Abstract
Obesity is a chronic inflammation with increased serum levels of insulin, insulin-like growth factor 1 (IGF1), and interleukin-17 (IL-17). The objective of this study was to test a hypothesis that insulin and IGF1 enhance IL-17-induced expression of inflammatory chemokines/cytokines through a glycogen synthase kinase 3β (GSK3B)-dependent mechanism, which can be inhibited by melatonin. We found that insulin/IGF1 and lithium chloride enhanced IL-17-induced expression of C-X-C motif ligand 1 (Cxcl1) and C-C motif ligand 20 (Ccl20) in the Gsk3b+/+ but not Gsk3b−/− mouse embryonic fibroblast (MEF) cells. IL-17 induced higher levels of Cxcl1 and Ccl20 in the Gsk3b−/− MEF cells, compared to the Gsk3b+/+ MEF cells. Insulin and IGF1 activated Akt to phosphorylate GSK3B at serine 9, thus inhibiting GSK3B activity. Melatonin inhibited Akt activation, thus decreasing P-GSK3B at serine 9 (i.e., increasing GSK3B activity) and subsequently inhibiting expression of Cxcl1 and Ccl20 that was induced either by IL-17 alone or by a combination of insulin and IL-17. Melatonin's inhibitory effects were only observed in the Gsk3b+/+ but not Gsk3b−/− MEF cells. Melatonin also inhibited expression of Cxcl1, Ccl20 and Il-6 that was induced by a combination of insulin and IL-17 in the mouse prostatic tissues. Further, nighttime human blood, which contained high physiologic levels of melatonin, decreased expression of Cxcl1, Ccl20 and Il-6 in the PC3 human prostate cancer xenograft tumors. Our data support our hypothesis and suggest that melatonin may be used to dampen IL-17-mediated inflammation that is enhanced by the increased levels of insulin and IGF1 in obesity.
Keywords: Melatonin, insulin, IGF1, IL-17, GSK3B, inflammation, prostate cancer
Introduction
Melatonin (also known chemically as N-acetyl-5-methoxytryptamine) is an endogenous indolamine that is synthesized mainly by the pineal gland during the dark phase of circadian rhythm in mammals, including humans [1]. Melatonin acts through two G protein-coupled melatonin receptors (MT1 and MT2) on the cytoplasmic membrane [2], a cytosolic melatonin receptor (MT3) [3], or directly as a free radical scavenger/antioxidant molecule [4]. Melatonin plays an important role in many physiological and pathological processes, thus it has been tested in the treatments of a variety of diseases [5]. Among them, melatonin has been shown to improve the symptoms of inflammatory bowel diseases. The anti-inflammatory actions of melatonin are mediated through multiple molecular mechanisms, including down-regulation of inducible nitric oxide synthase and cyclooxygenase, reduction of pro-inflammatory cytokines and chemokines (tumor necrosis factor-α (TNF-α), interferon-γ, interleukin-1 (IL-1), IL-6, IL-8, and IL-12), and increase of anti-inflammatory cytokines (IL-10 and IL-1 receptor antagonist)(see a recent review [6]). We noted that TNF-α, IL-1, IL-6, and IL-8 are the downstream target genes of IL-17 [7], which led us to investigate melatonin's action on IL-17 signaling in the present study.
IL-17 (including IL-17A, IL-17F, and IL-17A/F) binds to a heterodimer of IL-17RA/IL-17RC receptor complex, leading to recruitment of nuclear factor-κB (NF-κB) activator 1(Act1) through SEFIR (similar expression to fibroblast growth factor genes, IL-17 receptors and Toll–IL-1R) domains that exist in IL-17RA, IL-17RC, and Act1 proteins [8-12]. Act1 is also named as connection of IκB kinase and stress-activated protein kinase/Jun kinase (CIKS) [13]. Act1 is an E3 ubiquitin ligase that activates tumor necrosis factor receptor-associated factor 6 (TRAF6) through lysine-63-linked ubiquitination [14]. The polyubiquitinated TRAF6 activates transforming growth factor –β-activated kinase 1 (TAK1) and subsequently IκB kinase (IKK) complex, leading to activation of NF-κB pathway that induces transcription of a variety of cytokines, chemokines and growth factors [13, 15-21]. While the IL-17-driven NF-κB signaling pathway has been clearly defined, it is not quite clear how IL-17 activates the extracellular signal-regulated kinase ½ (ERK1/2), thus stabilizing the mRNAs of the IL-17 downstream target genes [22]. In addition, it has been demonstrated that IL-17 stabilizes downstream C-X-C motif ligand 1 (CXCL1) mRNA through an inducible kinase IKKi-dependent Act1-TRAF2-TRAF5 complex, which binds to splicing factor 2 (SF2, also named alternative splicing factor - ASF) and prevents SF2/ASF-mediated mRNA degradation [23, 24].
Insulin is a hormone produced by the β cells of the pancreas. Hyperinsulinemia occurs in obesity and type 2 diabetes mellitus with insulin resistance. Under the hyperinsulinemic status, insulin upregulates expression of growth hormone receptor in the liver, thus enhancing hepatic production of insulin-like growth factor 1 (IGF1) [25]. Insulin binds to insulin receptor (IR-A and IR-B) and IGF1 binds to IGF1 receptor (IGF1R). IGF1 can also bind to a heterodimer of IGF1R and IR-A (or IR-B). Ligand binding leads to autophosphorylation of the β subunit of IR or IGF1R, resulting in recruitment of insulin receptor substrates (IRS) 1 to IRS4 and other adaptor proteins. Consequently, phosphatidylinositol 3-kinase (PI3K)/Akt pathway and ERK1/2 pathway are activated [26]. One of the major substrates of Akt is glycogen synthase kinase 3β (GSK3B). Insulin-stimulated Akt phosphorylates GSK3B at serine 9, thus inactivating GSK3B [27, 28]. A decrease in GSK3B activity leads to reduced phosphorylation of glycogen synthase, thus glycogen synthase activity is increased, resulting in increased glycogen synthesis.
GSK3B is one of the two GSK3 isoforms (GSK3A and GSK3B) of serine/threonine protein kinases that are ubiquitously expressed in all cell types. GSK3B is constitutively active and it phosphorylates more than 50 substrates [29]. Among these substrates, CAAT enhancer binding protein β (C/EBPβ) is closely associated with IL-17 signaling. IL-17 induces expression of C/EBPβ and C/EBPδ mRNA and protein [9, 20, 30]. C/EBPβ and C/EBPδ transcription factors are essential for transcription of IL-17-downstream target genes such as IL-6 and 24p3/lipocalin 2 [31]. However, IL-17 also initiates a negative feedback mechanism by activation of ERK1/2 to phosphorylate C/EBPβ at threonine 188, followed by phosphorylation of C/EBPβ at threonine 179 by GSK3B. Phosphorylation of C/EBPβ inhibits expression of IL-17-downstream target genes, thus GSK3B negatively regulates IL-17 signaling through phosphorylation of C/EBPβ [32]. Indeed, inhibition of GSK3 activity by GSK3 inhibitor I can enhance IL-17-induced expression of IL-6, 24p3/lipocalin 2, CXCL5, C-C motif ligand 2 (CCL2), CCL7, and NF-κB inhibitor zeta, whereas overexpression of GSK3B can inhibit IL-17-induced IL-6-promoter and 24p3-promoter activities in a mouse stromal ST2 cell line [32]. Therefore, GSK3B functions as an intrinsic negative regulator of IL-17-mediated inflammatory responses.
