Abstract
Background
This study followed on findings from a recent genome-wide association study of PTSD that implicated the retinoid-related orphan receptor alpha (RORA) gene (Logue et al, 2012) by examining its relationship to broader array of disorders.
Methods
Using data from the same cohort (N = 540), we analyzed patterns of association between 606 single nucleotide polymorphisms (SNPs) spanning the RORA gene and comorbidity factors termed fear, distress (i.e., internalizing factors) and externalizing.
Results
Results showed that rs17303244 was associated with the fear component of internalizing (i.e., defined by symptoms of panic, agoraphobia, specific phobia, and obsessive-compulsive disorder) at a level of significance that withstood correction for gene-wide multiple testing.
Limitations
The primary limitations were the modest size of the cohort and the absence of a replication sample.
Conclusions
Results add to a growing literature implicating the RORA gene in a wide range of neuropsychiatric disorders and offer new insight into possible molecular mechanisms of the effects of traumatic stress on the brain and the role of genetic factors in those processes.
Keywords: retinoid-related orphan receptor alpha, RORA, single nucleotide polymorphism, gene, PTSD internalizing, externalizing, fear, distress
A growing number of genome-wide association studies (GWAS) have identified the retinoid-related orphan receptor alpha (RORA) gene as a psychiatric risk locus. Investigators have linked the gene to attention-deficit hyperactivity disorder (Neale et al, 2008), bipolar disorder (Le-Niculescu et al, 2009), major depression (Garriock et al, 2010; Terracciano et al, 2010), autism (Sarachana et al, 2011) and, most recently, posttraumatic stress disorder (PTSD; Logue et al, 2012). In the latter GWAS, we found an association between a lifetime diagnosis of PTSD and a SNP in the RORA gene (rs8042149) that met both genome-wide and Bonferroni-corrected levels of significance in a Caucasian sample. Five other RORA SNPs showed suggestive evidence of association with PTSD (p< 10-5) in that sample and nominally significant associations between other RORA SNPs and PTSD were also observed in an African American subsample from the same study and a second independent African American cohort. The association between rs8042149 and PTSD has since been replicated by an independent team of investigators (Amstadter et al, in press).
RORA is an interesting new candidate gene for PTSD because of the role that it plays in neuroprotection. The RORA protein is widely expressed in psychiatrically-relevant regions of the brain including the cerebral cortex, thalamus, hypothalamus (Ino, 2004) and has been shown to protect neurons and glial cells from the degenerative effects of oxidative stress (Boukhtouche et al, 2006; Jolly et al, 2012)—a process that has been identified as a mechanism of neurodegenerative effects of traumatic stress (Oosthuizen et al, 2005; Pall, 2001; Richards et al, 2011; Schiavone et al, in press).Based on this, we hypothesized that individuals with the functional RORA risk variant may be less capable of mounting a neuroprotective response to the neurotoxic elements of oxidative stress which contributes to the functional and structural brain alterations that putatively underlie PTSD (c.f., Logue et al, 2012).
Given that RORA has been linked to a broad array of psychiatric disorders, it seems untenable to conceptualize variants in this gene as posing risk for PTSD specifically. It is more likely that polymorphisms of the gene confer a general (i.e., non-disorder-specific) risk for the development of neurobehavioral conditions broadly, or that variants in different regions of this very large gene (which spans 741,010 base pairs and includes 11 exons)are associated with risk for different forms of psychopathology. In this study, we aimed to examine these alternatives by analyzing patterns of association between 606 SNPs spanning the RORA gene and three broad classes of psychiatric illness modeled as latent dimensions of comorbidity.
One of the fundamental challenges of psychiatric genetics research is the problem of heterogeneity within the phenotypes of interest, a primary manifestation of which is diagnostic comorbidity (i.e., the presence of two or more diagnoses in the same individual). Co-occurring disorders are the rule, rather than the exception, in psychiatric samples, and PTSD shows considerable overlap and covariation with neighboring disorders. One implication of this is that multiple overlapping psychiatric phenotypes may be present in any given sample of individuals with PTSD which can potentially obscure the search for genetic risk factors.
