Abstract
Colonization of the stomach by Helicobacter pylori affects about half of the world population and is associated with the development of gastritis, ulcers and cancer. Polymorphisms in the IL1B gene are linked to an increased risk of H. pylori-associated cancer, but the bacterial and host factors that regulate IL-1β production in response to H. pylori infection remain largely unknown. Using murine bone marrow-derived DCs, we show that the virulence bacterial factors cagPAI and CagL, but not VacA or CagA, regulate the induction of pro-IL-1β and secondarily the production of mature IL-1β in response to H. pylori infection. We further show that the host receptors, TLR2 and NOD2, but not NOD1, are required for induction of pro-IL-1β and NLRP3 in DCs infected with H. pylori. In contrast, NLRP3 and the adaptor ASC, were essential for the activation of Caspase-1, processing of pro-IL-1β into mature IL-1β and secretion of IL-1β. Finally, we provide evidence that mutant mice deficient in Caspase-1, IL-1β and IL-1 receptor, but not NLRP3, are impaired in the clearance of the CagA-positive SPM326 H. pylori strain in the stomach when compared to wild-type mice. These studies identify bacterial cagPAI and the cooperative interaction among the host innate receptors TLR2, NOD2 and NLRP3 as important regulators of IL-1β production in H. pylori-infected DCs.
Keywords: Helicobacter pylori, IL-1β, Inflammasome, Nod2
Introduction
Detection of microbes by the immune system is mediated by the activation of host soluble factors and germ-line encoded pattern-recognition receptors (PRRs) by microbial moieties or endogenous molecules generated in the setting of infection [1]. PRRs including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and RIG-like helicases are activated by conserved and unique microbial structures [1, 2]. TLRs mediate recognition of several molecules including LPS and lipopeptides at the cell surface as well as microbial nucleic acids in endosomes [1]. In contrast, NLRs and RIG-like helicases induce innate immune responses through cytosolic sensing of bacterial and viral components [1, 2]. Two NLR family members, NOD1 and NOD2, are activated by molecules produced during the synthesis and/or degradation of bacterial peptidoglycan [3–6]. Nod2 is activated by muramyl dipeptide (MDP), a structure present in all Gram-negative and -positive bacteria [3–6]. In response to infection, TLRs and NOD2 induce transcription of immune response genes through the NF-κB transcription factor and MAPKs that ultimately culminate in host defense responses to eliminate microbial invasion.
A major inflammatory pathway induced in response to microbial infection is the inflammasome, a multi-protein platform that activates Caspase-1 in phagocytes and mast cells [7, 8]. Once activated, Caspase-1 cleaves pro-interleukin-1β (IL-1β) and pro-IL-18 into their biologically active secreted forms [7]. To date, several inflammasomes have been identified including those triggered by the activation of NLR family members, NLR Caspase domain-containing 4 (NLRC4) and NLR pyrin domain-containing 3 (NLRP3) [7, 8]. Activation of the NLRC4 is induced by the release of bacterial flagellin or PrgJ-like rod proteins into the host cell cytosol in response to infection with several bacterial pathogens including Salmonella typhimurium, Legionella pneumophila and Pseudomonas aeuruginosa [9]. In mouse macrophages, activation of NLRP3 requires two signals. The first signal, referred to as priming, is NF-κB-dependent transcription of pro-IL-1β and Nlrp3, through the stimulation of PRRs by microbial products or certain cytokines such as tumor necrosis factor-α (TNF-α) or IL-1β [10, 11]. The second signal activates NLRP3 and is induced by ATP, certain bacterial toxins, or particulate matter [7–9]. In response to activating stimuli, NLRP3 recruits the adapter protein ASC (apoptosis-associated speck-like protein containing a Caspase recruitment and activation domain) to drive the activation of Caspase-1 [7].