Approximately 35% of adult Americans are obese [33]. It is well known that obesity is associated with type 2 diabetes mellitus with increased serum levels of insulin and IGF1, and that obesity results in a chronic inflammatory state with increased serum levels of inflammatory mediators TNFα and IL-6 [34]. Recently, it has been found that serum and tissue levels of IL-17 are increased in obese mice [35, 36] and humans [37]. Given that insulin and IGF1 can inhibit GSK3B activity through PI3K/Akt pathway [27, 28], we hypothesized that insulin and IGF1 might enhance IL-17-induced expression of inflammatory chemokines/cytokines through a GSK3B-dependent mechanism, which can be inhibited by melatonin. In the current study, we tested this hypothesis. Our data demonstrated that insulin and IGF1 indeed enhanced IL-17-induced expression of CXCL1, CCL20, and IL-6 in the in vitro cultured mouse embryonic fibroblast (MEF) cell lines and ex vivo cultured mouse prostatic tissues, which was dependent on GSK3B. We also tested the postulate that melatonin, an endogenous indolamine molecule with anti-inflammatory actions [6], blocks the action of insulin and IGF1 through inhibition of Akt-mediated GSK3B phosphorylation.
Materials and methods
Cell and tissue culture
Mouse Gsk3b knockout (Gsk3b−/−) and wild-type (Gsk3b+/+) MEF cells were described previously [38]. Cells were maintained in Dulbecco's Modified Eagles Medium (DMEM, Mediatech, Inc., Manassas, VA, USA) containing 10% fetal bovine serum (FBS, Mediatech, Inc., Manassas, VA, USA) and 100 IU/ml penicillin/streptomycin in a 37°C, 5% CO2 humidified incubator. Six-week-old mice were euthanized by CO2 asphyxiation. Mouse prostatic glands were dissected out, cut into cubes of approximately 1 to 2 mm3 in size, and washed three times with phosphate buffered saline (PBS). The prostatic tissues were kept in 60-mm cell culture dishes in serum-free DMEM in the incubator and immediately treated with the reagents. The animal studies were approved by the Animal Care and Use Committee of Tulane University.
Treatment of cells and tissues
Cells were grown in 60-mm cell culture dishes to approximately 90% confluency and changed into serum-free DMEM for 16 hours prior to the treatments. The cells or mouse prostatic tissues were treated separately or in combination with 20 ng/ml recombinant mouse IL-17A (R&D Systems, Inc., Minneapolis, MN, USA), 10 nM recombinant human insulin, 50 ng/ml recombinant human IGF1, 5 ng/ml TNFα, 10 nM melatonin, and/or 20 mM lithium chloride (LiCl) (Sigma-Aldrich, St. Louis, MO, USA). Insulin, IGF1, melatonin, and LiCl were added 30 minutes prior to addition of IL-17A in the combined treatments. At different time points as indicated, the cells or tissues were harvested for RNA or protein isolation.
Real-time quantitative reverse transcriptase PCR
After 2-hours treatment with IL-17A, the cells and tissues were collected in lysis buffer and homogenized with a 1-ml syringe connected to a 21-gauge needle. Total RNA was isolated according to the instructions of RNeasy Mini Kit (QIAGEN, Valencia, CA, USA) with on-membrane DNase I digestion to avoid genomic DNA contamination. cDNA was made from total RNA using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). Mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were obtained from Applied Biosystems (Foster City, CA, USA). The PCR primers specific for each mouse gene are as follows: Cxcl1 Forward (5′-CACCCAAACCGAAGTCATAG-3′), Cxcl1 Reverse (5′-AAGCCAGCGTTCACCAGA-3′), Ccl20 Forward (5′-AACTGGGTGAAAAGGGCTGT-3′), Ccl20 Reverse (5′-GTCCAATTCCATCCCAAAAA-3′), Il-6 Forward (5′-CTACCCCAATTTCCAATGCT-3′), Il-6 Reverse (5′-ACCACAGTGAGGAATGTCCA-3′). Real-time quantitative PCR (qRT-PCR) was performed in triplicates with an iQ5®iCycler and iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) following the recommended protocols. Results were normalized to GAPDH levels using the formula ΔCt (Cycle threshold) = Ct of target gene – Ct of GAPDH. The mRNA level of the control group was used as the baseline; therefore, ΔΔCt was calculated using the formula ΔΔCt = ΔCt of target gene - ΔCt of the baseline. The fold change of mRNA level was calculated as fold = 2−ΔΔCt.
Western blot analysis
Proteins were extracted from the treated cells in RIPA lysis buffer [50 mM sodium fluoride, 0.5% Igepal CA-630 (NP-40), 10 mM sodium phosphate, 150 mM sodium chloride, 25 mM Tris pH 8.0, 1mM phenylmethylsulfonyl fluoride, 2 mM ethylenediaminetetraacetic acid (EDTA), 1.2 mM sodium vanadate] supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Equal amount of proteins was subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. The membranes were blocked with 5% nonfat dry milk in TBST buffer (25 mM Tris-HCl, 125 mM NaCl, 0.1% Tween 20) for 2 hours and probed with the indicated primary antibodies overnight and then IRDye®800CW- or IRDye®680-conjugated secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) for 1 hour. The results were visualized by using an Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE, USA). For loading control, the membranes were stripped and probed for unphosphorylated proteins and/or GAPDH. The antibodies used are as follows: rabbit anti-P-Akt (S473), rabbit anti-Akt, rabbit anti-P-GSK3B (S9), rabbit anti-GSK3B, rabbit anti-P-GSK3A (S21), rabbit anti-GSK3A, rabbit anti-ERK1/2, rabbit anti-P-STAT3(Y705), mouse anti-STAT3, rabbit anti-P-IκBα, mouse anti-IκBα, and rabbit anti-P-C/EBPβ (human T235 and mouse T188) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA); mouse anti-P-ERK1/2, rabbit anti-C/EBPβ, and rabbit anti-p65 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); mouse anti-GAPDH antibodies were ordered from Millipore Corporation (Billerica, MA, USA).
