Abstract
Chemotherapy plus granulocyte colony stimulating factor (G-CSF) (C+G) and G-CSF alone are two of the most common methods of mobilizing CD34+ cells for autologous hematopoietic stem cell transplantation (AHSCT). In order to compare and determine real-world outcomes and costs of these strategies, we performed a retrospective study of 226 consecutive patients at 11 medical centers (64 lymphoma, 162 multiple myeloma), of whom 55% and 66% received C+G. Patients with C+G collected more CD34+ cells/day than G-CSF alone (lymphoma: average 5.51x106 cells/kg on day 1 vs. 2.92x106 cells/kg, p=0.0231; myeloma: 4.16x106 cells/kg vs. 3.69x106 cells/kg, p<0.00001) and required fewer days of apheresis (lymphoma: average 2.11 days vs. 2.96, p=0.012; myeloma: 2.02 vs. 2.83 days, p=0.0015), though nearly all patients ultimately reached the goal of 2x106 cells/kg. With the exception of higher rates of febrile neutropenia in myeloma patients with C+G (17% vs. 2%, p<0.05), toxicities and other outcomes were similar. Mobilization with C+G cost significantly more (lymphoma: median $10,300 vs. $7,300, p<0.0001; myeloma: $8,800 vs. $5,600, p<0.0001), though re-mobilization adds $6,700 for drugs alone. Our results suggest that while both C+G and G-CSF alone are effective mobilization strategies, C+G may be more cost-effective for patients at high risk of insufficient mobilization.
Keywords: Autologous hematopoietic stem cell transplantation, Mobilization, Chemo-mobilization, G-CSF, Lymphoma, Multiple myeloma
Introduction
Autologous hematopoietic stem cell transplation (AHSCT) is an important treatment for hematologic malignancies. In patients with relapsed lymphoma or multiple myeloma in first or second remission, it can improve progression free survival as well as overall survival1–4.
Key to the feasibility of AHSCT is the number of CD34+ cells/kg body weight transplanted. Higher cell doses, particularly >5x106 CD34+ cells/kg, accelerate recovery of marrow function; conversly, lower cell doses, particularly <2x106 CD34+ cells/kg, delay count recovery, increasing the risk of infection and other complications, and may even result in engraftment failure5–8. Many factors influence the number of CD34+ cells collected, including advanced age, previous radiation or chemotherapy, hypocellular marrow, marrow involvement, history of mobilization failure, and mobilization method9–12.
Administration of growth factors and or chemotherapy are two of the most common methods of mobilizing hematopoietic cells. Granulocyte colony-stimulating factor (G-CSF) induces myeloid hyperplasia and release of CD34+ cells into circulation through proteolytic cleavage of adhesion molecules13. This can be enhanced with the addition of chemotherapy, though trade-offs exist in terms of efficiency, safety, and cost14. For example, mobilization with chemotherapy + G-CSF may improve CD34+ cell collections15, 16 but increase the incidence of neutropenic fever17 and infections18.
Our purpose is to better characterize the outcomes of mobilization with chemotherapy + G-CSF (C+G) versus G-CSF alone. Previous reports in the literature primarily focus on a single institution or rely on resource use data from the 1990s. Our goal is to gain a sense of real-world practices and outcomes by reviewing the recent experiences of 11 institutions across the United States with patients with lymphoma or multiple myeloma who underwent AHSCT. In particular, we are interested in the number of cells collected, number of days of apheresis required, need for re-mobilization, time to engraftment, toxicity, and resource utilization and cost.
Materials and Methods
Patient selection
We performed a retrospective chart review at 11 medical centers that conduct AHSCT for patients with lymphoma and multiple myeloma. At each center, we looked at consecutive patients with lymphoma and myeloma aged at least 18 years who underwent peripheral blood stem cell mobilization between January 1, 2006 and December 31, 2007. By focusing on these years, we hope to describe modern practices independent of the use of plerixifor. The goal was to enroll 5 patients with each disease from each site – due to cost limitiations, we felt including more sites would be more representative than including more subjects from each site. Patients were selected chronologically (e.g. starting January 1, 2006) independent of mobilization method or other factors. If a site contributed additional patients, selection continued in chronologically. Individuals were excluded if they participated in an AHSCT trial or if they received plerixafor (Mozobil®) between January 1, 2006 and December 31, 2007. Additionally, chemo-mobilization regimens that made use of induction/salvage chemotherapy or multiple cycles of chemotherapy for mobilization purposes were excluded due to selection bias and difficulties in comparing outcomes between these groups. Chemo-mobilization refers to the administration of chemotherapy (typically a cyclophosphamide-based regimen) with G-CSF for the primary purpose of mobilization, as opposed to chemotherapy administered for purposes of re-induction or salvage. Protocols were at the discretion of individual institutions. Stem cell collection practices and related outcomes were analyzed separately by disease and mobilization method, i.e. lymphoma patients mobilized with C+G (L:C+G) vs. G-CSF alone (L:G) and myeloma patients mobilized with C+G (M:C+G) vs. G-CSF alone (M:G).
