion molecules. Importantly, these molecules could treat and prevent UTI, and had a synergistic effect when combined with TMP/SMX treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwardt O, Rabbani S, Hartmann M, et al. Design, synthesis and biological evaluation of mannosyl triazoles as FimH antagonists. Bioorg Med Chem. 2011;19:6454–6473. doi: 10.1016/j.bmc.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 63.Jiang X, Abgottspon D, Kleeb S, et al. Antiadhesion therapy for urinary tract infections--a balanced PK/PD profile proved to be key for success. J Med Chem. 2012;55:4700–4713. doi: 10.1021/jm300192x. [DOI] [PubMed] [Google Scholar]
- 64.Scharenberg M, Schwardt O, Rabbani S, Ernst B. Target selectivity of FimH antagonists. J Med Chem. 2012;55:9810–9816. doi: 10.1021/jm3010338. [DOI] [PubMed] [Google Scholar]
- 65.Pinkner JS, Remaut H, Buelens F, et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci U S A. 2006;103:17897–17902. doi: 10.1073/pnas.0606795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chorell E, Pinkner JS, Bengtsson C, et al. Design and synthesis of fluorescent pilicides and curlicides: bioactive tools to study bacterial virulence mechanisms. Chemistry. 2012;18:4522–4532. doi: 10.1002/chem.201103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aberg V, Fallman E, Axner O, et al. Pilicides regulate pili expression in E. coli without affecting the functional properties of the pilus rod. Mol Biosyst. 2007;3:214–218. doi: 10.1039/b613441f. [DOI] [PubMed] [Google Scholar]
- 68.Cegelski L, Pinkner JS, Hammer ND, et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Brumbaugh AR, Mobley HL. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines. 2012;11:663–676. doi: 10.1586/erv.12.36. This work is an excellent, comprehensive review of advancements made in UTI vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alteri CJ, Hagan EC, Sivick KE, et al. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5:e1000586. doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wieser A, Romann E, Magistro G, et al. A multiepitope subunit vaccine conveys protection against extraintestinal pathogenic Escherichia coli in mice. Infect Immun. 2010;78:3432–3442. doi: 10.1128/IAI.00174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langermann S, Palaszynski S, Barnhart M, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 73.Poggio TV, La Torre JL, Scodeller EA. Intranasal immunization with a recombinant truncated FimH adhesin adjuvanted with CpG oligodeoxynucleotides protects mice against uropathogenic Escherichia coli challenge. Can J Microbiol. 2006;52:1093–1102. doi: 10.1139/w06-065. [DOI] [PubMed] [Google Scholar]
- 74.Langermann S, Mollby R, Burlein JE, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 75.Karam MR, Oloomi M, Mahdavi M, et al. Assessment of immune responses of the flagellin (FliC) fused to FimH adhesin of uropathogenic Escherichia coli. Mol Immunol. 2013;54:32–39. doi: 10.1016/j.molimm.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Nesta B, Spraggon G, Alteri C, et al. FdeC, a novel broadly conserved Escherichia coli adhesin eliciting protection against urinary tract infections. MBio. 2012;3 doi: 10.1128/mBio.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roberts JA, Kaack MB, Baskin G, et al. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J Urol. 2004;171:1682–1685. doi: 10.1097/01.ju.0000116123.05160.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alteri CJ, Smith SN, Mobley HL. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009;5:e1000448. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donovan GT, Norton JP, Bower JM, Mulvey MA. Adenylate cyclase and the cyclic AMP receptor protein modulate stress resistance and virulence capacity of uropathogenic Escherichia coli. Infect Immun. 2013;81:249–258. doi: 10.1128/IAI.00796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torres AG, Redford P, Welch RA, Payne SM. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun. 2001;69:6179–6185. doi: 10.1128/IAI.69.10.6179-6185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henderson JP, Crowley JR, Pinkner JS, et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009;5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia EC, Brumbaugh AR, Mobley HL. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun. 2011;79:1225–1235. doi: 10.1128/IAI.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russo TA, McFadden CD, Carlino-MacDonald UB, et al. The siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect Immun. 