Tissue-isolated PC3 human prostate cancer xenografts
Fifteen xenograft tumor samples were obtained from our previous study [39]. Briefly, PC3 human prostate cancer xenografts were first grown subcutaneously in nude mice, then, implanted in nude rats in a tissue-isolated manner, and finally, perfused with human venous blood for 1 hour as previously described [40]. The blood was drawn from healthy male donors during the daytime, nighttime, and nighttime after 90 minutes of ocular, bright, white light exposure at 580 μW/cm2 (i.e., 2800 lux) (so called light-at-night). It has been previously determined that the physiological levels of melatonin were the lowest in the daytime (12:50 PM) blood (approximately 12 ∼ 14 pg/ml), the highest in the nighttime (2:00 AM) blood (approximately 55 ∼ 60 pg/ml), and intermediate in the nocturnal blood collected after ocular light exposure (approximately 28 ∼ 29 pg/ml) [40]. The collection and use of human blood was approved by the Institutional Review Board at Thomas Jefferson University and each donor signed a consent form. Three xenograft tumors per each type of blood were perfused in situ with the daytime blood, nighttime blood, light-at-night blood, nighttime blood supplemented with 1 nM of melatonin receptor antagonist S20928, or light-at-night blood supplemented with 500 pM of melatonin (Sigma-Aldrich, St. Louis, MO, USA). Approximately 100 mg of each tumor was frozen in liquid nitrogen and pulverized. Total RNA was isolated by using RNeasy Mini Kit (QIAGEN) according to the instructions. Real-time quantitative PCR was performed as described above.
Statistical analysis
All experiments were repeated at least three times and consistent results were obtained. The mRNA levels represent means ± standard deviations of three independent experiments or three different tumor samples in the PC3 human prostate cancer xenograft study. The Student's t test was used to analyze the quantitative data. P-value < 0.05 was considered statistically significant.
Results
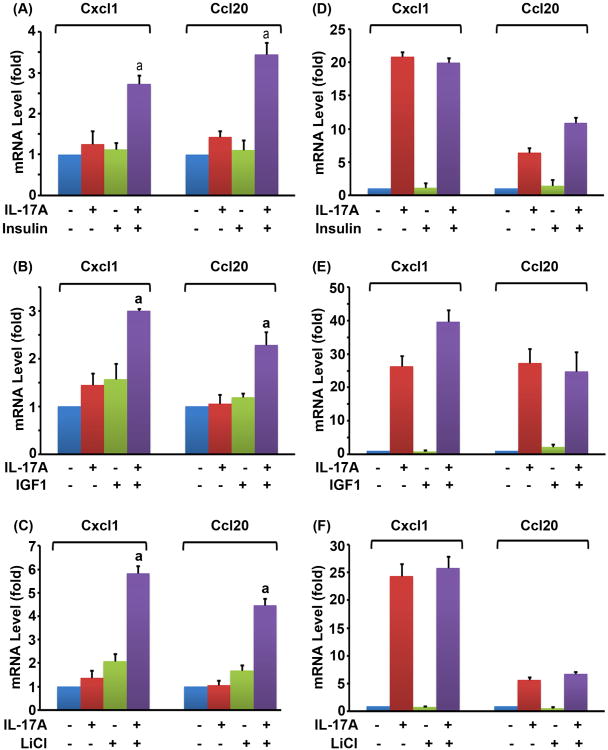
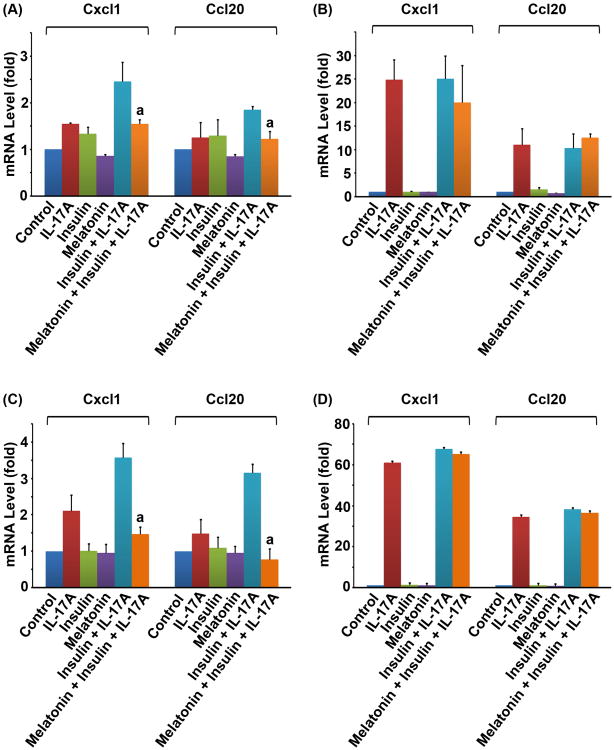
As shown in Fig. 1A and B, we found that IL-17A alone, insulin alone, or IGF1 alone only slightly induced expression of Cxcl1 and Ccl20, two of the IL-17-downstream target genes, in the Gsk3b+/+ MEF cells. A combination of IL-17A and insulin (or IGF1), however, significantly increased the mRNA levels of Cxcl1 and Ccl20 (P < 0.05) (Fig. 1A,B). The classic GSK3 inhibitor LiCl also enhanced IL-17-induced gene expression (Fig. 1C). To determine if GSK3B was involved, we performed the same experiments in the Gsk3b−/− MEF cells. We found that, in the absence of GSK3B, IL-17A alone induced more than 20-fold higher levels of Cxcl1 and more than 5-fold higher levels of Ccl20 in the Gsk3b−/− MEF cells, compared to the Gsk3b+/+ MEF cells (Fig.1D-F versus A-C). When insulin, IGF1, or LiCl was combined with IL-17A, they did not further enhance IL-17-induced expression of Cxcl1 and Ccl20 in the Gsk3b−/− MEF cells (Fig. 1D-F), suggesting that GSK3B is required to mediate the synergy between insulin/IGF1 and IL-17A.
Fig. 1.
Insulin, IGF1, and LiCl enhanced IL-17-induced chemokine expression in the Gsk3b+/+ MEF cells, but not in the Gsk3b−/− MEF cells. (A-C) Gsk3b+/+ MEF cells. (D-F) Gsk3b−/− MEF cells. Insulin (10 nM), IGF1 (50 ng/ml), or LiCl (20 mM) was added 30 min prior to addition of IL-17A (20 ng/ml). Two hours after IL-17A treatment, the relative mRNA levels of Cxcl1 and Ccl20 were determined by qRT-PCR. The data are presented as mean ± standard deviation of three experiments. aP < 0.05, compared to the IL-17A alone group. When compared to the untreated control group, only the combined treatment groups showed P < 0.05 in panels A to C; the IL-17A alone groups and the combined treatment groups showed P < 0.05 in panels D to F; the other treatment groups showed P > 0.05 in panels A to F.