Institutional review board approval was obtained at City of Hope National Medical Center, Duarte, California; Fred Hutchinson Cancer Research Center, Seattle, Washington; Duke University Medical Center, Durham, North Carolina; Huntsman Cancer Institute, University of Utah, Salt Lake City, Utah; Indiana Blood and Marrow Transplantation, Beech Grove, Indiana; Rush University Medical Center, Chicago, Illinois; Texas Transplant Institute, San Antonio, Texas; Shands at the University of Florida, Gainesville, Florida; Strong Memorial Hospital, University of Rochester, Rochester, New York; University of Minnesota Medical Center, Minneapolis, Minnesota; and The Methodist Hospital, Baylor College of Medicine, Houston, Texas.
Cell dose
For the purpose of this study, the target cell dose target for transplantation was at least 2×106 CD34+ cells/kg, and the ideal cell dose was considered to be at least 5×106 CD34+ cells/kg. However, goals were subject to individual physician discretion, and in some cases, if 2×106 CD34+ cells/kg were not available despite repeat mobilizations, patients may still proceed to transplant. Peripheral blood CD34+ cell count was not always checked prior to initiating apheresis.
Engraftment
Neutrophil engraftment is defined as the first of 3 consecutive days in which the absolute neutrophil count exceeded 500 cells/μL. Platelet engraftment was defined as the first of 7 days in which the platelet count exceeded 20,000/μL without transfusion.
Costs
The costs of mobilization, apheresis, transplant, and complications were calculated using Center for Medicare and Medicaid Standards, as adapted from Shaughnessy et al.19. Transplantation costs were separated for inpatient and outpatient transplants. Specific drug costs not available there were obtained from the 2010 Red Book20. Chemotherapy used specifically for mobilization purposes (e.g. cyclophosphamide) was included in the cost of mobilization; chemotherapy that was part of a salvage regimen was excluded. Table 1 lists these costs in detail.
Table 1.
Costs of stem cell mobilization and transplant
| Average costs | |
|---|---|
| Mobilization | |
| Chemotherapy | |
| Cyclophosphamide | $0.05/mg |
| Rituximab | $6.92/mg |
| Etoposide | $1.58/mg |
| Chemotherapy infusion | $143.44/hr |
| G-CSF | $0.70/mcg |
| Physician time | $167.50/hr |
| Apheresis | |
| Catheter insertion and removal | $1228.55 |
| Pheresis | $2048.32/day |
| Blood work | $132.20 for CD34+ monitoring $50.50 for platelets $135 for WBC |
| Lab processing of cells | $101.67/day |
| Cryopreservation | $1361.54 |
| Transplant | |
| Stem cell infusion | $4493 |
| Inpatient hospitalization | $2957/day |
| Outpatient hotel stay | $80/day |
| Clinic visits | $459/visit |
| Blood work | $402 for chemistries $76.50 for Mg/P $50.50 for platelets $135 for WBC |
| G-CSF | $0.70/mcg |
| Transfusions | $538.46 per unit of Platelets (Single) $252 per unit of Platelets (Random) $538.46 per unit of Packed RBC |
| Complications | |
| Additional catheter insertion/removal | $1228.55 |
| Hospitalization | $943.02/day |
| Additional clinic visits, eg for nausea and vomiting | $459/visit |
| Antimicrobials | |
| Oral antibiotics (e.g. ciprofloxacin) | $4.98/day |
| Intravenous antibiotics (e.g. vancomycin) | $84.50/day |
| Antifungals (e.g. fluconazole) | $1.13/day |
| Antivirals (e.g. acyclovir) | $0.18/day |
Statistical methods
Statistical differences between chemo-mobilized patients and patients mobilized with G-CSF alone were determined by the Yates chi-squared test for categorical variables and by an unpaired, two-tailed t-test and Mann-Whitney for continuous variables. 95% confidence intervals were calculated as the mean±1.96(s.d./√n).
Results
Patients
Data were collected for 226 consecutive patients across 11 centers. Details are provided in Table 2. Sixty-four had lymphoma (28%) and 162 had multiple myeloma (72%); lymphoma subtypes include Hodgkin disease (9 patients treated with C+G, 10 with G-CSF alone), anaplastic large cell (2, 0), Burkitt’s (1, 0), diffuse large cell (6, 8), follicular (8, 2), mantle cell (5, 7), peripheral T cell (2, 1), primary central nervous system (1, 0), Waldenstrom’s (0, 1), and other (1, 0). Overall, thirty-five lymphoma patients (55%) and 108 multiple myeloma patients (66%) received C+G; the remainder received G-CSF alone. The majority of patients were male (132, 58%) and caucasian (178, 79%). Seventy percent of patients with lymphoma and 36% of patients with multiple myeloma had relapsed/refractory disease.
Table 2.