One solution to this problem is for theoretical conceptualizations and data analytic approaches to move towards the use of hierarchical structural models in which groups of symptoms are classified at varying levels of specificity with each syndrome containing common (i.e., higher-order) and unique components. Along these lines, factor analytic studies of the structure of comorbidity have shown that the covariation of the most common mental disorders can be accounted for primarily by two broad dimensions: externalizing and internalizing. Externalizing has been defined as the latent dimension of psychopathology that explains the covariation observed between substance-related and antisocial personality disorders in adults (e.g., Krueger, 1999; Kendler et al, 2011) and the co-occurrence of conduct disorder, oppositional defiant disorder, and ADHD in children (e.g., Dick et al, 2005). Internalizing is the dimension that underlies the co-occurrence of the anxiety and unipolar mood disorders (Krueger, 1999). In many studies it has been subdivided into conceptually distinct, yet correlated, factors termed “distress” (defined by major depression, dysthymia, generalized anxiety disorder) and “fear” (comprised of panic and phobic disorders; Cox et al, 2002; Krueger, 1999; Slade and Watson, 2006).
Twin studies have shown these dimensions to have substantial heritabilities (Krueger et al, 2002; Young et al, 2000; Wolf et al, 2010) with estimates generally higher for these traits than for the individual DSM disorders that define them. For example, while estimates o fthe heritability of individual externalizing disorders have generally fallen in the .18 - .66 range (Kendler et al, 2003), published heritability estimates for externalizing are in the .43 - .84 range (Krueger et al, 2002; Wolf et al, 2010; Young et al, 2000; Young et al, 2009). Furthermore, studies that have directly compared estimates of the heritability of internalizing versus externalizing have found stronger genetic contributions to externalizing and, conversely, higher estimates of non-shared environmental influences on internalizing (e.g., Kendler et al, 2003; Wolf et al, 2010).
Internalizing and externalizing can be conceptualized as endophenotypic traits which, in theory, are expected to map more directly and completely onto their underlying genetic substrate compared to individual DSM disorders. Building on this, the primary objective of this study was to apply this model in a genetic association analysis of RORA. Given RORA’s established role in neuroprotection, along with evidence for a greater contribution of non-shared environmental influences (e.g., adverse life events) to internalizing, we hypothesized that RORA (as a moderator of the molecular stress response) would be more strongly associated with measures of internalizing than with externalizing. Though the extant literature did not suggest differential predictions for the relationship of RORA o the fear versus distress components of internalizing, we modeled them separately on the basis of research and theory pointing to their distinct etiologies and mechanisms (e.g., McTeague and Lang, 2012; Patrick et al, in press; Vaidyanathan et al, 2009; Watson, 2005).
Methods and Materials
Participants
Eight hundred fifty-two participants enrolled in one of two VA studies. The first enrolled trauma-exposed military veterans who screened positive for PTSD; the second included military veterans with trauma histories and their cohabitating partners. Both studies involved comprehensive psychiatric diagnostic assessments and blood sample collection. See additional details in Logue et al (2012). The statistical program STRUCTURE (Falush et al, 2003; Pritchard et al, 2000) was used to identify a subgroup of 540 White, non-Hispanic participants on the basis of a Bayesian cluster analysis of 10,000 randomly chosen SNPs with minor allele frequency (MAF)> .05 from the full sample. The Caucasian sample was comprised of 375 veterans and 165partners. The majority was male (60.4%) and the mean age was 51.88 years (range: 21 – 75, SD: 11.09). Participants reported exposure to a wide variety of traumatic events on the Traumatic Life Events Questionnaire (Kubany et al., 2000) with exposure to multiple events over the course of the lifespan the norm. The events most frequently endorsed by male participants were sudden death of a loved one (56.8%), combat (52.9%), and accidents other than those involving motor vehicles (44%). For women, the most frequently endorsed events were sudden death of a loved one (61.2%), a life threatening incident involving a loved one (45.2%), and childhood and/or adult sexual assault (44.9%).
Measures
Structured Clinical Interview for DSM-IV (SCID-IV; First et al, 1994)
Lifetime Axis I disorders were assessed with the SCID-IV and dimensional scores for each diagnosis by summing scores across symptoms within a module. All interviews were videotaped for the purposes of evaluating diagnostic reliability.
Adult Antisocial Behavior
Adult antisocial behavior was assessed in the veteran-only study using the International Personality Disorder Examination (IPDE; Loranger, 1999). In the couples study, it was assessed using the SCID-II (SCID-II; First et al, 1995). To create a single adult antisocial scale across the two measures, the summary score from matching items on each measure were standardized and then combined. In the subset of participants who completed the IPDE (n = 181), this variable correlated .99 with the full adult antisocial behavior severity score on the IPDE and.71 with total antisocial personality disorder symptom severity (i.e., adult symptoms + child conduct disorder symptoms).