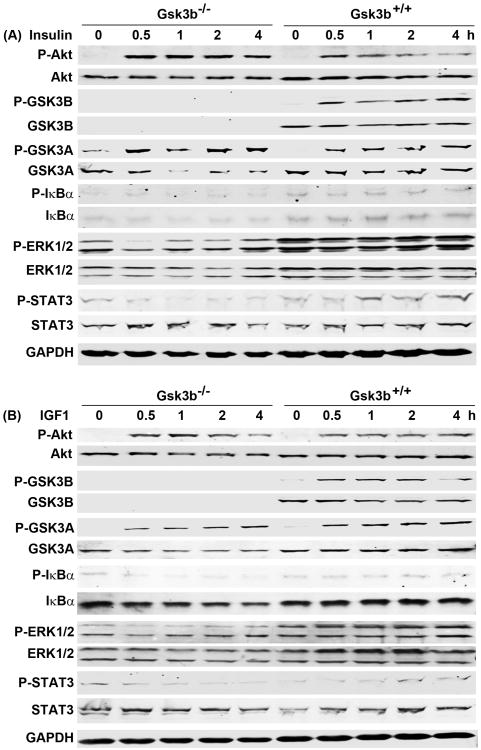
We next assessed the signaling pathways activated by insulin and IGF1 in the Gsk3b−/− and Gsk3b+/+ MEF cells. We found that insulin increased phosphorylated Akt (P-Akt at serine 473) from 0.5 to 4 hours in the Gsk3b−/− MEF cells, whereas P-Akt levels were slightly lower in the Gsk3b+/+ MEF cells (Fig. 2A). This slight difference between the two cell lines was reflected in P-GSK3A (at serine 21) levels. Nevertheless, we confirmed that GSK3B protein was not expressed in the Gsk3b−/− MEF cells and P-GSK3B (at serine 9) was only increased by insulin in the Gsk3b+/+ MEF cells (Fig. 2A). We found that insulin alone did not increase P-IκBα levels in either the Gsk3b−/− or Gsk3b+/+ MEF cells. Although the basal levels of ERK1/2 and P-ERK1/2 were slightly higher in the Gsk3b+/+ MEF cells than the Gsk3b−/− MEF cells, insulin did not increase P-ERK1/2 levels in either cell line (Fig. 2A). We found that insulin only slightly increased P-STAT3 (Signal transducer and activator of transcription 3) levels from 1 to 4 hours in the Gsk3b+/+ but not the Gsk3b−/− MEF cells (Fig. 2A). IGF1 increased P-Akt and P-GSK3A levels to the same magnitude in both Gsk3b−/− and Gsk3b+/+ MEF cells, but only increased P-GSK3B levels in the Gsk3b+/+ MEF cells (Fig. 2B). IGF1 did not increase P-IκBα, P-ERK1/2, or P-STAT3 levels in either Gsk3b−/− or Gsk3b+/+ MEF cells (Fig. 2B). These findings suggest that insulin and IGF1 activate Akt pathway in both Gsk3b−/− and Gsk3b+/+ MEF cells, which inactivates GSK3B through phosphorylation at serine 9 in the Gsk3b+/+ MEF cells.
Fig. 2.
Effects of insulin and IGF1 on intracellular signaling pathways. The Gsk3b−/− and Gsk3b+/+ MEF cells were treated with 10 nM insulin (A) or 50 ng/ml IGF1 (B) for the indicated time. The levels of phosphorylated and unphosphorylated proteins were determined by Western blot analysis. Equal loading of proteins was confirmed by reprobing the blot for GAPDH.
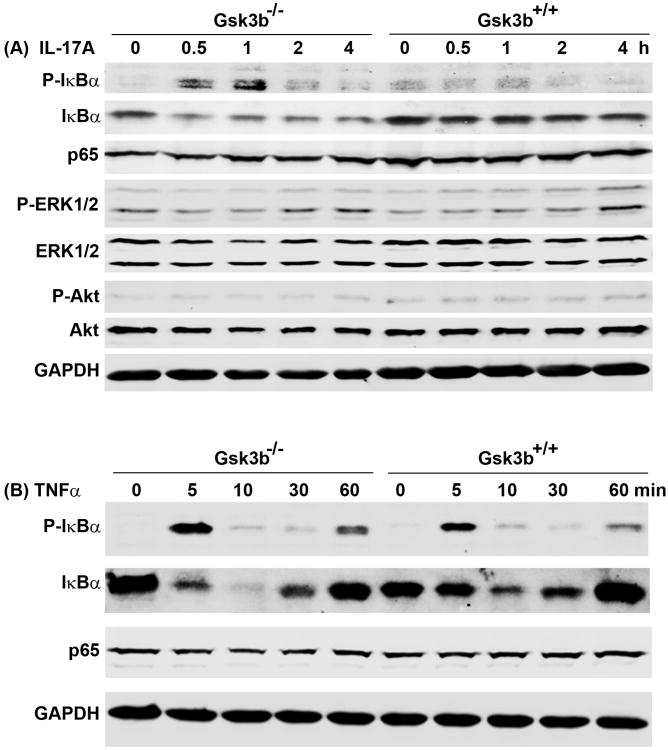
Since we observed that IL-17A alone dramatically increased expression of Cxcl1 and Ccl20 in the Gsk3b−/− but not the Gsk3b+/+ MEF cells, we investigated the differences in IL-17-activated NF-κB and ERK1/2 pathways. We found that IL-17A dramatically increased the P-IκBα levels at 0.5 to 1 hour in the Gsk3b−/− MEF cells, whereas such an increase was absent in the Gsk3b+/+ MEF cells (Fig. 3A). The increase of P-IκBα was accompanied by a decrease of IκBα, implicating that NF-κB is released from the NF-κB:IκBα complex and becomes activated. There were not any obvious changes in NF-κB p65 (RELA) levels after IL-17A treatment (Fig. 3A). On the other hand, there was not much difference in IL-17A-induced activation of P-ERK1/2 between the Gsk3b−/− and Gsk3b+/+ MEF cells, except that there was a little higher level of P-ERK2 at 2 hours in the Gsk3b−/− MEF cells than the Gsk3b+/+ MEF cells (Fig. 3A). No increase of P-Akt levels was observed in either Gsk3b−/− or Gsk3b+/+ MEF cells. These findings suggest that, in the absence of GSK3B, IL-17A stimulates higher levels of NF-κB activation to transcribe the downstream genes. To examine whether the enhanced NF-κB activity is specific to IL-17 signaling, we treated the MEF cells with TNFα, a classic activator of NF-κB pathway. We found that TNFα induced similar time-dependent changes of P-IκBα and IκBα in both Gsk3b−/− and Gsk3b+/+ MEF cells, without any changes in NF-κB p65 (RELA) levels (Fig. 3B). These findings suggest that GSK3B negatively regulates IL-17-induced phosphorylation of IκBα, but not TNFα-induced phosphorylation of IκBα.
Fig. 3.
Gsk3b knockout enhanced phosphorylation of IκBα induced by IL-17 but not by TNFα. The Gsk3b−/− and Gsk3b+/+ MEF cells were treated with 20 ng/ml IL-17A (A) or 5 ng/ml TNFα (B) for the indicated time. The levels of phosphorylated and unphosphorylated proteins were determined by Western blot analysis. Equal loading of proteins was confirmed by reprobing the blot for GAPDH.