Patient characteristics
| L:C+G (n=35) |
L:G (n=29) |
M:C+G (n=108) |
M:G (n=54) |
|
|---|---|---|---|---|
| Median age (range) | 56 (21–75) | 52 (19–71) | 60 (27–77) | 62 (33–76) |
| Gender | ||||
| Female | 9 (26%) | 13 (45%) | 45 (42%) | 27 (50%) |
| Male | 26 (74%) | 16 (55%) | 63 (58%) | 27 (50%) |
| Ethnicity | ||||
| Caucasian | 31 (89%) | 27 (93%) | 83 (77%) | 37(68%) |
| African american | 1 (3%) | 2 (7%) | 13 (12%) | 13 (24%) |
| Asian | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) |
| Hispanic | 3 (9%) | 0 (0%) | 1 (1%) | 3 (6%) |
| Other | 0 (0%) | 0 (0%) | 10 (9%) | 1 (2%) |
| Number of previous treatments | ||||
| 0 | 10 (29%) | 9 (31%) | 66 (61%) | 38 (70%) |
| 1 | 20 (57%) | 17 (59%) | 35 (32%) | 14 (26%) |
| 2 | 22 (6%) | 1 (3%) | 2 (2%) | 2 (4%) |
| 3 | 3 (9%) | 2 (7%) | 2 (2%) | 0 (0%) |
| unknown | 0 (0%) | 0 (0%) | 3 (3%) | 0 (0%) |
Mobilization regimens
Mobilization protocols were at the discretion of individual sites and physicians. For chemomobilization, almost all patients were treated with a cyclophosphamide-based regimen (97% lymphoma, 83% of myeloma), the majority at a dose of 3 g/m2 (71% and 49%), occasionally in combination with rituximab or etoposide, with some receiving dexamethasone. Median time to when G-CSF was started post-chemotherapy was 3 days for lymphoma patients and 4 days for myeloma patients. A median dose of 10 ug/kg G-CSF was used in all groups, given for a median of 10 days with chemomobilization and 6 days with G-CSF alone (p<0.001).
Stem cell mobilization and apheresis
Both C+G and G-CSF alone successfully mobilized stem cells, with nearly all patients collecting at least 2×106 CD34+ cells/kg (Table 3). Patients mobilized with C+G had to wait a week longer between mobilization and apheresis than patients mobilized with G-CSF alone (median wait for L:C+G: 11 days vs. L:G: 4 days, p<0.00001; M:C+G: 12 days vs. M:G: 4 days, p<0.00001). However, the median number of CD34+ cells collected was similar for lymphoma patients mobilized with C+G and G-CSF alone (6.6×106 cells/kg vs. 5.5 × 106 cells/kg, p=0.30) and higher for multiple myeloma patients mobilized with C+G vs. G-CSF alone (13.8×106 cells/kg vs. 6.8×106 cells/kg, p<0.001). A median of 10 L blood volume/patient were processed for L:C+G, 20 L for L:G (p<0.001), 12 L for M:C+G, and 18.65 for M:G (p<0.001).
Table 3.
Total cells collected
| L:C+G n=35 |
L:G n=29 |
M:C+G n=108 |
M:G n=54 |
|
|---|---|---|---|---|
| <2×106 cells/kg | 0 (0%) | 1 (3%) | 2 (2%) | 1 (2%) |
| 2–5×106 cells/kg | 15 (42%) | 12 (40%) | 11 (10%) | 17 (31%) |
| >5×106 cells/kg | 20 (58%) | 16 (57%) | 95 (88%) | 36 (67%) |
After 2 days, 69% of L:C+G patients met their collection goal vs. 53% of L:G (p>0.05), and 77% of M:C+G vs. 57% of M:G (p<0.05) (Table 4). Patients mobilized with C+G collected more cells/kg/day on average (L:C+G: 5.51×106 cells/kg collected on day 1 vs. L:G: 2.92×106 cells/kg, p=0.0231; MM:C+G: 14.16×106 cells/kg vs. M:G: 3.69×106 cells/kg, p<0.00001) and required fewer days of apheresis on average (L:C+G: 2.11 days vs. L:G: 2.96 days, p=0.012; M:C+G: 2.02 days vs.M:G: 2.83 days, p=0.0015).
Table 4.
Average number of cells collected and cumulative percentage of patients reaching goal per day*
| Day | L:C+G | L:G | M:C+G | M:G |
|---|---|---|---|---|
| 1 | 5.51 (29%) | 2.92 (7%) | 14.16 (41%) | 3.69 (7%) |
| 2 | 3.25 (69%) | 2.11 (53%) | 11.59 (77%) | 2.91 (57%) |
| 3 | 0.91 (89%) | 0.68 (71%) | 3.64 (91%) | 1.22 (77%) |
| 4 | 0.89 (100%) | 1.05 (82%) | 1.76 (93%) | 1.07 (90%) |
| 5 or more | NA (100%) | 1.24 (100%) | 1.13 (100%) | 0.83 (100%) |
106 cells/kg (percent of patients requiring pheresis that day)
Patients mobilized with C+G tended to require fewer rounds of mobilization: 97% of L:C+G required only one mobilization vs. 83% of L:G and 95% of M:C+G vs. 85% of M:G. Few patients required three or more rounds of mobilization: 3% of L:C+G vs. 7% of L:G; 1% of M:C+G required 3 or more rounds vs. 4% M:G. None of these differences was statistically significant (all p>0.05).