The Clinician Administered PTSD Scale (CAPS; Blake et al, 1990)
The CAPS is a 30-item structured diagnostic interview that assesses the frequency and severity of the 17 DSM-IV PTSD symptoms, 5 associated features, and functional impairment. Dimensional lifetime severity scores were calculated by summing the frequency and intensity ratings (each range from 0-4) for each of the 17 items (possible range: 0-136; Weathers et al, 1999).
Procedure
This research was approved and reviewed annually by the appropriate human subjects and institutional review boards. Participants were recruited through medical databases, flyers, clinician referrals, and a database of veterans who had previously consented to be contacted for research. All participants provided written informed consent and were compensated for their time. Interviewers were advanced psychology graduate students, post-doctoral clinical psychology trainees, and licensed clinical psychologists all of whom received extensive training prior to data collection. All interviews were video-recorded and approximately 25% were later viewed by blind independent raters for purposes of maintaining quality control and evaluating inter-rater reliability. Reliability statistics for diagnostic variables used in these analyses are listed in Table 1.
Table 1. Lifetime Diagnostic Prevalence and Symptom Severity Grouped by Psychopathology Factor.
| Diagnosis | Prevalence (%) | Mean Severity (SD) | Severity Range | Reliability Kappa | Intraclass Correlation |
|---|---|---|---|---|---|
| Distress Disorders | |||||
| Major Depressive | 51.5 | 9.69 (5.84) | 0-18 | .86 | .96 |
| Dysthymia* | 15.7 | 4.90 (4.62) | 0-14 | .78 | .94 |
| Generalized Anxiety | 9.9 | 5.90 (5.16) | 0-16 | .84 | .94 |
| Fear Disorders | |||||
| Panic | 15.7 | 10.87 (9.72) | 0-32 | .71 | .97 |
| Agoraphobia | 13.4 | .81 (1.56) | 0-4 | .69 | .88 |
| Specific Phobia | 11.9 | 3.53 (3.90) | 0-10 | .73 | .96 |
| Obsessive-Compulsive | 3.5 | 1.03 (2.69) | 0-14 | .72 | .88 |
| Externalizing Disorders | |||||
| Adult Antisocial Behavior | 5.6 | 3.06 (3.46) | 0-16 | .89 | .95 |
| Alcohol Abuse/Dependence | 55.6 | 7.69 (7.63) | 0-24 | .87 | .98 |
| Cannabis Abuse/Dependence | 17.0 | 1.13 (2.52) | 0-12 | .77 | .90 |
| Cocaine Abuse/Dependence | 14.0 | 1.49 (3.77) | 0-22 | .97 | .89 |
| Not Modeled in the Factor Analysis | |||||
| Posttraumatic Stress Disorder | 55.0 | 55.46 (34.64) | 0-132 | .87 | .97 |
Note. Prevalence and Severity information based on the subset of n = 540 White, non-Hispanic participants who were included in these analyses. Reliability statistics were based on approximately 25% of the full sample.
Measure assesses symptoms within the past two years only.
Genotyping
DNA was isolated from peripheral blood samples on a Qiagen AutoPure instrument with Qiagen reagents and samples normalized using PicoGreen assays (Invitrogen). Samples were run on an Illumina OMNI 2.5-8 array and scanned using an Illumina HiScan System according to the manufactures protocol. Details on call rates, elimination of participants, and evaluation of biological sex using X-chromosome homozygosity are described in detail elsewhere (Logue et al, 2012).
SNPs on the RORA gene were eliminated if they yielded greater than 5% missing genotypes or if they were rare (< 5% MAF). Of the 973 SNPs genotyped within 5 kb of RORA, 607 had minor allele frequencies greater than 5%. Of these, one SNP failed the Hardy-Weinberg Equilibrium (HWE) test (i.e., p< 8.24x10-05, the Bonferroni corrected p-value for 607 SNPs) and was excluded from further analyses. Linkage disequilibrium (LD) across the remaining 606 SNPs was evaluated using Haploview (Barrett et al, 2005).
