Abstract
Rationale
The α4β2 subtype of nicotinic acetylcholine receptors (nAChRs) plays a central role in the mediation of nicotine reinforcement. Positive allosteric modulators (PAMs) at α4β2 nAChRs facilitate the intrinsic efficiency of these receptors although they do not directly activate the receptors. α4β2 PAMs are hypothesized to reduce nicotine self-administration in subjects engaged in routine nicotine consumption. The present study tested this hypothesis using a rat model of nicotine self-administration.
Methods
Male Sprague-Dawley rats were trained in daily 1 h sessions to intravenously self-administer nicotine (0.03 mg/kg/infusion, free base) on a fixed-ratio 5 schedule. Effects of the α4β2 PAM desformylflustrabromine (dFBr), α4β2 agonist 5-iodo-A-85380, and acetylcholinesterase inhibitor galantamine on nicotine intake were examined. The ability of dFBr and 5-iodo-A-85380 to substitute for nicotine was also assessed.
Results
dFBr and 5-iodo-A-85380 dose-dependently reduced nicotine self-administration without changing lever responses for food. Galantamine decreased self-administration of nicotine and food at high doses. Unlike 5-iodo-A-85380, dFBr failed to substitute for nicotine in supporting self-administration behavior.
Conclusions
These results demonstrated the effectiveness of dFBr in reducing nicotine intake and the inability of dFBr to support self-administration behavior. These findings suggest that positive allosteric modulation of α4β2 nAChRs may be a promising target for the treatment of nicotine addiction. Moreover, α4β2 PAMs, in contrast to agonist medications, may have clinical advantages because they may have little liability for abuse because of their lack of reinforcing actions on their own.
Keywords: 5-iodo-A-85380, desformylflustrabromine, galantamine, nicotine, nicotinic acetylcholine receptors (nAChRs), positive allosteric modulator, self-administration
Introduction
Tobacco smoking is a leading preventable cause of death in the United States. Currently, approximately 22% of adults and 24% of youths smoke tobacco (CDC 2008). Although most smokers want to quit smoking, only very few smokers can remain abstinent in their attempts to quit without treatment. Even when treated with the three medications approved by the U.S. Food and Drug Administration (i.e., nicotine replacement, bupropion, and varenicline), long-term abstinence rates remain unsatisfactorily low (Aubin et al. 2008; Gonzales et al. 2006; Jorenby et al. 2006). Therefore, greater effort is needed to develop new strategies to curb the intake of nicotine and other hazardous chemicals contained in tobacco smoke.
Nicotine exerts its reinforcing actions by activating nicotinic acetylcholine receptors (nAChRs). nAChRs have 12 subunits: nine α-subunits (α2–α10) and three β-subunits (β2–β4). These subunits assemble nAChRs into hetero- and homo-pentameric ion channels. In the mammalian brain, the most abundant and widespread nAChRs are heteromeric α4β2- and homomeric α7-containing receptors (Sargent 1993; Zoli et al. 1998). α4β2 nAChRs play a pivotal role in mediating the rewarding effects of nicotine and the nicotine addiction process (Exley and Cragg 2008; Mineur and Picciotto 2008; Tapper et al. 2004; Vieyra-Reyes et al. 2008; Wonnacott et al. 2005). Recent studies have shown that the nAChRs containing other subunits such as α3, α5, α6 also participate in nicotine reinforcement (Brunzell 2012; Picciotto and Kenny 2012; Toll et al. 2012; Tuesta et al. 2011). In contrast, research on involvement of the α7 nAChRs in nicotine reward has produced mixing results (Besson et al. 2012; Brunzell and McIntosh 2012; Grabus et al. 2006; Grottick et al. 2000; Markou and Paterson 2001; Pons et al. 2008; Stolerman et al. 2004; van Haaren et al. 1999; Walters et al. 2006). Up to date, the α4β2 subtype of nAChRs has been targeted for the development of nicotinic agents for the treatment of nicotine addiction. Varenicline is a partial agonist at α4β2 AChRs (Cahill et al. 2011; Coe et al. 2005). Bupropion exerts its effects via antagonism of α4β2 nAChRs, in addition to its inhibition of monoamine transporter reuptake (Fryer and Lukas 1999; Li et al. 2002; Nomikos et al. 1989; Slemmer et al. 2000).