Helicobacter pylori chronically colonizes the gastric mucosa of more than 50% of the world population and can persist for life [12]. H. pylori infection can induce chronic gastritis, peptic ulcer disease, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [12–15]. H. pylori expresses virulence factors that include vacuolating cytotoxin A (VacA) and cytotoxin-associated genes pathogenicity island (cagPAI) [16, 17]. The cagPAI encodes components of a type IV secretion system (T4SS) capable of injecting into the host cell the CagA protein and other factors that are associated with more severe inflammatory disease [17]. A major cytokine induced in response to gastric H. pylori infection is IL-1β [18]. The importance of IL-1β in H. pylori infection is underscored by the observation that polymorphisms in the IL1B gene are associated with an increased predisposition to gastric cancers in infected individuals [18, 19]. Furthermore, transgenic mice overproducing IL-1β in the stomach develop gastric inflammation and carcinoma [20]. In dendritic cells (DCs), TLR2 is the major TLR that regulates cytokine responses to H. pylori infection [21] whereas in epithelial cell, NOD1 recognizes H. pylori peptidoglycan resulting in NF-κB activation and subsequent IL-8 production [22]. H. pylori induces the activation of Caspase-1 in dendritic cells [23]. However, little is known about the microbial molecules and host PRRs that mediate the production of IL-1β in response to H. pylori infection. In this study, we demonstrate that secretion of IL-1β in DCs infected with H. pylori is regulated by cagPAI and requires host TLR2, NOD2 and the NLRP3 inflammasome. Specifically, H. pylori stimulation via TLR2 and NOD2 in dendritic cells primes the NLRP3 inflammasome by inducing pro-IL-1β and NLRP3, which enables the activation of Caspase-1 via NLRP3 and production of mature IL-1β. Finally, we provide evidence that IL-1β signaling regulates the colonization of H. pylori in vivo.
Materials and Methods
Ethics statement
Animal studies were carried out in accordance with in accordance with the recommendations in the Guide for the Care and Use of laboratory Animals of the National Institutes of Health. The protocol was approved by the University of Michigan Committee on Use and Care of Animals (Approved Protocol Number: 09716).
Mice
Nod1−/−, Nod2−/−, Casp1−/−, Asc−/−, Nlrp3−/−, and Nlrc4−/− in C57BL/6J background have been previously described [24–26]. Mice deficient in TLR2 in C57BL/6J background were a gift of Dr. Shizuo Akira (Osaka University, Japan). C57BL/6J mice were originally purchased from The Jackson Laboratory and maintained in our laboratory. Mice deficient in both NOD2 and TLR2 were generated by crossing Nod2−/− and Tlr2−/− mice and intercrossing the F1 generation. Mice were housed in a pathogen-free facility.
Reagents and bacterial culture
Ultrapure LPS from Escherichia coli O111:B4 was purchased from InvivoGen. H. pylori strain 26695, P1 WT, isogenic mutant P1 ΔcagL (cagL deficient), P12 WT, isogenic mutant P12 ΔvacA (VacA deficient), P12 ΔcagPAI (cagPAI deficient), and P12 ΔcagL have been described [27]. H. pylori strain G27 WT and isogenic mutant G27 ΔcagPAI were gifts from Dr. Scott Merrell (Uniformed Services University of the Health Sciences, Bethesda, MD) and SPM326 from Dr. Lesley Smythies (University of Alabama, Birmingham, AL). H. pylori was routinely grown on Campylobacter agar plates or Brucella broth containing 10% of fetal bovine serum, 10 μg/ml of vancomycin (Sigma), 5 μg/ml of trimethoprim (Sigma), and 1 μg/ml of nystatin (Sigma) at 37°C under microaerobic conditions. H. pylori was isolated from gastric homogenates cultured on plates contained 200 μg/ml of Bacitracin (Sigma), 6 μg/ml of Vancomycin (Sigma), 16 μg/ml of cefsulodin (Sigma), and 20 μg/ml of trimethoprim (Sigma) to inhibit the growth of normal gastric flora.
Preparation of bone marrow-derived dendritic cells (BMDCs) and infection with H. pylori
BMDCs were prepared as previously described [11]. Briefly, bone marrow cells were cultured with GM-CSF (20ng/ml), with fresh GM-CSF added on days 3 and 5. After 7 days, non-adherent cells were collected by vigorous aspiration. BMDCs were seeded in 48-well plates (2×105/well) for ELISA or 6-well plates (5×106/well) for immunoblotting and quantitative PCR and infected with H. pylori overnight or 6 h respectively.
Bacterial invasion assay
The invasion efficiency of H. pylori strains was evaluated using a gentamicin protection assay. Briefly, BMDCs were infected for 20 minutes and then incubated for 20 minutes at 37°C in medium containing gentamicin (100 μg/ml) to kill extracellular bacteria. The infected cells were then washed in PBS, lysed in 0.5% TritonX-100/PBS, and the number of intracellular bacteria was determined by plating.