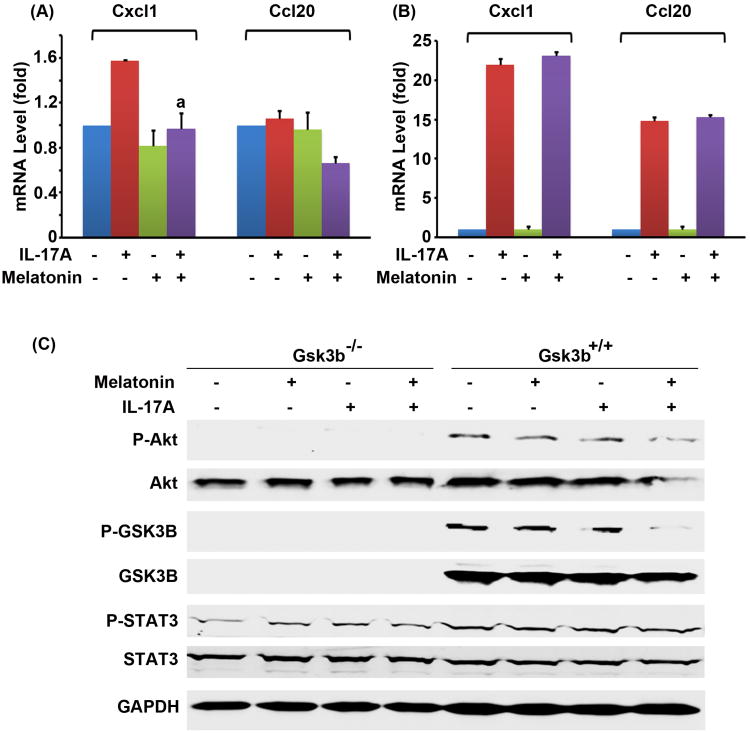
Since phosphorylation of GSK3B at serine 9 inhibits GSK3B activity, melatonin can enhance GSK3B activity through decreasing P-GSK3B at serine 9. Having shown that GSK3B is a negative regulator of IL-17 signaling, we tested if melatonin could inhibit IL-17-induced gene expression through enhancing GSK3B. We found that melatonin inhibited IL-17A-induced Cxcl1 expression in the Gsk3b+/+ MEF cells (Fig. 4A). Melatonin did not inhibit IL-17A-induced expression of Cxcl1 and Ccl20 in the Gsk3b−/− MEF cells (Fig. 4B), suggesting that melatonin's action is mediated by GSK3B. Further, we found that, although IL-17A did not activate Akt, melatonin reduced the basal levels of P-Akt and Akt, thus reduced the level of P-GSK3B at serine 9 in the Gsk3b+/+ MEF cells (Fig. 4C). The basal levels of P-STAT3 were not affected by melatonin (Fig. 4C), suggesting that melatonin's effect is specific to the Akt-GSK3B pathway.
Fig. 4.
Melatonin inhibited IL-17-induced chemokine expression in the Gsk3b+/+ MEF cells but not in the Gsk3b−/− MEF cells. (A) The Gsk3b+/+ MEF cells or (B) the Gsk3b−/− MEF cells were treated with 10 nM melatonin 30 min prior to addition of 20 ng/ml IL-17A. Two hours after IL-17A treatment, the relative mRNA levels of Cxcl1 and Ccl20 were determined by qRT-PCR. The data are presented as mean ± standard deviation of three experiments. aP < 0.05, compared to the IL-17A alone group. When compared to the untreated control group, the IL-17A alone group in induction of Cxcl1 in panel A showed P < 0.05; the IL-17A alone groups and the combined treatment groups in panel B showed P < 0.05; the other treatment groups showed P > 0.05 in panels A and B. (C) Effects of melatonin on intracellular signaling pathways: The Gsk3b−/− and Gsk3b+/+ MEF cells were treated with 10 nM melatonin 30 min prior to addition of 20 ng/ml IL-17A for 2 hours. The levels of phosphorylated and unphosphorylated proteins were determined by Western blot analysis. The loading of proteins was confirmed by reprobing the blot for GAPDH.
Since insulin activates PI3K/Akt pathway to inhibit GSK3B activity and subsequently enhance IL-17-induced gene expression, we tested if melatonin could antagonize insulin's action by inhibiting Akt activation. We found that melatonin indeed significantly reduced the mRNA levels of Cxcl1 and Ccl20 that were induced by a combination of insulin and IL-17A in the Gsk3b+/+ MEF cells (P < 0.05) (Fig. 5A). Since melatonin did not show any effects in the Gsk3b−/− MEF cells (Fig. 5B), it suggests that melatonin's action is dependent on GSK3B. In order to rule out the possibility that 10 nM melatonin is not sufficient to inhibit IL-17A's effects in the Gsk3b−/− MEF cells, we treated the cells with 500 nM of melatonin. We found that 500 nM of melatonin significantly inhibited induction of Cxcl1 and Ccl20 by insulin and IL-17A in the Gsk3b+/+ MEF cells (P < 0.05) (Fig. 5C), but this high dose of melatonin still had no effects in the Gsk3b−/− MEF cells (Fig. 5D). These findings further confirm that melatonin's action is GSK3B-dependent.
Fig. 5.
Melatonin inhibited the synergy between insulin and IL-17 in induction of Cxcl1 and Ccl20 in the Gsk3b+/+ MEF cells, but not in the Gsk3b−/− MEF cells. The Gsk3b+/+ MEF cells (A & C) or the Gsk3b−/− MEF cells (B & D) were treated with melatonin (10 nM in A-B, 500 nM in C-D) and/or 10 nM insulin 30 min prior to addition of IL-17A (20 ng/ml in A-B, 100 ng/ml in C-D). Two hours after IL-17A treatment, the relative mRNA levels of Cxcl1 and Ccl20 were determined by qRT-PCR. The data are presented as mean ± standard deviation of three experiments. aP < 0.05, compared to the insulin + IL-17A group. When compared to the control group, the insulin + IL-17A groups in panels A and C showed P < 0.05; the IL-17A alone group in induction of Cxcl1 in panel C showed P < 0.05; the IL-17A alone groups, the insulin + IL-17A groups, and melatonin + insulin + IL-17A groups in panels B and D showed P < 0.05; the other treatment groups showed P > 0.05 in panels A to D.