Statistical Analyses
We first performed confirmatory factor analysis using Mplus 6.12 (Muthén and Muthén, 2011)to model the fear, distress, and externalizing latent variables using symptom summary (i.e., dimensional) scores for each diagnosis. The indicators for Fear were lifetime panic disorder, agoraphobia, specific phobia, and obsessive-compulsive disorder. Distress was defined by depression, dysthymia, and generalized anxiety disorder. Externalizing indicators were adult antisocial personality disorder and alcohol, cannabis, and cocaine abuse/dependence. The residuals for cannabis and cocaine abuse/dependence were allowed to correlate because they were based on items with virtually identical structure and wording. PTSD was not modeled because we have already reported its association with RORA (Logue et al, 2012).
Possible factor-associated population substructure within the Caucasian subsample was examined using principal components (PC) analysis in the program EIGENSTRAT (Price et al., 2006) and these components were included in initial analyses predicting each of the three phenotypes.
Association between each of the three latent variables and the 606 SNPs in RORA that passed minor allele frequency and HWE test filters was then assessed in three separate analyses in PLINK (Purcell et al., 2007). In each analysis, the max(T) permutation procedure with 5000 replications was used to correct for multiple testing across the 606 RORA SNPS. SNPs that showed association after correction for multiple testing in PLINK were then further evaluated using the PLINK linear regression test to examine effects of relevant covariates.
Finally, we also performed a multiple-testing correction that took into account comparisons for the 606 RORA SNPs across all three factor scores simultaneously. A permutation simulation to determine significance was computed in R (R Development Core Team, 2008) similar to that performed by PLINK's Max(T) procedure (i.e., genotypes for all subjects were permuted 5,000 times) except additionally correcting over all of the examined phenotypes. That is, for each permutation, association was assessed for all SNPs and all 3 traits and the minimum p-value was recorded. The corrected p-value was computed by determining the percentile of the observed (single-SNP single trait) p-value when compared to this empirical minimum p-value distribution. This procedure is less conservative than a Bonferroni correction, as it properly accounts for the correlation between SNPs (linkage disequilibrium) and the correlated nature of the three traits examined.
Results
Prevalence of the various lifetime diagnoses along with means and SDs for symptom severity scores and inter-rater reliability statistics for each diagnosis is listed in Table 1.
Confirmatory Factor Analysis
A3-factor confirmatory factor analysis of the 11 SCID diagnoses yielded excellent model fit (X2= 53.11 (df = 40), p= .08; RMSEA = .03; SRMR = .03; CFI = .99; TLI = .98).1 All diagnostic indicators loaded significantly on their respective latent variables (all p< .001), with standardized factor loadings ranging from .36 for obsessive-compulsive disorder symptoms to .85 for panic disorder symptoms. Fear was high correlated with Distress (r = .74, p< .001) but was less strongly associated with Externalizing (r = .35, p< .001); Distress was moderately associated with Externalizing (r = .47, p< .001).
Associations between RORA SNPs and Fear, Distress and Externalizing Factor Scores
Prior to testing associations between RORA SNPs and the three psychopathology traits, we examined possible effects of population substructure using principal components (PC) analysis in the program EIGENSTRAT. None of the top 10 PCs were significantly associated with the Fear, Distress or Externalizing factor scores.
Fear
16 of the606 RORA SNPs were associated with Fear factor scores at the p< .01 level (See Supplementary Table). The Manhattan Plot depicting results for all 3 traits is shown in Figure 1. One SNP withstood correction for multiple testing across the gene. Specifically, rs17303244 evidenced a significant association with Fear with the minor allele (G) related to higher levels of fear disorder symptomatology (unstandardized β = 3.303, R2 = .03, T = 4.065, unadjusted p = .000055, adjusted p = .021; MAF = 8%). Homozygosity for the minor allele was present in only 3 participants (0.06%) so to ensure that these rare cases were not exert undue influence on results, we eliminated these cases and reran the analysis. Results were unchanged from that for the full sample so these three cases were retained in subsequent analyses.
Figure 1.