Quantitative real-time PCR
RNA was extracted using the RNeasy Mini kit (Qiagen) and cDNA was prepared from 0.1 μg of RNA using High Capacity RNA-to-cDNA kit (Applied Biosystems) according to the manufacturer’s instruction. Quantitative real-time PCR was performed by the StepOne Real-Time PCR System using SYBR green buffer according the manufacturer’s instruction (Applied Biosystems). β-actin was used for normalization. The following primer sequences were used; IL-1β forward: 5′-GATCCACACTCTCCAGCTGCA-3′; IL-1 β reverse: 5′-CAACCAACAAGTGATATTCTCCATG; Nlrp3 forward: 5′-ATGGTATGCCAGGAGGACAG-3′; Nlrp3 reverse: 5′-ATGCTCCTTGACCAGTTGGA-3′; Actb forward: 5′-CAATAGTGATGACCTGGCCGT-3′; Actb reverse: 5′-CAATAGTGATGACCTGGCCGT-3′.
Measurement of cytokines
Mouse cytokines were measured in culture supernatants using the enzyme-linked immunosorbent assay (ELISA) kit from R&D systems.
Immunoblotting
Cells were lysed together with the cell supernatant by the addition of 1% Nonidet P-40, complete protease inhibitor cocktail (Roche), and 2 mM dithiothreitol. After centrifugation at 20,000 × g for 15 min, the supernatant was mixed with 5x SDS buffer and boiled for 10 min, and samples were separated by SDS-PAGE and transferred to polyvinyldifluoride membranes. Membranes were incubated with rabbit antibody to mouse Caspase-1 (a gift from P. Vandenabeele, University of Ghent, Ghent, Belgium), goat antibody to mouse IL-1β (R&D systems), and mouse antibody to mouse GAPDH (Millipore). Proteins were detected by ECL kit.
Mouse infection
Mice were inoculated three times by oral gavage with 500 μl of H. pylori strain SPM326 (2–8 × 109/ml) with 1 day separating each inoculation. After 4 weeks mice were euthanized by CO2 and stomachs were removed and washed with sterile water. Washed stomachs were homogenized, plated onto agar plates, and incubated under microaerobic conditions at 37 C for 5–7 days.
Statistical analysis
Statistical significance between groups was determined by the two tailed Student’s t test or one-way analysis of variance (ANOVA) followed by post hoc anlaysis (Newman-Keuls multiple comparison test) (Graphpad Prism 5). Differences were considered significant at p < 0.05.
Results
H. pylori cagPAI and CagL, but not VacA or CagA, enhance IL-1β production in DCs
H. pylori has two major virulence factors, VacA and cagPAI [16, 17]. The cagPAI encodes components of the T4SS [17], so we determined whether the T4SS is involved in the regulation of IL-1β. We compared the ability of wild-type H. pylori and an isogenic mutant deficient in CagL, a critical component of the T4SS and cag PAI-associated pili [17]. To determine whether VacA, cagPAI and/or CagL regulate IL-1β production in H. pylori-infected DCs, we infected DCs with wild-type H. pylori and isogenic mutants deficient in VacA, cagPAI or CagL IL-1β secretion elicited in DCs infected with either wild-type or the H. pylori VacA mutant were comparable (Figure 1A). However, DCs infected with cagPAI or CagL mutants had reduced IL-1β secretion when compared to cells infected with wild-type H. pylori (Figure 1A). The reduction in IL-1β secretion was not explained by impaired uptake of the mutant strains by DCs (Figure 1C). The role of cagPAI in eliciting IL-1β production was confirmed in a second H. pylori strain (Figure 1C). Likewise, impaired IL-1β release was also observed in another H. pylori strain deficient in CagL (Figure 1D). In contrast, a CagA mutant induced comparable IL-1β production to its isogenic wild-type strain (Figure 1E). Consistently, the induction of IL-1β mRNA by the cagPAI and CagL mutants was impaired compared to the wild-type bacterium (Figure 1F). In contrast, the induction of Nlrp3 mRNA by the cagPAI and CagL mutants was comparable to that of the wild-type bacterium (data not shown). Consistent with a role of cagPAI and CagL in the induction of IL-1β, the defective ability of the mutants to induce Il1b mRNA and to elicit IL-1β secretion were rescued by pre-treatment of DCs with LPS (Figure 1F). These results indicate that cagPAI and CagL, but not VacA or CagA, regulate the induction of pro-IL-1β and secondarily the production of IL-1β in H. pylori-infected DCs.
Figure 1. H. pylori cagPAI and CagL, but not VacA, enhances IL-1 β induction in DCs.