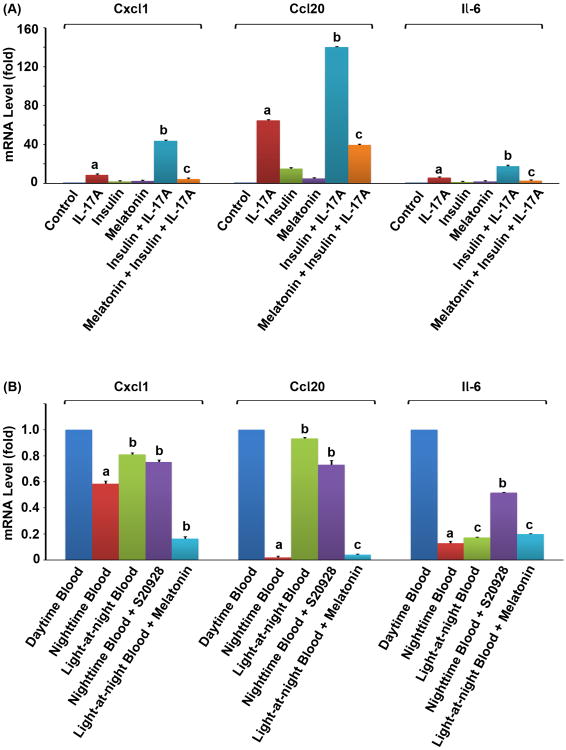
We tested if our findings obtained from the cultured MEF cell lines would be reproducible in mouse prostatic tissues. We harvested fresh mouse prostatic glandular tissues and treated them in the ex vivo cultures. We found that IL-17A alone significantly induced expression of Cxcl1, Ccl20 and Il-6 in the mouse prostatic tissues (P < 0.05, compared to the control group) and a combination of insulin and IL-17A induced expression of these genes to significantly higher levels (P < 0.01, compared to the IL-17A alone group) (Fig. 6A). Melatonin significantly reduced expression of these genes that was induced by insulin and IL-17A (P < 0.01, compared to the insulin + IL-17A group) (Fig. 6A). These findings suggest that insulin can enhance IL-17-induced gene expression in the mouse prostatic tissues, which can be antagonized by melatonin.
Fig. 6.
Melatonin inhibited expression of IL-17-downstream target genes in mouse prostatic tissues and PC3 human prostate cancer xenograft tumors. (A) The ex vivo cultured mouse prostatic tissues were treated with 10 nM insulin and/or 10 nM melatonin 30 min prior to addition of 20 ng/ml IL-17A. Two hours after IL-17A treatment, the relative mRNA levels of Cxcl1, Ccl20, and Il-6 were determined by qRT-PCR. The data are presented as mean ± standard deviation of three experiments. aP < 0.05, compared to the control group; bP < 0.01, compared to the IL-17A alone group; cP < 0.01, compared to the insulin + IL-17A group. (B) The PC3 human prostate cancer xenograft tumors were perfused in situ with the daytime blood, nighttime blood, light-at-night blood, nighttime blood supplemented with 1 nM of melatonin receptor antagonist S20928, or light-at-night blood supplemented with 500 pM of melatonin, for 1 hour. The relative mRNA levels of Cxcl1, Ccl20, and Il-6 were determined by qRT-PCR. The data are presented as mean ± standard deviation of three tumors. aP < 0.05, compared to the daytime blood group; bP < 0.05, compared to the nighttime blood group; cP > 0.05, compared to the nighttime blood group.
We found that the nighttime blood significantly reduced expression of Cxcl1, Ccl20, and Il-6 in the xenograft tumor tissues, compared to the tumor tissues perfused with the daytime blood (P < 0.05) (Fig. 6B). On the other hand, the levels of gene expression in the tumor tissues perfused with the light-at-night blood were moderate compared to the daytime blood and nighttime blood groups (Fig. 6B). Since the blood contains many other factors that may regulate expression of the genes examined, we did two additional experiments to assess if the observed changes in gene expression were dependent on melatonin. First, we added melatonin receptor antagonist S20928 to the nighttime blood. As expected, addition of S20928 significantly increased the levels of gene expression (P < 0.05), compared to the nighttime blood group (Fig. 6B). Next, we added melatonin to the light-at-night blood. We found that addition of melatonin significantly reduced expression of Cxcl1 and Ccl20 (P < 0.05), compared to the light-at-night blood alone group (Fig. 6B). Collectively, these findings suggest that physiological levels of melatonin in the nighttime blood decrease expression of IL-17-downstream target genes in the tissue-isolated PC3 human prostate cancer xenografts.
Discussion
Melatonin has been shown to reduce mRNA levels of IL-6, IL-1β, TNF-α, and matrix metalloproteinase-9 (MMP-9) in the rabbit liver infected with rabbit hemorrhagic disease viruses, thus attenuating inflammation and promoting liver regeneration [41]. Melatonin has also been demonstrated to reduce methamphetamine-induced overexpression of IL-6, IL-1β, and TNF-α in microglial cell lines [42]. It is known that IL-17 cytokine induces expression of proinflammatory chemokines, cytokines, growth factors, and MMPs, including IL-6, IL-1β, TNF-α, and MMP-9 [7, 43]. Therefore, we were prompted to investigate if melatonin can regulate IL-17-induced expression of chemokines and cytokines.
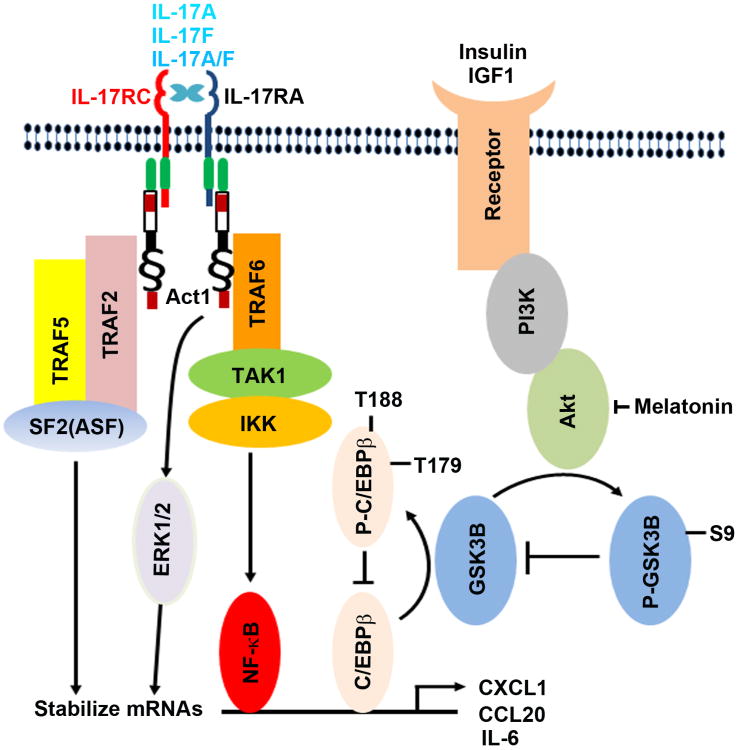
IL-17 acts on the IL-17RA/IL-17RC receptor complex to recruit Act1 and then TRAF6, leading to activation of TAK1 and IKK kinases, thus IκBα is phosphorylated and degraded. Subsequently, NF-κB is released from the NF-κB:IκBα complex and enters into the nucleus to initiate transcription of downstream target genes [44]. In addition, IL-17 activates ERK1/2 pathway and Act1-TRAF2-TRAF5-SF2/ASF complex to stabilize the mRNAs of IL-17-downstream target genes [22-24]. Thus, IL-17 increases the expression levels of the downstream target genes through mechanisms of transcription and mRNA stabilization via distinct signaling pathways (Fig. 7).