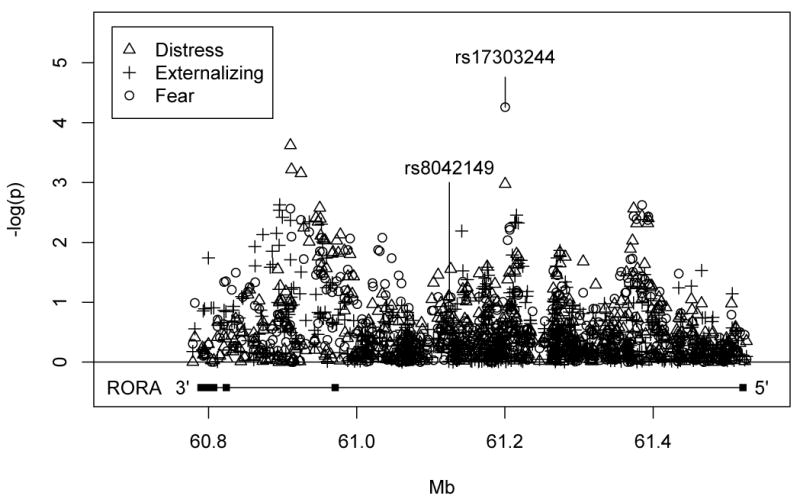
Manhattan plot depicting association results across the RORA gene for the 3 traits tested.
Next, we examined the association between rs17303244 and Fear factor scores with PTSD severity and Sex included as covariates in the model. Results showed that both PTSD severity (unstandardized β = .11, p = 1.204 X 10-34) and Sex (unstandardized β = -1.36, p= .02) were were significant covariates of Fear, and rs17303244 remained significant (unstandardized β ½ 2.52, p ν .0004) with them included in the model.2
Distress
Twenty-one of the 606 SNPs on RORA showed initial evidence of association with Distress factor scores at the p< .01 level, but none of these withstood permutation testing.
Externalizing
Fifteen SNPs on RORA showed initial evidence of association with the Externalizing factor scores at the p<.01 level, but again, none withstood correction for multiple testing.
rs17303244 Associations with Individual Fear Disorders
As noted in the introduction, we focused our analyses on latent dimensions of psychopathology because, in theory, they can be expected to map more directly and completely onto their genetic substrate compared to individual DSM disorders. We tested this assumption by comparing the proportion of variance in Fear factor scores explained by rs17303244to the proportion of variance in each of the individual disorders that comprised the Fear factor accounted for by the SNP. Results showed thatrs17303244 explained 3% of the variance in Fear factor scores (p = .000055), 2.5% of the variance in panic disorder severity (p = .003182), 2.0% of the variance in OCD severity (p = .001176), 1.8% of the variance in specific phobia severity (p = .002007), and 1.2% of the variance in agoraphobia severity (p = .010610).
Permutation testing simultaneously adjusting for all three trait
Finally, when we performed a permutation test that adjusted for comparisons across the three latent phenotypes(Fear, Distress, Externalizing) simultaneously we found that the association between fear factor scores and rs17303244 narrowly missed this highly conservative multiple-testing corrected level of significance (p=0.057).
Discussion
We recently reported findings from a GWAS of PTSD that revealed a genome-wide significant association between a SNP on the RORA gene (rs8042149) and lifetime PTSD (Logue et al, 2012). Since then, this finding has been replicated by other investigators (Amstadter et al, in press). Though pointing to a novel and potentially fruitful avenue for future research on the genetics of traumatic stress, these studies were limited by their exclusive focus on PTSD leaving open the question of whether RORA’s association is specific to this disorder or perhaps linked to other comorbid conditions as well. We addressed this question by analyzing patterns of association between 606 SNPs spanning the RORA gene and latent dimensions of psychopathology termed fear and distress (internalizing factors) and externalizing. Building on prior research on the structure of PTSD comorbidity (e.g., Miller et al, 2012; Miller et al, 2008; Wolf et al, 2010) and our hypotheses about the molecular mechanism of RORA’s relationship to PTSD (Logue et al, 2012), we expected to find stronger associations between RORA and internalizing than externalizing.
Results supported this hypothesis. Analyses revealed that RORA SNP rs17303244 was associated with factor scores representing the fear spectrum disorders(i.e., defined by panic, agoraphobia, specific phobia, and obsessive-compulsive disorder) at a level of significance that withstood correction for multiple testing across 606 SNPs. The fear-associated SNP, rs17303244, was not significantly associated with either distress or externalizing, and though nominally significant associations between other RORA SNPs and the three latent traits were observed, none of them withstood multiple testing corrections. Given the high prevalence of lifetime PTSD in this sample (55%) and the moderate positive correlations between PTSD and fear spectrum disorders in this and other studies (e.g., Miller et al, 2008; Miller et al, 2012), we then examined the specificity of the fear-rs17303244 association by including PTSD and sex as covariates in the analysis. rs17303244remained a significant predictor of fear factor scores even after controlling for the effects of these variables. Furthermore, secondary analyses that examined rs17303244’s associations with each of the individual fear disorders showed this SNP to be more strongly associated with the fear factor than with any of the individual disorders that defined it. Though the magnitudes of these differences were modest, this finding points to the potential value of incorporating hierarchical structural models of psychopathology into future psychiatric molecular genetic studies.