A, DCs were infected with wild-type P12 H. pylori and isogenic mutants deficient in VacA, cagPAI, or CagL at a multiplicity of infection (MOI) of 1/20 overnight. IL-1β production was determined by ELISA. B, uptake of wild-type P12 H. pylori and isogenic mutants by DCs. C–E, DCs were infected with indicated wild-type H. pylori and isogenic mutants at a multiplicity of infection (MOI) of 1/20 overnight. F–G, DCs were infected with wild-type H. pylori and indicated isogenic mutant strains with or without LPS priming (6 hr). IL-1β production was determined by ELISA. The mRNA expression of Il1b was evaluated by real-time PCR at 6 hr after infection and fold increase (arbitrary unit) was obtained by comparison to the level of uninfected DCs. Data shown are means ± SD of triplicate samples of one experiment representative of three independent experiments. ** p < 0.01
TLR2 and NOD2 induce pro-IL-1β expression and require for IL-1β release upon H. pylori infection in DCs
We next evaluated host factors required for IL-1β secretion in response to H. pylori infection. Specifically, we tested the ability of bone-marrow derived DCs from wild-type, Tlr2−/−, Nod1−/− and Nod2−/− mice to secrete IL-1β in response to infection with H. pylori 26695. IL-1β secretion was reduced in DCs from Nod2−/ and Tlr2−/−, but not Nod1−/− mice, when compared to DCs from WT mice (Figure 2A). To determine whether TLR2 and NOD2 were redundant in the secretion of IL-1β, we generated mice doubly deficient in TLR2 and Nod2 and infected DCs from the mutant mice with H. pylori. The release of IL-1β in response to H. pylori was much lower in DCs from Nod2−/−Tlr2−/− mice than in DCs from either Tlr2−/− or Nod2−/− mice (Figure 2B). To determine whether TLR2 and Nod2 regulate the induction of pro-IL-1β, DCs from wild-type, Tlr2−/−, Nod1−/−, Nod2−/− and Nod2−/−Tlr2−/− mice were infected with H. pylori and levels of pro-IL-1β induction were assessed by immunoblotting. We found that pro-IL-1β was induced upon infection in wild-type DCs. However, levels of induction was reduced to a greater extent in doubly-deficient Nod2−/−Tlr2−/− DCs compared to single deficient DCs (Figure 2C). To determine whether TLR2 and Nod2 regulate the transcriptional induction of pro-IL-1β, we prepared mRNA from wild-type and Nod2−/−Tlr2−/− DCs before and after H. pylori infection and measured Il1b mRNA by quantitative real-time PCR. Consistent with the results shown in Figure 2C, the Il1b mRNA levels were significantly reduced in Nod2−/−, Tlr2−/−, and Nod2−/−Tlr2−/− DCs (Figure 2D) after H. pylori infection. These results indicate that NOD2 and TLR2 have a redundant role in H. pylori-induced IL-1β secretion such that pro-IL-1β induction is significantly impaired in the absence of both receptors in DCs.
Figure 2. TLR2 and NOD2 induce pro-IL-1β expression and IL-1β release upon H. pylori infection in DCs.
A–D, DCs from wild-type, Tlr2−/−, Nod1−/−, Nod2−/− and Nod2−/−Tlr2−/− mice were infected with H. pylori at a MOI of 1/20 overnight (A,B) or for 6 hr (C,D). IL-1β secretion was determined by ELISA (A–B) and induction of pro-IL-1β was analyzed by immunoblotting (C). mRNA expression of Il1b was evaluated by real-time PCR and fold increase (arbitrary units) was obtained by comparison to the level of uninfected DCs (D). Immunoblotting for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. For A, B, and D, data shown are means ± SD of triplicate samples of one experiment representative of three independent experiments. * p < 0.05, ** p < 0.01.
Processing of IL-1β upon H. pylori infection depends on Caspase-1
IL-1β is synthesized as an inactive precursor and can be proteolytically cleaved by several proteases including Caspase-1 into its biologically active mature form [28]. To determine whether H. pylori-induced IL-1β processing is dependent on Caspase-1, we first evaluated the secretion of IL-1β in H. pylori-infected DCs from WT or Casp1−/− mice. The release of IL-1β, but not TNF-α, was impaired in DCs from Casp1−/− mice (Figure 3A and 3B). As a control for pro-IL-1β processing, we also stimulated DCs with LPS and ATP, a stimulus that potently induces cleavage of pro-IL-1β into its mature (p17) form via NLRP3 and Caspase-1 [11]. Although pro-IL-1β induction was comparable in H. pylori infected-DCs from WT and Casp1−/− mice, the processing of pro-IL-1β into IL-1β (p17) was impaired in DCs from Casp1−/− mice (Figure 3C). In addition, infection of DCs with another H. pylori strain SPM326 that can colonize mice showed the same dependency on Caspase-1 for pro-IL-1β and IL-1β secretion (Figure 3D, 3E and 3F). These results indicate that processing and secretion of IL-1β in H. pylori-infected DCs requires Caspase-1.