Engraftment
Eight patients did not proceed to transplant: 3 due to inadequate CD34 collection (1 L:G, 2 M:G), 1 due to sepsis (L:G), 1 due to complications from COPD (M:C+G), 1 due to a knee replacement (M:C+G), and 2 patient decided they did not want transplant (both M:G). For those who did go to transplant, all except one patient in the M:C+G engrafted; that patient received only 1.4×106 CD34+ cells/kg. All other patients received at least 2.0×106 CD34+ cells/kg (median L:C+G: 5.6×106, L:G 5.3×106, M:C+G 6.6×106, M:G 4.1×106, p <0.001). The median number of days to neutrophil engraftment was 10 for L:C+G, 12 for L:G, 11 for M:C+G, and 12 for M:G (all p>0.05). The median number of days to platelet engraftment was 16 for L:C+G, 17 for L:G, 14 for M:C+G, and 17 for M:G (all p>0.05).
Morbidity
During the mobilization period, patients with multiple myeloma mobilized with C+G were more likely to experience febrile neutropenia (L:C+G 6% vs. L:G 0%, p>0.05; M:C+G 17% vs. M:G 2%, p<0.05), need iv antibiotics (L:C+G 6% vs. L:G 10%, p>0.05; M:C+G 26% vs. M:G 7%, p<0.05), and require hospitalization (L:C+G 14% vs. L:G 0%, p>0.05; M:C+G 36% vs. M:G 9%, p<0.05). However, there were no significant differences in documented infections or sepsis (L:C+G 0% vs. L:G 4%; M:C+G 6% vs. M:G 14%, all p>0.05) (note: some patients may have had a documented infection without having a fever and neutropenia). Requirement for a new apheresis catheter were also similar (L:C+G 2% vs. L:G 0%; M:C+G 6% vs. M:G 4%, all p>0.05).
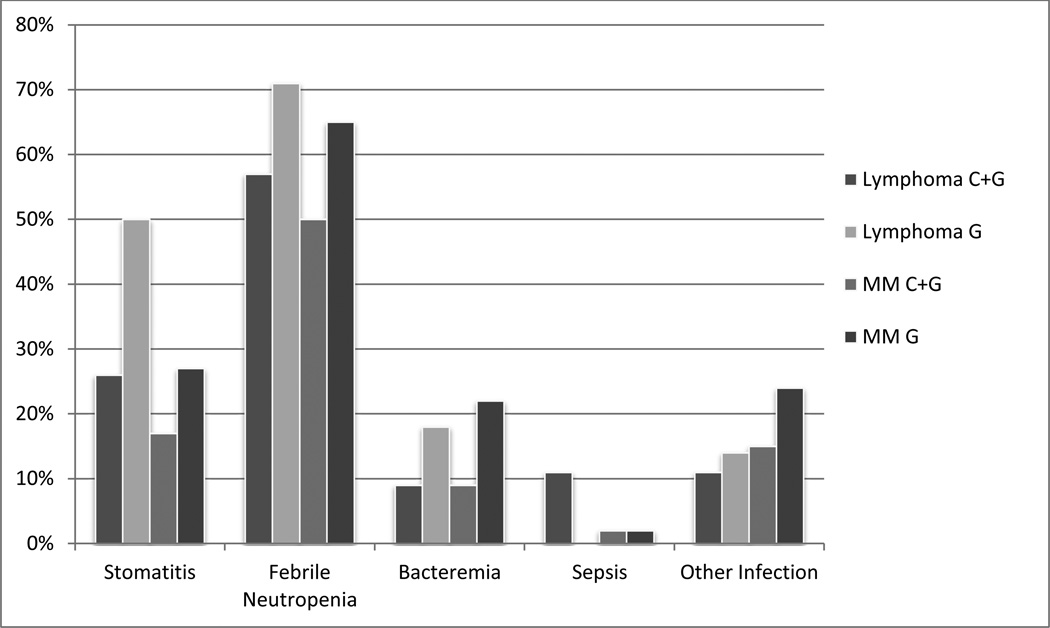
Interestingly, patients mobilized with C+G tended to have less complicated transplant courses, though most of these differences were not statistically significant (Figure 1). There was a trend toward less mucositis (L:C+G 26% vs. L:G 50%; M:C+G 17% vs. M:G 27%, p>0.05 for both), though similar numbers of days of pain medication were required (median 7 days for L:C+G vs. 11 for L:G, p=0.56, 10.5 M:C+G vs. 9 for M:G, p=0.52), a trend toward less febrile neutropenia (L:C+G 57% vs. L:G 71%; M:C+G 50% vs. M:G 65%, p>0.05 for both) and bacteremia (L:C+G 9% vs. L:G 18%, p>0.05; M:C+G 9% vs. M:G 22%, p<0.05), though sepsis (L:C+G 11% vs. L:G 0%; M:C+G 2% vs. M:G 2%, p>0.05 for both) and other infections (L:C+G 11% vs. L:G 14%; M:C+G 15% vs. M:G 24%, p>0.05 for both) were similar.