rs17303244 is located at 61,200,300 bp on chromosome 15 (419,817 bp from the start of the gene) in an intronic region and it is 75,347 bp away from the PTSD GWAS SNP (rs8042149). Examination of the LD structure for rs17303244based on data from Europeans in 1,000 genomes (The 1000 Genomes Project, 2008-2012) revealed that it was not in high LD with rs8042149 (i.e., D’ = .12, R2 = .002) nor with any of approximately two dozen other RORA SNPs that have been linked to psychiatric and neurological disorders or structural brain parameters in prior studies (details available from first author). This implies that rs17303244 is a novel psychopathology risk locus and that the association observed in this study is not simply reiterating effects seen previously with other SNPs that are in LD with this one.
Although the functional variant(s) within RORA that is responsible for the associations with fear-related psychopathology and/or PTSD is unknown, knowledge of the role that RORA plays in neuroprotection points to a possible mechanism by which RORA might confer risk for the development of trauma- and stress-related disorders. Specifically, we hypothesize that RORA moderates the effects of traumatic stress though its influence on oxidative stress—the process that occurs when excessive reactive oxygen species (ROS) and free radicals, including nitric oxide, damage cells causing apoptosis (programmed cell death). Production of excessive ROS is a normal process that occurs in response to inflammatory stimuli because of the ability of ROS to kill bacteria and virus. Abnormalities in this process have been implicated in neurodegenerative conditions such as Parkinson’s disease, Alzheimer’s disease and Multiple Sclerosis.
Emerging evidence suggests that oxidative stress may also play a role in the effects of traumatic stress on the brain (Oosthuizen et al, 2005; Richards et al, 2011; Schiavone et al, in press). Glucocorticoids are anti-inflammatory hormones released during stress to prevent the inflammatory response from becoming pathologically over activated and they exert and inhibitory influence on activity of the hypothalamic-pituitary-adrenal axis (Sapolsky et al, 2000) At the same time, glucocorticoids induce glucose levels that when metabolized, leads to increases in ROS. In neurons they also trigger the release of inducible nitric-oxide synthase (Sato et al, 2010) leading to the formation of long-lived neurotoxic oxidant species such as peroxynitrite.
The RORA protein has four isoforms, one of which is expressed primarily in the central nervous system and found in cell nuclei in brain regions including the frontal cortex, hippocampus, and hypothalamus (Ino, 2004; Nguyen et al, 2010). Its expression is activated during oxidative stress (Zhu et al, 2006) and it protects neurons from apoptosis by increasing the expression of genes involved in the clearance of reactive oxygen species (Gpx1 and Prx6; Boukhtoche et al, 2006). We suspect that the neurons of individuals carrying the RORA risk variant(s) mount an abnormal response to oxidative stress leading to neurodegeneration and functional abnormalities in regions of the brain subserving fear- and anxiety-related psychopathology. Consistent with this, RORA SNP variants have been linked in genetic-imaging studies to global measures of cortical thickness and fractional anisotropy of cerebral white matter (Kochunovet al, 2011) as well as to volume of the entorhinal cortex, the main interface between the hippocampus and neocortex (Furneyet al, 2011). Moreover, in the latter study, rs17237318, which was nominally associated with all 3 phenotypes examined in this study, was highly correlated with Alzheimer’s disease related atrophy (p = 9.63x10-6). Thus, we believe it will be usefulin future research to examine the role that RORA plays in moderating the effects of traumatic stress on the volume and integrity of brain regions where the RORA protein is expressed. That said, the function of the RORA gene is very complex and the protein encoded by it involved in a variety of other behaviorally- and psychiatrically-relevant processes including the regulation of circadian rhythms and steroid hormones (for a review, see Jetten, 2009). Evidence suggests that these processes are dysregulated in patients with PTSD (Germain, 2013; Rasmusson et al., 2010) and may, therefore, represent alternative mechanisms by which RORA confers risk for the disorder.