Figure 3. Processing of IL-1β upon H. pylori infection depends on Caspase-1.

A–F, DCs from wild-type and Casp1−/− mice were infected with H. pylori (HP 29965 or HP SPM326) at the indicated MOI overnight (A,B,D,E) or at a MOI of 1/100 for 6 hr (C,F). IL-1β and TNF-α production was determined by ELISA (A,B,D,E) and induction of pro-IL-1β and p17 was analyzed by immunoblotting (C,F). For A, B, D, and E, data shown are means ± SD of triplicate samples of one experiment representative of three independent experiments. * p < 0.05, ** p < 0.01.
TLR2 and NOD2 induce NLRP3 expression and pro-IL-1β processing upon H. pylori infection
We showed in Figure 2 that TLR2 and NOD2 regulate the induction of pro-IL-1β and IL-1β secretion in H. pylori-infected DCs. To determine whether TLR2 and NOD2 also regulate Caspase-1 activation, wild-type and Nod2−/−Tlr2−/− DCs were infected with H. pylori and Caspase-1 activation was assessed by immunoblotting. The production of the p20 subunit of Caspase-1 was induced by H. pylori infection in wild-type DCs, but was greatly impaired in Nod2−/−Tlr2−/− DCs (Figure 4A). Similarly, the amount of processed IL-1β (p17) was reduced in Nod2−/−Tlr2−/− DCs when compared to wild-type cells (Figure 4A). Activation of Caspase-1 via the NLRP3 inflammasome is regulated, in part, by a priming step that involves the induction of Nlrp3 [21]. Notably, infection of DCs by H. pylori increased the level of Nlrp3 mRNA, which was reduced in Nod2−/−, Tlr2−/−, and Nod2−/−Tlr2−/− DCs (Figure 4B). To further assess a role for NOD2/TLR2 in inflammasome priming (signal 1), we pretreated DCs with LPS, a stimulus that primes the Nlrp3 inflammasome, prior to H. pylori infection. Pre-treatment of DCs with LPS enhanced the production of IL-1β in H. pylori-infected cells and rescued the defective ability of DCs to secrete IL-1β in response to H. pylori infection (Figure 4C). These results indicate that NOD2 and TLR2 contribute to Caspase-1 activation via priming of the inflammasome.
Figure 4. TLR2 and NOD2 induce Nlrp3 expression and pro-IL-1β processing upon H. pylori infection.

A, DCs from WT and Nod2−/−Tlr2−/− mice were infected with H. pylori at a MOI of 1/100 for 6 hr. The production of the p20 subunit of Caspase-1 and processed IL-1β (p17) were analyzed by immunoblotting. B, DCs from WT and indicated WT and mutant DCs were infected with H. pylori at a MOI of 1/20 for 6 hr. mRNA expression of Nlrp3 was evaluated by real-time PCR and fold increase (arbitrary units) was obtained by comparison to the level of uninfected DCs. C, DCs from wild-type and indicated mutant DCs were infected with H. pylori overnight with or without LPS priming (6 hr). IL-1β production was determined by ELISA. For B and C, data shown are means ± SD of triplicate samples of one experiment representative of three independent experiments. * p < 0.05, ** p < 0.01.