One unique class of agents is referred to as positive allosteric modulators (PAMs). They bind nAChRs at specific motifs (allosteric sites) that are distinct from the orthosteric sites for nicotine and endogenous acetylcholine. The binding of PAMs at nAChRs substantially enhances receptor activity, including the affinity for ligands, intrinsic efficacy of the receptors, and probability of channel opening induced by nicotine and acetylcholine (Ehlert 2005). Thus, PAMs can substantially enhance and prolong the responses of nAChRs to both nicotine from tobacco smoke and endogenous acetylcholine. Based on the unique mechanism by which PAMs facilitate the activity of nAChRs, these PAMs may reduce the amount of nicotine self-administered through smoking in subjects engaged in routine nicotine consumption. A smoker treated with a PAM may experience the same level of the subjective reinforcing effects of nicotine while consuming less nicotine, thereby reducing tobacco intake because of their decreased need for nicotine intake.
To test this hypothesis, the present study used an intravenous nicotine self-administration paradigm to examine the effects of a PAM, desformylflustrabromine (dFBr), at α4β2 nAChRs on nicotine intake in rats. Desformylflustrabromine was isolated from the marine bryozoan Flustra foliacea (Lysek et al. 2002) in 2002 and then found to potentiate the actions of human nAChRs (Sala et al. 2005). In its subsequent characterization, dFBr proved to be a highly selective PAM at α4β2 nAChRs and substantially increase ionic current through α4β2 nAChRs. In in vitro systems, dFBr increased the electrophysiological response to nicotine and acetylcholine by approximately three- to seven-fold (Kim et al. 2007; Weltzin and Schulte 2010). In the present study, the effects of dFBr on nicotine intake were examined after rats were trained to regularly self-administer nicotine, thus modeling human subjects who are engaged in routine nicotine consumption. In a separate groups of rats, the effects of the α4β2 nAChR-selective agonist 5-iodo-A-85380 (Mukhin et al. 2000) and galantamine, a PAM at α7 nAChRs and acetylcholinesterase inhibitor (Maelicke et al. 2000; Schrattenholz et al. 1996), were also examined. Because the half-life of these compounds ranged from 1.9 to 8.6 hours (Goh et al. 2011; Ueda et al. 2004; van Beijsterveldt et al. 2004; present study), the drug test sessions were scheduled on every other day in order to eliminate any carry-over effect of these compounds and for lever responses to return to baseline level prior to each test session.
Given that PAMs at α4β2 nAChRs do not directly activate the receptors (Fant et al. 2009; Houtsmuller et al. 2002; Hughes 1989; West et al. 2000), they should not be able to produce reinforcing actions on their own and therefore have little liability for abuse or dependence. To support such a prediction, the present study also examined whether dFBr is able to substitute for nicotine in nicotine-trained rats, and comparisons were made with the α4β2 agonist 5-iodo-A-85380. Based on our previous work showed that operantly trained rats readily acquired self-administration of 5-iodo-A-85380 (Liu et al. 2003), 5-iodo-A-85380 was predicted to substitute for nicotine in self-administration behavior, demonstrating its reinforcing actions.
Materials and methods
Animals
Male Sprague-Dawley rats (Charles River, Portage, MI), 201–225 g upon arrival, were used. The animals were individually housed in a humidity- and temperature-controlled (21–22°C) colony room maintained on a 12 h/12 h reverse light/dark cycle with the lights off at 8:00 AM. After 1 week of habituation, the rats were placed on a food-restriction regimen (20 g chow/day) throughout the experiments, which allowed them to have consistent but low weight gain of approximately 85% of the free-fed condition. The rats had unlimited access to water. The training and experimental sessions were conducted during the dark phase at the same time each day (9:00 AM–3:00 PM). All of the experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Self-administration apparatus
Experimental sessions were conducted in standard operant conditioning chambers located inside sound-attenuating, ventilated cubicles (Med Associates, St. Albans, VT). The chambers were equipped with two retractable response levers on one side panel, a 28-V white light above each lever, and a red house light on top of the chambers. Between the two levers was a food pellet trough. Intravenous nicotine injections were delivered by a drug delivery system with a syringe pump (Med Associates, model PHM100-10 rpm). Experimental events and data collection were automatically controlled by a computer and software (Med Associates, Med-PC version IV).
Lever-press training
To facilitate the learning of operant responding for nicotine self-administration (see below), the rats underwent lever-press training. One day after the start of the food-restriction regimen, the rats were placed in the experimental chambers, and the training sessions began with introduction of the levers. Responding on the active lever was rewarded with the delivery of a food pellet (45 mg). The sessions lasted 1 h with a maximum delivery of 45 food pellets on a fixed-ratio 1 (FR1) schedule. After the rats learned to respond, the reinforcement schedule was increased to FR5. The training finished once rats earned 45 food pellets on the FR5 schedule in a single session. Successful lever-press training was achieved within 2–5 sessions.