2003;71:7164–7169. doi: 10.1128/IAI.71.12.7164-7169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durant L, Metais A, Soulama-Mouze C, et al. Identification of candidates for a subunit vaccine against extraintestinal pathogenic Escherichia coli. Infect Immun. 2007;75:1916–1925. doi: 10.1128/IAI.01269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaturvedi KS, Hung CS, Crowley JR, et al. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol. 2012;8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 87.Kostakioti M, Hadjifrangiskou M, Pinkner JS, Hultgren SJ. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol Microbiol. 2009;73:1020–1031. doi: 10.1111/j.1365-2958.2009.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kostakioti M, Hadjifrangiskou M, Cusumano CK, et al. Distinguishing the contribution of type 1 pili from that of other QseB-misregulated factors when QseC is absent during urinary tract infection. Infect Immun. 2012;80:2826–2834. doi: 10.1128/IAI.00283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Hadjifrangiskou M, Kostakioti M, Chen SL, et al. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol Microbiol. 2011;80:1516–1529. doi: 10.1111/j.1365-2958.2011.07660.x. This paper links both virulence and metabolism of UPEC with one two-component system and suggests the potential for using this system as a new target for antimicrobials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Debnath I, Norton JP, Barber AE, et al. The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli. Infect Immun. 2013;81:1450–1459. doi: 10.1128/IAI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hung DL, Raivio TL, Jones CH, et al. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. Embo J. 2001;20:1508–1518. doi: 10.1093/emboj/20.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hernday AD, Braaten BA, Broitman-Maduro G, et al. Regulation of the pap epigenetic switch by CpxAR: phosphorylated CpxR inhibits transition to the phase ON state by competition with Lrp. Mol Cell. 2004;16:537–547. doi: 10.1016/j.molcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 93.Winberg J, Mollby R, Bergstrom J, et al. The PapG-adhesin at the tip of P-fimbriae provides Escherichia coli with a competitive edge in experimental bladder infections of cynomolgus monkeys. J Exp Med. 1995;182:1695–1702. doi: 10.1084/jem.182.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lane MC, Mobley HL. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007;72:19–25. doi: 10.1038/sj.ki.5002230. [DOI] [PubMed] [Google Scholar]
- 95.Schwan WR, Lee JL, Lenard FA, et al. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect Immun. 2002;70:1391–1402. doi: 10.1128/IAI.70.3.1391-1402.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rentschler AE, Lovrich SD, Fitton R, et al. OmpR regulation of the uropathogenic Escherichia coli fimB gene in an acidic/high osmolality environment. Microbiology. 2013;159:316–327. doi: 10.1099/mic.0.059386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 98.Hryckowian AJ, Welch RA. RpoS contributes to phagocyte oxidase-mediated stress resistance during urinary tract infection by Escherichia coli CFT073. MBio. 2013;4:e00023–00013. doi: 10.1128/mBio.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lundberg JO, Ehren I, Jansson O, et al. Elevated nitric oxide in the urinary bladder in infectious and noninfectious cystitis. Urology. 1996;48:700–702. doi: 10.1016/S0090-4295(96)00423-2. [DOI] [PubMed] [Google Scholar]
- 100.Poljakovic M, Svensson ML, Svanborg C, et al. Escherichia coli-induced inducible nitric oxide synthase and cyclooxygenase expression in the mouse bladder and kidney. Kidney Int. 2001;59:893–904. doi: 10.1046/j.1523-1755.2001.059003893.x. [DOI] [PubMed] [Google Scholar]
- 101.Bower JM, Mulvey MA. Polyamine-mediated resistance of uropathogenicEscherichia coli to nitrosative stress. J Bacteriol. 2006;188:928–933. doi: 10.1128/JB.188.3.928-933.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 103•.Norton JP, Mulvey MA. Toxin-antitoxin systems are important for niche-specific colonization and stress resistance of uropathogenic Escherichia coli. PLoS Pathog. 2012;8:e1002954. doi: 10.1371/journal.ppat.1002954. This work identifies several toxin-antitoxin pairs as being important for stress resistance and bladder and kidney persistence in UPEC. [DOI] [PMC free article] [PubMed] [Google Scholar]