Limitations and Conclusion
These conclusions should be weighed in light of the study’s strengths and limitations. The primary limitations were the modest size of the cohort and the absence of a replication sample. In addition, though we applied a conservative correction for repeated-testing based on the large number of RORA SNPs that we examined (606), as in any study of this type, it is possible that the rs17303244 association with fear-related psychopathology was a false positive result due to chance. Therefore, findings from this study should be considered provisional until evidence of replication is obtained. With respect to study strengths, our focus on three well-established latent psychopathology traits was novel. The study also featured a clinical sample with a high base-rate of PTSD and other disorders and a comprehensive diagnostic assessment based on clinician-administered interviews. The dense coverage of SNPs spanning the RORA gene from a high-density array enabled more comprehensive analyses than most targeted gene association studies. To conclude, results of this study add to a growing literature implicating the RORA gene in the development of neuropsychiatric disorders and offers new insight into possible molecular mechanisms of the effects of traumatic stress on the brain and the role of genetic factors in moderating those processes.
Supplementary Material
Table 2.
Bivariate Correlations between Symptom Severity Scores for Disorders Modeled in the Factor Analysis
| Disorder | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Major Depressive | -- | |||||||||
| 2. Dysthymia | .36 | -- | ||||||||
| 3. Generalized Anxiety | .38 | .41 | -- | |||||||
| 4. Panic | .36 | .40 | .43 | -- | ||||||
| 5. Agoraphobia | .24 | .29 | .27 | .57 | -- | |||||
| 6. Specific Phobia | .12 | .12 | .16 | .30 | .27 | -- | ||||
| 7. Obsessive-Compulsive | .24 | .26 | .23 | .26 | .28 | .17 | -- | |||
| 8. Adult Antisocial Behavior | .23 | .22 | .23 | .20 | .14 | .07 | .10 | -- | ||
| 9. Alcohol Abuse/Dependence | .20 | .21 | .12 | .26 | .13 | .03 | .12 | .49 | -- | |
| 10.Cannabis Abuse/Dependence | .17 | .15 | .11 | .14 | .04 | -.02 | .09 | .32 | .32 | -- |
| 11. Cocaine Abuse/Dependence | .18 | .19 | .12 | .19 | .08 | .04 | .10 | .37 | .31 | .44 |
Footnotes
We also modeled a 2-factor (Internalizing versus Externalizing) structure and found significantly poorer fit compared to the 3-factor one so retained the latter for the genetic association analyses.
Though Fear disorders tend to be more prevalent among women than in men in epidemiological samples, in this clinical sample of veterans and their intimate partners, all diagnoses were more prevalent among men than women.
References
- Amstadter AB, Sumner JA, Acierno R, Ruggiero KJ, Koenen KC, Kilpatrick DG, et al. Support for association of RORA Variant and Posttraumatic Stress Symptoms in a Population-Based Study of Hurricane Exposed Adults. Mol Psychiatry. doi: 10.1038/mp.2012.189. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Charney D, Keane T. The Clinician-Administered PTSD Scale - IV. National Center for PTSD, Behavioral Sciences Division; Boston, MA: 1990. [Google Scholar]
- Boukhtouche F, Vodjdani G, Jarvis CI, Bakouche J, Staels B, Mallet J, et al. Human retinoic acid receptor-related orphan receptor alpha1 overexpression protects neurons against oxidative stress-induced apoptosis. J Neurochem. 2006;96:1778–1789. doi: 10.1111/j.1471-4159.2006.03708.x. [DOI] [PubMed] [Google Scholar]
- Cox BJ, Clara IP, Enns MW. Posttraumatic stress disorder and the structure of common mental disorders. Depress Anxiety. 2002;15:168–171. doi: 10.1002/da.10052. [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: Genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. J Abnorm Child Psychol. 2005;2005:219–229. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders. Biometrics Research; New York, NY: 1994. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part I: Description. J Personal Disord. 1995;9:83–91. [Google Scholar]
- Furney SJ, Simmons A, Breen G, Pedroso I, Lunnon K, Proitsi P, et al. Genome-wide association with MRI atrophy measures as a quantitative trait locus for Alzheimer’s disease. Mol Psychiatry. 2011;16:1130–1138. doi: 10.1038/mp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock HA, Kraft JB, Shyn SI, Peters EJ, Yokoyama JS, Jenkins GD, et al. A genome-wide association study of citalopram response in major depressive disorder. Biol Psychiatry. 2010;67:133–138. doi: 10.1016/j.biopsych.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170:372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino H. Immunohistochemical characterization of the orphan receptor ROR alpha in the mouse nervous system. J Histochem Cytochem. 2004;52:311–323. doi: 10.1177/002215540405200302. [DOI] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nuclear Receptor Signaling. 2009;7 doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S, Journiac N, Vernet-der Garabedian B, Mariani J. RORalpha, a key to the development and functioning of the brain. Cerebellum. 