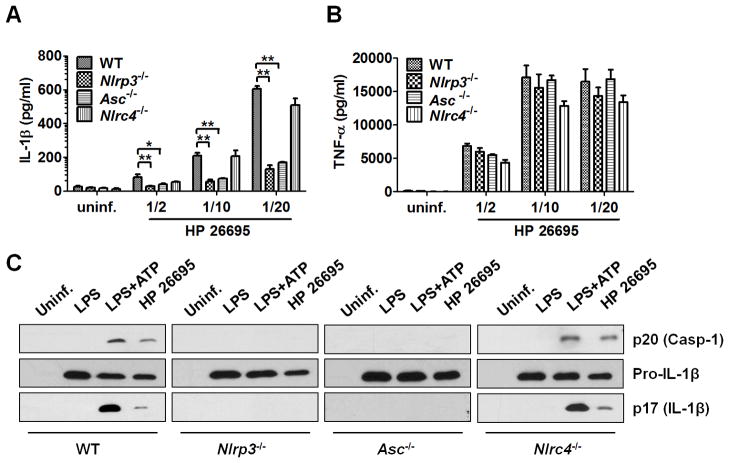
H. pylori infection activates Caspase-1 via the NLRP3 inflammasome in DCs
We next determined which inflammasome was involved in IL-1β secretion induced by H. pylori infection. DCs from wild-type and mice deficient in Nlrp3, Nlrc4, or the common adaptor Asc were infected with H. pylori. IL-1β secretion induced by infection was reduced in DC-deficient in Nlrp3 or Asc, but not in cells deficient in Nlrc4 (Fig. 5A). The impairment in IL-1β secretion in Nlrp3−/− or Asc−/− DCs was specific in that the production of TNF-α in response to H. pylori infection was unimpaired (Figure 5B). Consistently, activation of Caspase-1 and production of mature IL-1β (p17) were abrogated in Nlrp3−/− and Asc−/− DCs, but not in Nlrc4−/− DCs (Figure 5C). These results indicate that Caspase-1-dependent IL-1β processing and secretion requires the NLRP3 inflammasome in H. pylori-infected DCs.
Figure 5. H. pylori infection activates Caspase-1 via the NLRP3 inflammasome in DCs.
A–C, DCs from wild-type and Nlrp3−/−, Nlrp4−/−, Asc−/− and Nlrc4−/− mice were infected with H. pylori at the indicated MOI overnight (A–B) or at a MOI of 1/100 for 6 hr (C). IL-1β and TNF-α production was determined by ELISA (A–B) and activated Caspase-1 (p20) and processed IL-1β (p17) was analyzed by immunoblotting (C). For AB, data shown are means ± SD of triplicate samples of one experiment representative of three independent experiments. * p < 0.05, ** p < 0.01.
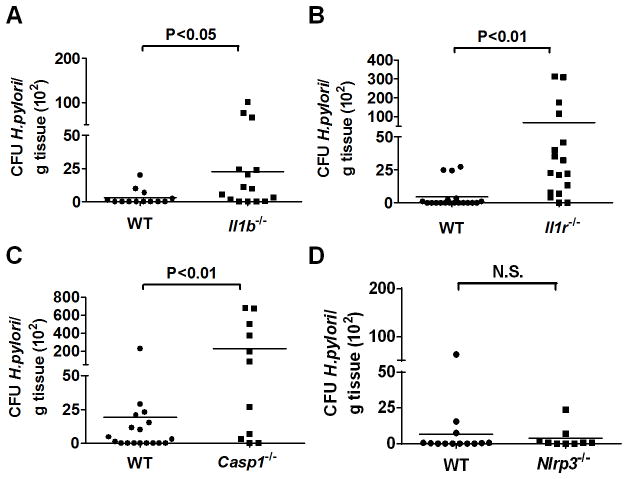
IL-1β signaling regulates H. pylori colonization in vivo
We next determined whether IL-1β signaling regulates the extent of H. pylori colonization in mice. To assess this, wild-type and mice deficient in IL-1β, IL-1 receptor Caspase-1, and NLRP3 were orally infected with H. pylori and bacterial loads were determined in gastric tissue after infection. The SS1 H. pylori strain is widely used for in vivo studies. However, the mouse-adapted SS1 strain lacks a functional Cag T4SS [29] which we have found to be important for IL-1β production (Figure 1). Therefore, we used in these experiments SPM326, a cagA-positive H. pylori strain that induces robust IL-1β production (Figure 3D), expresses a functional Cag T4SS, and colonizes mice at low levels [30]. Consistently, low or undectable pathogen colonization was found in the stomach of wild-type mice four weeks after infection (Figure 6). Notably, the bacterial loads in the stomach were increased in Il1b−/−, Il1r−/−, and Casp1−/− mice when compared to wild-type mice (Figure 6A, 6B and 6C). In contrast, we found comparable pathogen colonization in Nlrp3−/− and wild-type mice (Figure 6D). These results indicate that IL-1β signaling can limit colonization of H. pylori, but this is NLRP3-independent, in vivo.
Figure 6. IL-1β signaling regulates H. pylori colonization in vivo.
A–D, H. pylori colonization was determined in the stomach of wild-type, Il1b−/−, l1r−/−, Casp1−/− and Nlrp3−/− mice 4 weeks post-infection by quantitative culture. Values are expressed as colony forming units (CFUs) per gram stomach tissue. Each dot represents one animal. N. S., not significant.