Figure 1. Complications of transplant.
Rates of different complications of transplantation after mobilization with chemotherapy + granulocyte colony stimulating factor (C+G) or granulocyte colony stimulating factor alone (G) for patients with lymphoma and multiple myeloma (MM).
Costs
Mobilization with C+G was more resource-intensive (Table 5), particularly with regard to antibiotics, antifungals, antivirals, and pain medications (note: we did not distinguish between prophylactic and therapeutic use). However, use of these medications did not contribute significantly to cost; rather, the primary drivers the increased cost of chemomobilization were chemotherapy and extra days of G-CSF (Figure 2S): median cost for L:C+G was $10,300 vs. $7,300 for L:G (p<0.001); median cost for M:C+G was $8,800 vs. $5,600 for M:G (p<0.001) (Table 6). For the 19 patients who required re-mobilization, median costs were increased by $6,700 (mean $5,500, 95% CI $4,500–$6,400) on top of additional apheresis costs. Overall median costs of apheresis were similar: L:C+G $8,200 vs. L:G $7,800 (p=0.422) and M:C+G $8,200 vs. M:G $8,300 (p=0.456). While C+G was associated with fewer days of apheresis on average, the median number of days of apheresis was 2 for both C+G and G, therefore not affecting median costs.
Table 5.
Peri-mobilization Resource Utilization
| L:C+G n=35 |
L:G n=29 |
p-value | M:C+G n=108 |
M:G n=54 |
p-value | |
|---|---|---|---|---|---|---|
| Pain medication | ||||||
| % of patients (n) | 17% (6) | 21% (6) | P>0.05 | 36% (39) | 26% (14) | P>0.05 |
| Median days | 29 | 2.5 | P=0.009 | 12 | 2.5 | P=0.001 |
| Antivirals | ||||||
| % of patients (n) | 63% (22) | 3% (1) | NA | 37% (40) | 6% (3) | P<0.05 |
| Median days | 12.5 | 13 | NA | 13.5 | 20 | P=0.684 |
| Antifungals | ||||||
| % of patients (n) | 69% (24) | 10% (3) | P<0.05 | 47% (51) | 4% (2) | P<0.05 |
| Median days | 12 | 15 | P=0.562 | 11 | 7.5 | P=0.496 |
| IV antibiotics | ||||||
| % of patients (n) | 6% (2) | 10% (3) | P>0.05 | 26% (28) | 7% (4) | P<0.05 |
| Median days | 2.5 | 12 | P=0.196 | 7 | 2.5 | P=0.039 |
| Oral antibiotics | ||||||
| % of patients (n) | 83% (29) | 24% (7) | P<0.05 | 77% (83) | 19% (10) | P<0.05 |
| Median days | 14 | 13 | P=0.78 | 10 | 10 | P=0.195 |
| Hospitalization | ||||||
| % of patients (n) | 14% (5) | 0% (0) | NA | 36% (39) | 9% (5) | P<0.05 |
| Median days | 1 | NA | NA | 2 | 2 | P=0.76 |
Table 6.
Costs of Mobilization, Apheresis, and Transplant
| L:C+G n=35 |
L:G n=29 |
p-value | M:C+G n=108 |
M:G n=54 |
p-value | |
|---|---|---|---|---|---|---|
|
Mobilization | ||||||
| Median | $10,300 | $7,300 | P<0.001 | $8,800 | $5,600 | P<0.001 |
| (range) | (5,500–20,600) | (1,800–14,100) | (2,200–21,700) | (2,100–16,100) | ||
| Mean | $11,600 | $7,400 | $9,700 | $6,900 | ||
| (95% CI) | (10,200–13,000) | (6,200–8,600) | (9,000–10,400) | (5,900–7,800) | ||
|
Apheresis | ||||||
| Median | $8,200 | $7,800 | P=0.422 | $8,200 | $8,300 | P=0.456 |
| (range) | (4,000–14,600) | (3,600–26,800) | (4,900–28,600) | (4,000–22,400) | ||
| Mean | $8,400 | $10,200 | $9,800 | $9,600 | ||
| (95% CI) | (7,500–9,200) | (8,300–12,100) | (8,900–10,700) | (8,600–10,600) | ||
| Transplant | n=5 | n=0 | P>0.05 | n=63 | n=15 | P<0.05 |
| (outpatient) | (14%) | (0%) | (60%) | (29%) | ||
| Median | $22,900 | NA | NA | $28,000 | $36,900 | P=0.017 |
| (range) | (18,900–37,400) | (8,900–65,400) | (22,300–52,800) | |||
| Mean | $26,600 | NA | 28,700 | $34,900 | ||
| (95% CI) | (19,200–34,000) | (26,500–31,000) | (30,300–39,500) | |||
| Transplant | n=30 | n=28 | P>0.05 | n=42 | n=36 | P<0.05 |
| (inpatient) | (86%) | (100%) | (40%) | (71%) | ||
| Median | $65,600 | $70,600 | P=0.549 | $60,700 | $60,900 | P=0.449 |
| (range) | (33,200–454,900) | (36,800–120,800) | (41,700–120,200) | (46,000–124,500) | ||
| Mean | $77,900 | $69,700 | $62,200 | $65,400 | ||