2012;11:451–452. doi: 10.1007/s12311-011-0339-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am J Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Nichols TE, Winkler AM, Hong EL, Holcomb HH, et al. Genetic analysis of cortical thickness and fractional anisotropy of water diffusion in the brain. Front Neurosci. 2011;5:120. doi: 10.3389/fnins.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief-broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychol Asess. 2000;12:411–424. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Patel SD, Bhat M, Kuczenski R, Faraone SV, Tsuang MT, et al. Convergent functional genomics of genome-wide association data for bipolar disorder: comprehensive identification of candidate genes, pathways and mechanisms. Am J Med Genet. 2009;150B:155–181. doi: 10.1002/ajmg.b.30887. [DOI] [PubMed] [Google Scholar]
- Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, et al. A genome-wide association study of posttraumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.113. e-pub ahead of print 7 August 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger AW. International Personality Disorder Examination: DSM-IV and ICD-10 Interviews. Psychological Assessment Resources, Inc; Lutz, FL: 1999. [Google Scholar]
- McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety. 2012;29:264–281. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Fogler J, Wolf EJ, Kaloupek DG, Keane TM. The internalizing and externalizing structure of psychiatric comorbidity in combat veterans. J Trauma Stress. 2008;21:58–65. doi: 10.1002/jts.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Wolf EJ, Reardon AF, Greene A, Ofrat S, McInerney S. Personality and the latent structure of PTSD comorbidity. J Anxiety Disord. 2012;26:599–607. doi: 10.1016/j.janxdis.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 6. Muthén & Muthén; Los Angeles, CA: 2011. [Google Scholar]
- Neale BM, Lasky-Su J, Anney R, Franke B, Zhou K, Maller JB, et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet. 2008;147B:1337–1344. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010;24:3036–3051. doi: 10.1096/fj.10-154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen F, Wegener G, Harvey BH. Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): evidence from an animal model. Neuropsychiatr Dis Treat. 2005;1:109–123. doi: 10.2147/nedt.1.2.109.61049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall ML. Common etiology of posttraumatic stress disorder, fibromyalgia, chronic fatigue syndrome and multiple chemical sensitivity via elevated nitric-oxide / peroxynitrite. Med Hypotheses. 2001;57:139–145. doi: 10.1054/mehy.2001.1325. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. J Abnorm Psychol. doi: 10.1037/a0032807. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . The R Project for Statistical Computing 2008 [Google Scholar]
- Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adaptation to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med. 2010;10:1150–1162. doi: 10.1258/ebm.2010.009334. [DOI] [PubMed] [Google Scholar]
- Richards RS, Nwose EU, Bwititi P. Biochemical basis of circadian rhytms and diseases: With emphasis on post-traumatic stress disorder. Med Hypotheses. 2011;77:605–609. doi: 10.1016/j.mehy.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sarachana T, Xu M, Wu RC, Hu VW. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PloS One. 2011;6:e17116. doi: 10.1371/journal.pone.0017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function in rats. J Clin Biochem Nutr. 2010;47:224–243. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone S, Jaquet V, Trabace L, Krause K. Severe life stress and oxidative stress in the brain: From animal models to human pathology. AntioxidRedox Signal. doi: 10.1089/ars.2012.4720. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade T, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychol Med. 2006;36:1593–1600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Tanaka T, Sutin AR, Sanna S, Deiana B, Lai S, et al. Genome-wide association scan of trait depression. Biol Psychiatry. 2010;68:811–817. doi: 10.1016/j.biopsych.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 1,000 Genomes Project (2008-2012) 1000 Genomes: A Deep Catalog of Human Genetic Variation. http://www1000genomesorg/
- Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychol Bull. 2009;135:909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. J Abnorm Psychol. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assess. 1999;11:124–133. [Google Scholar]
- Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, Koenen KC. Posttraumatic stress disorder and the genetic structure of comorbidity. J Abnorm Psychol. 2010;119:320–331. doi: 10.1037/a0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, et al. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, McAvoy S, Kuhn R, Smith DI. RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene. 2006;25:2901–2908. doi: 10.1038/sj.onc.1209314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