Discussion
It is known that the mucosal levels of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α are significantly higher in H. pylori-positive than H. pylori-negative specimens. Furthermore, the production of pro-inflammatory cytokines is important in the pathogenesis of H. pylori infection and development of H. pylori-associated complications such as cancer [31, 32]. Among these pro-inflammatory cytokines, IL-1β can increase the expression of other cytokines, such as IL-6 and TNF-α, and regulate the expression of adhesion molecules and influx of inflammatory cells [33]. However, the mechanism by which IL-1β is produced in response to H. pylori infection remains poorly understood. In the present study, we showed that IL-1β secretion in H. pylori-infected DCs is mediated by cooperative interaction between TLR2/NOD2 and NLRP3. TLR2 and NOD2 are both required for the transcriptional induction of pro-IL-1β and the priming of the inflammasome that is mediated by the upregulation of NLRP3. Based on the analysis of single and double deficient cells, the results indicate that TLR2 is more critical, but NOD2 also contributes to the induction of pro-IL1β and NLRP3 which is consistent with the ability of these PRRs to induce gene expression via NF-κB and MAPK activation [1, 34]. In contrast, NLRP3 was required for Caspase-1 activation, processing of pro-IL-1β, and the release of mature IL-1β. These results are in agreement with the current view of NLRP3 activation that relies on two signals for the assembly of the inflammasome. While TLR2 and NOD2 provide signal 1 through the induction of NLRP3, the identity of signal 2, that activates NLRP3 and is presumably provided by a molecule produced by H. pylori remains to be determined. Although NLRP3 was required for IL-1β secretion in DCs infected with H. pylori in vitro, NLRP3 was not essential to clear the pathogen in the stomach. Because the clearance of H. pylori was impaired in mice deficient in IL-1β, IL-1R1 or Caspase-1, the results suggest that the regulation of IL-1β in response to H. pylori infection in vivo is complex and may involve additional inflammasomes which is not revealed in studies with DCs in vitro.
H. pylori possesses two major major virulence factors, VacA and cagPAI. We provide evidence that cagPAI promotes IL-1β secretion by enhancing the transcriptional induction of IL-1β whereas VacA was dispensable. The cagPAI is a 40 kb stretch of DNA that encodes components of a T4SS that forms a pilus for the injection of virulence factors into host target cells [17]. The mechanism by which cagPAI regulates transcription of IL-1β is unclear. The observation that H. pylori deficient in CagL, an essential component of the Cag T4SS apparatus, is impaired in inducing IL-1β suggests that effector proteins or other bacterial molecules injected into the host cytosol via the T4SS might be involved in the regulation of IL-1β. Notably, CagA, a major effector that is translocated via the Cag T4SS was not involved in the regulation of IL-1β. Previous studies showed that peptidoglycan fragments can be delivered via the Cag T4SS to the cytosol of epithelial cells eliciting NOD1 activation [22]. Because NOD2 is involved in the induction of IL-1β, it is possible that that peptidoglycan molecules containing the muramyl dipeptide motif may be leaked into the cytosol of DCs leading to NOD2 activation. However, we found that Nod1−/−Nod2−/− DCs infected with the cagPAI mutant still elicited reduced IL-1β secretion when compared to mutant DCs infected with the wild-type bacterium (results not shown). These results suggest that the mechanism by which cagPAI regulates transcription of IL-1β is not via translocation of Nod1/Nod2 microbial agonists into the host cytosol. Because CagL also binds to β1 integrins on the target cell surface, it is also possible that interactions between CagL and host receptors on DCs regulate IL-1β production.
Single-nucleotide polymorphisms of the IL1B gene are associated with an increased risk for the development of gastric cancer in the setting of H. pylori infection [18]. The mechanism by which IL-1β gene variants promote cancer is poorly understood and controversial. For example, some authors have reported that these genetic polymorphisms are associated with increased production of IL-1β, which has been suggested to induce hypochlorhydria, progressive gastric atrophy and increased risk for gastric cancer [18]. Using a mouse model of IL-1β overexpression in the stomach, Tu et al. provided evidence that IL-1β induces the recruitment of myeloid-derived suppressor cells to the stomach and the activation of these cells may contribute to cancer development through the production of IL-6 and TNFα [20]. However, Sugimoto et al. reported that IL-1β gene polymorphisms are linked to lower production of IL-1β in the gastric mucosa of individuals infected with H. pylori and lower pathogen eradication rate [35]. Our results are consistent with the latter study in that we found that IL-1β signaling inhibits H. pylori colonization in mice. However, Hitzlet et al. reported that IL-1R-null had comparable colonization of the H. pylori SS1 strain [23]. Unlike the H. pylori CagA-positive SPM326 strain used in our in vivo studies, the H. pylori SS1 strain lacks a functional Cag T4SS [29]. Thus, differential expression of cagPAI encoded factors that regulate IL-1β production may account, in part, for the difference in results. Thus, it is possible that increased H. pylori colonization as a result of deficient host IL-1β production promotes enhanced inflammatory responses to the pathogen and increased risk for cancer development.