Fig. 7.
Illustration of the proposed mechanisms underlying the crosstalk between insulin/IGF1 and IL-17 signaling pathways. IL-17 acts on the IL-17RA:IL-17RC receptor complex to activate Act1-TRAF6-TAK1-IKK signaling cascade, thus activating NF-κB and C/EBPβ transcription factors for initiation of transcription of the downstream target genes. In addition, IL-17 activates ERK1/2 and Act1-TRAF2-TRAF5-SF2/ASF complex to stabilize mRNAs. Insulin and IGF1 activate PI3K/Akt pathway through their receptors; Akt phosphorylates GSK3B at serine 9 to inhibit GSK3B enzyme activity; GSK3B phosphorylates C/EBPβ at threonine 179 after a priming phosphorylation at threonine 188 by ERK1/2, thus inhibiting C/EBPβ's transcription function. Therefore, insulin/IGF1 signaling is linked with IL-17 signaling by GSK3B and C/EBPβ. Melatonin inhibits Akt activation, thus enhancing GSK3B activity and subsequently diminishing IL-17-induced gene expression by inhibiting C/EBPβ function.
In this study, we demonstrated that insulin and IGF1 enhance IL-17-induced expression of chemokines (Cxcl1 and Ccl20) and cytokine (Il-6). Insulin and IGF1 mainly act through the PI3K/Akt pathway to inhibit GSK3B activity by phosphorylation of GSK3B at serine 9, as it has been shown that phosphorylation of GSK3B at serine 9 decreases GSK3B enzyme activity [27, 28]. We also demonstrated that the crosstalk between insulin/IGF1 and IL-17 signaling pathways is dependent on GSK3B. We provided three lines of evidence to support this concept: first, the synergy between insulin/IGF1 and IL-17 is only found in the Gsk3b wild-type MEF cells, which is abolished in the Gsk3b knockout MEF cells; second, the synergy between GSK3 inhibitor LiCl and IL-17 is only found in the Gsk3b wild-type MEF cells, which is also abolished in the Gsk3b knockout MEF cells; and third, melatonin, an activator of GSK3B activity, can only inhibit the synergistic action of insulin and IL-17 in the Gsk3b wild-type MEF cells, but not in the Gsk3b knockout MEF cells.
GSK3B has been shown to act as a negative regulator of IL-17 signaling in the previous study [32]. The mechanism of GSK3B's inhibitory action on IL-17 signaling has been proposed as that GSK3B phosphorylates C/EBPβ at threonine 179 after C/EBPβ is phosphorylated at threonine 188 by ERK1/2, thereby the phosphorylated C/EBPβ inhibits the transcription function of the unphosphorylated C/EBPβ [32] (Fig. 7). In addition, it is possible that GSK3B may act on other unknown substrates to inhibit IL-17 signaling. For example, we demonstrated that Gsk3b knockout increases the levels of P-IκBα induced by IL-17A (Fig. 3A), suggesting that GSK3B may negatively regulate IL-17 signaling at the level of IKK or above in the signaling cascade (Fig. 7). Recently, it has been shown that GSK3B phosphorylates C/EBPδ at threonine 156, leading to degradation of C/EBPδ, thus attenuating Toll-like receptor 4-induced inflammation-associated genes in macrophages and tumor cells [45]. Given that IL-17 induces expression of C/EBPδ [9, 20, 30] and C/EBPδ is essential for transcription of IL-17-downstream target genes such as IL-6 and 24p3/lipocalin 2 [31], it is intriguing to investigate whether the GSK3B-C/EBPδ mechanism mediates GSK3B's inhibitory action in IL-17 signaling in future studies.
Two main implications of this study are identified. First, the synergy between insulin/IGF1 and IL-17 may explain the chronic inflammatory status found in obesity. It is known that obese people have increased serum levels of insulin and IGF1[34] and IL-17 [37]. Based on the current study, insulin and IGF1 can enhance IL-17-induced proinflammatory chemokines and cytokines, thus the increased levels of insulin, IGF1, and IL-17 may act together to build up the chronic inflammatory status in obesity. This is consistent with the findings that many of the obesity-associated inflammatory cytokines/chemokines are IL-17-downstream target genes, including IL-6, IL-8, IL-1β, TNFα, vascular endothelial growth factor, CCL2, and CCL5 [46]. Second, the synergy between insulin/IGF1 and IL-17 can be inhibited by melatonin. We found that melatonin at pharmacological concentrations (10 to 500 nM) inhibited chemokine expression induced by insulin and IL-17 in the cultured MEF cells and mouse prostatic tissues. We and other investigators have previously shown that human serum melatonin concentrations can reach 500 nM by oral administration of 80 mg melatonin or intravenous administration of 2 mg melatonin [47, 48]. Further, we found that melatonin at physiological concentrations inhibited chemokine expression in the tissue-isolated PC3 human prostate cancer xenografts. The nighttime blood significantly reduced expression of Cxcl1, Ccl20, and Il-6 in the xenograft tumor tissues, compared to the tumor tissues perfused with the daytime blood. In contrast, the levels of gene expression in the tumor tissues perfused with the light-at-night blood were moderate compared to the daytime blood and nighttime blood groups. These findings are consistent with the blood levels of melatonin, that is, the lowest in the daytime blood, the highest in the nighttime blood, and intermediate in the light-at-night blood [40]. Of note, because we used the archived tumor samples, we were unable to retrospectively measure the levels of insulin and IL-17 in the blood samples that were used in perfusion of the tumors. Nevertheless, we speculate that the physiological levels of insulin and IL-17 might exist in the blood samples [49, 50]. To our best knowledge, this is the first time that melatonin is found to inhibit IL-17-mediated inflammation through enhancing GSK3B activity.
Obesity has been associated with many co-morbidities, including type 2 diabetes and several cancers [34]. Light-at-night-induced suppression of melatonin production has been shown to contribute to obesity [51]. In a rabbit model of high-fat diet-induced obesity, intake of melatonin is associated with reduction of food consumption and weight gain, lowering of blood pressure and heart rate, and decrease of blood glucose and lipids [52]. In a rat model of high-calorie diet-induced obesity, melatonin treatment reduces weight gain, visceral adiposity, and serum levels of insulin and triglycerides [53]. In male young Zucker diabetic fatty rats, melatonin reduces weight gain, serum triglyceride level, and low-density-lipoprotein cholesterol, but increases high-density-lipoprotein cholesterol [54]. Furthermore, melatonin significantly reduces the plasma levels of IL-6 and TNF-α in Zucker diabetic fatty rats [55], though it is not clear if the reduction of IL-6 and TNF-α is mediated through inhibition of IL-17 signaling. Based on the findings in the present study and aforementioned previous studies, we speculate that melatonin is potentially able to dampen the inflammatory status in obesity. In conclusion, the present study has demonstrated that insulin and IGF1 enhance IL-17-induced expression of inflammatory chemokines/cytokines through a GSK3B-dependent mechanism, which can be inhibited by melatonin. The anti-inflammatory actions of melatonin may be utilized to treat obesity-associated chronic inflammation and co-morbidities such as type 2 diabetes and cancer.