| (95% CI) | (51,500–104,400) | (62,000–77,500) | (58,300–66,000) | (60,200–70,600) | ||
| Total Costs | n=5 | n=0 | n=63 | n=15 | ||
| (outpatient) | (14%) | (0%) | (60%) | (29%) | ||
| Median | $39,700 | N/A | N/A | $45,600 | $51,400 | P=0.403 |
| (range) | (32,700–59,700) | (25,800–98,600) | (32,300–69,000) | |||
| Mean | $44,600 | N/A | $48,400 | $50,100 | ||
| (95% CI) | (34,300–54,800) | (45,200–51,600) | (44,600–55,500) | |||
| Total Costs | n=30 | n=28 | n=42 | n=36 | ||
| (inpatient) | (86%) | (100%) | (40%) | (71%) | ||
| Median | $81,600 | $88,200 | P=0.703 | $78,100 | $77,200 | P=0.924 |
| (range) | (46,600–482,200) | (46,700–132,000) | (52,900–145,700) | (58,000–152,500) | ||
| Mean | $98,300 | $87,700 | $81,000 | $82,600 | ||
| (95% CI) | (71,300–125,300) | (79,900–95,500) | (76,300–85,900) | (76,500–88,700) | ||
Transplant costs were calculated separately for patients who received their transplants as inpatients vs. outpatients (Figure 3S). For outpatient transplants, median costs for L:C+G (n=5, 14%) were $22,900; no L:G patients were transplanted as oupatients. Median outpatient transplant costs for M:C+G (n=63, 60%) were $28,000 vs. $37,000 for M:G (n=15, 29%) (p=0.017). For inpatient transplants, median costs for L:C+G (n=30, 86%) were $65,600 vs. $70,600 for L:G (n=28, 100%) (p=0.549); median costs for M:C+G (n=42, 40%) were $60,700 vs. $60,900 for M:G (n=36, 71%) (p=0.449).
Overall, for outpatient transplants, median total costs combining mobilization, apheresis, and transplant for L:C+G (n=5, 14%) were $39,700; no L:G patients were transplanted as oupatients, and median total costs for outpatient transplants with M:C+G (n=63, 60%) were $45,600 vs. $51,400 for M:G (n=15, 29%) (p=0.403). For inpatient transplants, median costs for L:C+G (n=30, 86%) were $81,600 vs. $88,200 for L:G (n=28, 100%) (p=0.703); median costs for M:C+G (n=42, 40%) were $78,100 vs. $77,200 for M:G (n=36, 71%) (p=0.924).
Discussion
This retrospective, multi-institution review compared C+G versus G-CSF alone and found significant differences in mobilization results but similar overall outcomes. While C+G cost more and required patients to wait an additional 7–8 days before cells could be collected, an average of one less day of apheresis was required as more cells were collected per day, and although differences were not statistically significant, there was a trend toward lower frequency of re-mobilization with C+G. Outcomes with transplant including engraftment and cost tended to be similar regardless of mobilization method. Although there was a trend to less transplant-related toxicities with C+G, it is difficult to determine if there is a true difference as a result of mobilization method or if this is due to other confounders (e.g. disease burden, inpatient vs. outpatient transplant, etc.). Although outpatient transplants for myeloma after mobilization with C+G cost less than outpatient transplants after G-CSF alone, it is unclear from our study if this is an effect of mobilization method or other confounders, and there was no difference in total costs. Results were otherwise comparable whether patients had lymphoma or multiple myeloma.
Inadequate stem cell mobilization results in increased resource consumption and costs21. Although C+G increased yields compared to G-CSF, we found this advantage was more than offset by the increased resource utilization and costs of chemotherapy itself and increased complications like febrile neutropenia. However, as each additional day of apheresis may increase costs by as much as $6,60022 on top of the additional mobilization costs of chemotherapy and G-CSF as noted above, it may be more cost-effective to treat patients at risk of inadequate mobilization with C+G. Our findings are similar to other studies where the cost of mobilization with G-CSF was $5,76023 and the cost of C+G was $10,60524.