Acknowledgments
This work was supported by grants R01DK091191 and R01 DK61707 from the National Institutes of Health to G. N. D. K was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-E00012). D. K. and J. P. were supported by the World Class Institute (WCI) program (Grant No. WCI 2009-002) of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology of Korea (MEST). J. P. was also supported by a program (Grant No. 2011-0002726) for Basic Research in Science and Engineering. L. F. was supported by a Research Career Development Award from the Crohn’s and Colitis Foundation of America. We would like to thank Millennium Pharmaceuticals, Richard Flavell, Tak Mak and Shizuo Akira for providing mutant mice, Lesley Smythies and Timothy Cover for H. pylori strains, Grace Chen for review of manuscript and Sharon Koonse for animal husbandry.
Abbreviations used in this paper
- cagPAI
cytotoxin-associated genes pathogenicity island
- DCs
dendritic cells
- NLR
nucleotide-binding oligomerization domain-like receptors
- NLRP3
NLR pyrin domain-containing 3
- PRRs
pattern-recognition receptors
- TLRs
Toll-like receptors
- T4SS
type IV secretion system
- VacA
vacuolating cytotoxin
Footnotes
Disclosures
Luigi Franchi is an employee of Lycera, a biotech company working in the field of inflammation.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like Receptors in Host Defense and Disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 4.Girardin SE, Boneca IG, Carneiro LAM, Antignac A, Jéhanno M, Viala J, Tedin K, et al. Nod1 Detects a Unique Muropeptide from Gram-Negative Bacterial Peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 5.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, et al. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 6.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, et al. Host Recognition of Bacterial Muramyl Dipeptide Mediated through NOD2. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 7.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder K, Tschopp J. The Inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, Eigenbrod T, Núñez G. Cutting Edge: TNF-α Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 13.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 14.Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori Infection. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Cover TL. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 17.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Omar EM, Carrington M, Chow WH, McColl KEL, Bream JH, Young HA, Herrera J, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 19.Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, et al. Host and microbial constituents influence helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 20.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, et al. Overexpression of Interleukin-1β Induces Gastric Inflammation and Cancer and Mobilizes Myeloid-Derived Suppressor Cells in Mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rad R, Ballhorn W, Voland P, Eisenächer K, Mages J, Rad L, Ferstl R, et al. Extracellular and Intracellular Pattern Recognition Receptors Cooperate in the Recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 22.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 23.Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Müller A. Caspase-1 Has Both Proinflammatory and Regulatory Properties in Helicobacter Infections, Which Are Differentially Mediated by Its Substrates IL-1β and IL-18. J Immunol. 2012;188:3594–3602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 24.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 25.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1[beta] in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, et al. RICK/RIP2 Mediates Innate Immune Responses Induced through Nod1 and Nod2 but Not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 27.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 28.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, et al. A novel heterodimeric cysteine protease is required for interleukin-1[beta]processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 29.Kawazoe T, Sakagami T, Nakajima K, Hori K, Fukuda Y, Matsumoto T, Miwa H. Role of Bacterial Strain Diversity of Helicobacter pylori in Gastric Carcinogenesis Induced by N-Methyl-N-nitrosourea in Mongolian Gerbils. Helicobacter. 2007;12:213–223. doi: 10.1111/j.1523-5378.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 30.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 31.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1153–1159. doi: 10.3109/00365529509101624. [DOI] [PubMed] [Google Scholar]
- 32.Moss SF, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello CA. Interleukin-1beta. Crit Care Med. 2005;33:S460–462. doi: 10.1097/01.ccm.0000185500.11080.91. [DOI] [PubMed] [Google Scholar]
- 34.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugimoto M, Furuta T, Yamaoka Y. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J Gastroenterol Hepatol. 2009;24:1725–1732. doi: 10.1111/j.1440-1746.2009.06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]