Acknowledgments
This work was partly supported by grants from the National Institute of General Medical Sciences (8P20GM103518-09) and the National Cancer Institute (R01CA174714) of the National Institutes of Health, a grant from Department of Defense (PC121647), the Developmental Fund of Tulane Cancer Center (TCC), and Louisiana Cancer Research Consortium (LCRC) Fund (to Z.Y.). This work was also supported by Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science (to R.T.D. and D.E.B.), and in part by a Tulane University School of Medicine and LCRC Start-Up Grant (#631455) and NIH grant (1R21CA129875-01A1)(to D.E.B.) and the Institute for Integrative Health (Baltimore, MD) (to G.C.B). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. TCC and LCRC Core Facilities were used to conduct this study. The authors thank Dr. James R. Woodgett (Mount Sinai Hospital and the Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada M5G 1×5) for providing the Gsk3b wild-type and knockout cell lines.
Footnotes
Author Contributions: D Ge conducted the in vitro experiments and analyzed the data. RT Dauchy, L Mao, EM Dauchy, GC Brainard, JP Hanifin, KS Cecil, DE Blask, and SM Hill conceived and conducted the experiments on human xenograft tumors. S Liu, Q Zhang, BR Rowan, Z Xiong, and L Myers assisted in the design of experiments and analysis of data. Z You designed the experiments, analyzed the data, and prepared the manuscript. All authors contributed to the revision and approval of the article.
References
- 1.Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 2.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 200527:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 3.Nosjean O, Ferro M, Coge F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 4.Reiter RJ. Melatonin: Lowering the High Price of Free Radicals. News Physiol Sci. 200015:246–250. doi: 10.1152/physiologyonline.2000.15.5.246. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Barcelo EJ, Mediavilla MD, Tan DX, et al. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- 6.Mauriz JL, Collado PS, Veneroso C, et al. A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 7.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novatchkova M, Leibbrandt A, Werzowa J, et al. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 9.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 10.Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 11.Maitra A, Shen F, Hanel W, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho AW, Shen F, Conti HR, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonardi A, Chariot A, Claudio E, et al. CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proc Natl Acad Sci U S A. 2000;97:10494–10499. doi: 10.1073/pnas.190245697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Qian W, Qian Y, et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Z, Fanslow WC, Seldin MF, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Chen J, Huang A, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci U S A. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Deng L, Hong M, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 18.Kanayama A, Seth RB, Sun L, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 200726:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 20.Shen F, Ruddy MJ, Plamondon P, et al. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 22.Hata K, Andoh A, Shimada M, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–1044. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 23.Bulek K, Liu C, Swaidani S, et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. 2011;12:844–852. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun D, Novotny M, Bulek K, et al. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baxter RC, Bryson JM, Turtle JR. Somatogenic receptors of rat liver: regulation by insulin. Endocrinology. 1980;107:1176–1181. doi: 10.1210/endo-107-4-1176. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher EJ, Leroith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross DA, Alessi DR, Vandenheede JR, et al. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303(Pt 1):21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 29.Chiara F, Rasola A. GSK-3 and mitochondria in cancer cells. Front Oncol. 2013;3:16. doi: 10.3389/fonc.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 31.Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 32.Shen F, Li N, Gade P, et al. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 34.Cohen DH, Leroith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer. 2012;19:F27–45. doi: 10.1530/ERC-11-0374. [DOI] [PubMed] [Google Scholar]
- 35.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 36.Pini M, Fantuzzi G. Enhanced production of IL-17A during zymosan-induced peritonitis in obese mice. J Leukoc Biol. 201087:51–58. doi: 10.1189/jlb.0309188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 38.Hoeflich KP, Luo J, Rubie EA, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 39.Mao L, Dauchy RT, Blask DE, et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3beta. Mol Endocrinol. 2012;26:1808–1820. doi: 10.1210/me.2012-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blask DE, Brainard GC, Dauchy RT, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–11184. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 41.Laliena A, San Miguel B, Crespo I, et al. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012;53:270–278. doi: 10.1111/j.1600-079X.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- 42.Tocharus J, Khonthun C, Chongthammakun S, et al. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J Pineal Res. 2010;48:347–352. doi: 10.1111/j.1600-079X.2010.00761.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Liu S, Ge D, et al. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72:2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song X, QIAN Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Balamurugan K, Sharan S, Klarmann KD, et al. FBXW7alpha attenuates inflammatory signalling by downregulating C/EBPdelta and its target gene Tlr4. Nat Commun. 2013;4:1662. doi: 10.1038/ncomms2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 47.Demuro RL, Nafziger AN, Blask DE, et al. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000;40:781–784. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- 48.Waldhauser F, Waldhauser M, Lieberman HR, et al. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–313. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- 49.Garley M, Jablonska E, Grabowska SZ, et al. IL-17 family cytokines in neutrophils of patients with oral epithelial squamous cell carcinoma. Neoplasma. 2009;56:96–100. doi: 10.4149/neo_2009_02_96. [DOI] [PubMed] [Google Scholar]
- 50.Jafarzadeh A, Esmaeeli-Nadimi A, Nough H, et al. Serum levels of interleukin (IL)-13, IL-17 and IL-18 in patients with ischemic heart disease. Anadolu Kardiyol Derg. 2009;9:75–83. [PubMed] [Google Scholar]
- 51.Reiter RJ, Tan DX, Korkmaz A, et al. Obesity and metabolic syndrome: association with chronodisruption, sleep deprivation, and melatonin suppression. Ann Med. 2012;44:564–577. doi: 10.3109/07853890.2011.586365. [DOI] [PubMed] [Google Scholar]
- 52.Hussein MR, Ahmed OG, Hassan AF, et al. Intake of melatonin is associated with amelioration of physiological changes, both metabolic and morphological pathologies associated with obesity: an animal model. Int J Exp Pathol. 2007;88:19–29. doi: 10.1111/j.1365-2613.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nduhirabandi F, Du Toit EF, Blackhurst D, et al. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res. 2011;50:171–182. doi: 10.1111/j.1600-079X.2010.00826.x. [DOI] [PubMed] [Google Scholar]
- 54.Agil A, Navarro-Alarcon M, Ruiz R, et al. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res. 2011;50:207–212. doi: 10.1111/j.1600-079X.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- 55.Agil A, Reiter RJ, Jimenez-Aranda A, et al. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res. 2013;54:381–388. doi: 10.1111/jpi.12012. [DOI] [PubMed] [Google Scholar]