This study is limited by its retrospective design. It is possible that the differences in stem cell collection were due to biases related to patient selection, patient referral, center experience and practice, plans for single vs. tandem transplantation, as well as a number of other possible variables. Unfortunately, we were not able to capture data for all these potential confounders. In addition, the selection of chemo-mobilization may, in part, be for the treatment of the underlying malignancy, and as such its use may not be completely comparable to mobilization with G-CSF alone. We do not have data on how this data or other known predictors of poor mobilization such as thrombocytopenia may have impacted mobilization. This study did not follow relapse rates after transplant, so we are unable to comment as to whether C+G reduces the rate of relapse compared to G-CSF alone. Finally, the wide array of chemotherapy regimens used in this study makes it difficult to generalize outcomes. Nonetheless, this study provides a useful snapshot of practices and outcomes from multiple institutions across the country.
Although we did not look at plerixafor, a small molecule CXCR4 antagonist that can enhance the mobilization of peripheral blood stem cells, it is worth noting that the addition of this agent to G-CSF can increase CD34+ yields compared to G-CSF alone in patients with multiple myeloma25 and lymphoma26. Historical comparison to cyclophosphamide + G-CSF report similar numbers of cells collected, costs, and clinical outcomes27. Several studies have looked at the optimal use of plerixafor in terms of both timing and cost-effectiveness28–30.
In summary, C+G improves stem cell collection but there are tradeoffs, particularly with regard to cost. These may be offset in patients at high risk of inadequate mobilization; such a strategy has been advocated for the selective use of plerixafor. Although our study provides a real-world look at practices and outcomes across multiple institutions, prospective randomized studies looking at C+G compared to G-CSF alone as well as plerixafor + G-CSF would further our knowledge and understanding of optimal and cost-effective methods of mobilization.
Supplementary Material
Acknowledgments
Dr. Sung’s effort was supported by NIH T32 HL007057-37.
Footnotes
-
-Anthony D. Sung: No financial disclosures.
-
-Daniel T. Grima: No financial disclosures.
-
-Lisa M. Bernard: No financial disclosures.
-
-Stephen Brown: No financial disclosures.
-
-George Carrum: No financial disclosures.
-
-Leona Holmberg: Dr. Holmberg has received research funding from Sanofi/Genzyme.
-
-Mitchell E. Horwitz: No financial disclosures.
-
-Jane L. Liesveld: No financial disclosures.
-
-Junya Kanda: No financial disclosures.
-
-Brian McClune: No financial disclosures.
-
-Paul Shaughnessy: Dr. Shaughnessy has received research funding and honorarium from Sanofi/Genzyme and honorarium from Millenium.
-
-Guido J. Tricot: No financial disclosures.
-
-Nelson J. Chao: No financial disclosures.
-
-The chart review was funded by Genzyme Corporation, who provided input into the study design and analyses. The manuscript development was not funded and no author received remuneration for their contributions to the manuscript.
<Supplementary information is available at Bone Marrow Transplantation’s Website>
Conflicts of Interest
The chart review was funded by Genzyme Corporation, who provided input into the study design and analyses. The manuscript development was not funded and no author received remuneration for their contributions to the manuscript. Dr. Holmberg has received research funding from Sanofi/Genzyme and Dr. Shaughnessy has received research funding and honorarium from Sanofi/Genzyme and honorarium from Millenium.
References
- 1.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. The New England journal of medicine. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 2.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(36):9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 3.Oliansky DM, Gordon LI, King J, Laport G, Leonard JP, McLaughlin P, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of follicular lymphoma: an evidence-based review. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(4):443–468. doi: 10.1016/j.bbmt.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Oliansky DM, Czuczman M, Fisher RI, Irwin FD, Lazarus HM, Omel J, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(1):20–47. doi: 10.1016/j.bbmt.2010.07.008. e30. [DOI] [PubMed] [Google Scholar]
- 5.Allan DS, Keeney M, Howson-Jan K, Popma J, Weir K, Bhatia M, et al. Number of viable CD34(+) cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone marrow transplantation. 2002;29(12):967–972. doi: 10.1038/sj.bmt.1703575. [DOI] [PubMed] [Google Scholar]
- 6.Ashihara E, Shimazaki C, Okano A, Hatsuse M, Okamoto A, Shimura K, et al. Infusion of a high number of CD34+ cells provides a rapid hematopoietic recovery and cost savings in autologous peripheral blood stem cell transplantation. Japanese journal of clinical oncology. 2002;32(4):135–139. doi: 10.1093/jjco/hyf030. [DOI] [PubMed] [Google Scholar]
- 7.Faucher C, Le Corroller AG, Chabannon C, Viens P, Stoppa AM, Bouabdallah R, et al. Autologous transplantation of blood stem cells mobilized with filgrastim alone in 93 patients with malignancies: the number of CD34+ cells reinfused is the only factor predicting both granulocyte and platelet recovery. Journal of hematotherapy. 1996;5(6):663–670. doi: 10.1089/scd.1.1996.5.663. [DOI] [PubMed] [Google Scholar]
- 8.Scheid C, Draube A, Reiser M, Schulz A, Chemnitz J, Nelles S, et al. Using at least 5x10(6)/kg CD34+ cells for autologous stem cell transplantation significantly reduces febrile complications and use of antibiotics after transplantation. Bone marrow transplantation. 1999;23(11):1177–1181. doi: 10.1038/sj.bmt.1701748. [DOI] [PubMed] [Google Scholar]
- 9.Hosing C, Saliba RM, Ahlawat S, Korbling M, Kebriaei P, Alousi A, et al. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. American journal of hematology. 2009;84(6):335–337. doi: 10.1002/ajh.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micallef IN, Apostolidis J, Rohatiner AZ, Wiggins C, Crawley CR, Foran JM, et al. Factors which predict unsuccessful mobilisation of peripheral blood progenitor cells following G-CSF alone in patients with non-Hodgkin's lymphoma. The hematology journal : the official journal of the European Haematology Association / EHA. 2000;1(6):367–373. doi: 10.1038/sj.thj.6200061. [DOI] [PubMed] [Google Scholar]
- 11.Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M, Neben K, et al. Poor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(4):490–499. doi: 10.1016/j.bbmt.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(6):718–723. doi: 10.1016/j.bbmt.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Experimental hematology. 2002;30(9):973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 14.Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone marrow transplantation. 2009;43(3):181–195. doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- 15.Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C, et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood. 2001;98(7):2059–2064. doi: 10.1182/blood.v98.7.2059. [DOI] [PubMed] [Google Scholar]
- 16.Dazzi C, Cariello A, Rosti G, Argnani M, Sebastiani L, Ferrari E, et al. Is there any difference in PBPC mobilization between cyclophosphamide plus G-CSF and G-CSF alone in patients with non-Hodgkin's Lymphoma? Leukemia & lymphoma. 2000;39(3–4):301–310. doi: 10.3109/10428190009065829. [DOI] [PubMed] [Google Scholar]
- 17.Mahindra A, Bolwell BJ, Rybicki L, Elder P, Kalaycio M, Dean R, et al. Etoposide plus G-CSF priming compared with G-CSF alone in patients with lymphoma improves mobilization without an increased risk of secondary myelodysplasia and leukemia. Bone marrow transplantation. 2012;47(2):231–235. doi: 10.1038/bmt.2011.73. [DOI] [PubMed] [Google Scholar]
- 18.Meldgaard Knudsen L, Jensen L, Gaarsdal E, Nikolaisen K, Johnsen HE. A comparative study of sequential priming and mobilisation of progenitor cells with rhG-CSF alone and high-dose cyclophosphamide plus rhG-CSF. Bone marrow transplantation. 2000;26(7):717–722. doi: 10.1038/sj.bmt.1702609. [DOI] [PubMed] [Google Scholar]
- 19.Shaughnessy P, Islas-Ohlmayer M, Murphy J, Hougham M, MacPherson J, Winkler K, et al. Cost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(5):729–736. doi: 10.1016/j.bbmt.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Reuters T. Red Book 2010: Pharmacy's Fundamental Reference. PDR Network LLC; 2010. [Google Scholar]
- 21.Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone marrow transplantation. 2010;45(9):1396–1403. doi: 10.1038/bmt.2009.370. [DOI] [PubMed] [Google Scholar]
- 22.Hosing C, Smith V, Rhodes B, Walters K, Thompson R, Qazilbash M, et al. Assessing the charges associated with hematopoietic stem cell mobilization and remobilization in patients with lymphoma and multiple myeloma undergoing autologous hematopoietic peripheral blood stem cell transplantation. Transfusion. 2011;51(6):1300–1313. doi: 10.1111/j.1537-2995.2011.03176.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ, Hillner BE, Schmitz N, Linch DC, Dreger P, Goldstone AH, et al. Economic analysis of a randomized clinical trial to compare filgrastim-mobilized peripheral-blood progenitor-cell transplantation and autologous bone marrow transplantation in patients with Hodgkin's and non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(1):5–10. doi: 10.1200/JCO.1997.15.1.5. [DOI] [PubMed] [Google Scholar]
- 24.Jagasia MH, Savani BN, Neff A, Dixon S, Chen H, Pickard AS. Outcome, toxicity profile and cost analysis of autologous stem cell mobilization. Bone marrow transplantation. 2011;46(8):1084–1088. doi: 10.1038/bmt.2010.254. [DOI] [PubMed] [Google Scholar]
- 25.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 26.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(28):4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 27.Shaughnessy PI-OM, Murphy J, Hougham M, MacPherson J, Winkler K, Silva M, Steinberg M, Matous J, Selvey S, Maris M, McSweeney PA. Cost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamide. 2011;17(5):729–736. doi: 10.1016/j.bbmt.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2012;52(1):55–62. doi: 10.1111/j.1537-2995.2011.03206.x. [DOI] [PubMed] [Google Scholar]
- 29.Costa LJ, Alexander ET, Hogan KR, Schaub C, Fouts TV, Stuart RK. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone marrow transplantation. 2011;46(1):64–69. doi: 10.1038/bmt.2010.78. [DOI] [PubMed] [Google Scholar]
- 30.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106(5):1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.