Surgery
The rats were anesthetized with isoflurane (1–3%) in 95% O2 and 5% CO2. An indwelling intravenous catheter was inserted into the right external jugular vein. The catheters were constructed using a 15 cm piece of Silastic tubing (0.30 mm inner diameter, 0.64 mm outer diameter; Dow Corning Corporation, Midland, MI, USA) attached to a 22-gauge stainless-steel guide cannula. The latter was bent and molded onto durable polyester mesh (Plastics One, Roanoke, VA, USA) with dental cement and became the catheter base. Through an incision on the rat’s back, the base was anchored underneath the skin at the level of the scapulae, and the catheter passed subcutaneously to the ventral lower neck region and inserted into the right jugular vein (3.5 cm). The animals were allowed at least 7 days to recover from surgery. During the recovery period, the catheters were flushed daily with 0.1 ml sterile saline that contained heparin (30 U/ml) and timentin (66.7 mg/ml) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with heparinized saline before and after the experimental sessions.
Nicotine self-administration
After recovery from surgery, the rats were trained in daily 1 h sessions to self-administer nicotine at a unit dose of 0.03 mg/kg/infusion (free base). During the training sessions, the animals were placed in the experimental chambers and connected to a drug delivery system. The daily 1 h sessions were initiated by extension of the two levers and illumination of the red house light. Once the rats reached the FR requirement on the active lever, an infusion of nicotine was dispensed by the drug delivery system in a volume of 0.1 ml over approximately 1 s, depending on the rat’s body weight. Each nicotine infusion was paired with the presentation of a stimulus that consisted of 5 s tone and 20 s lever light on. Following each nicotine delivery was a 20 s timeout period, during which time responses were recorded but not reinforced. Responses on the inactivate lever had no programmed consequence. An FR1 schedule was used for days 1–5, an FR2 schedule was used for days 6–8, and an FR5 schedule was used for the remainder of the experiments. To establish stable nicotine self-administration, all of the rats received 20 daily self-administration training sessions before the start of drug treatment.
Food self-administration
To control for any possible nonspecific effects of the drugs under investigation on locomotor activity, arousal state, and goal-directed behavior, a separate group of rats was trained to self-administer food pellets. These animals were allowed to have free access to chow in their home cages. The food self-administration sessions were performed under condition identical to that of nicotine self-administration, except that responses on the active lever were reinforced on the FR5 schedule by deliveries of food pellets rather than nicotine infusions. Each food pellet (45 mg) delivery was paired with a presentation of the stimulus (5 s tone and 20 s lever light on), which signaled a 20 s timeout period.
Pharmacological tests
Because stable levels of nicotine self-administration were typically achieved in 20 daily sessions, the pharmacological tests to examine the effects of the drugs under investigation began on day 21.
Test 1. Effects of dFBr on nicotine self-administration
Twelve rats were used to test the effects of dFBr on nicotine self-administration. Thirty minutes before the test sessions, dFBr (0, 0.1, 1, 3, and 6 mg/kg) was subcutaneously administered using a within-subjects Latin-square design. The test sessions were performed every other day with one non-treatment session in between.
Test 2. Effects of 5-iodo-A-85380 on nicotine self-administration
Twelve rats were used to test the effects of 5-iodo-A-85380 on nicotine self-administration. Similar to the procedure used for the dFBr test described above, 30 min prior to the test sessions, the rats received an intraperitoneal injection of 5-iodo-A-85380 (0, 1, 2.5, and 5 mg/kg) in a within-subjects Latin-square design. The test sessions were performed every other day, with one non-treatment session in between.
Test 3. Effects of galantamine on nicotine self-administration
Eight rats were used to test the effects of galantamine on nicotine self-administration. Similar to the procedure used for the dFBr test described above, 30 min prior to the test sessions, the rats received a subcutaneous injection of galantamine (0, 0.3, 1, and 3 mg/kg) in a within-subjects Latin-square design. The test sessions were performed every other day, with one non-treatment session in between.
Test 4. Substitution of nicotine by dFBr and 5-iodo-A-85380
Three groups of rats (n = 8 per group) were used for this substitution test. After the establishment of stable nicotine self-administration in the 20 sessions, the rats were divided into three groups in a pseudo-random order so that each group had similar lever-responding and nicotine-intake profiles. Starting on day 21, in one group, nicotine was replaced by dFBr at a unit dose of 0.5 mg/kg/infusion. This dFBr dose was calculated based on the most effective dose (i.e., 6 mg/kg) that reduced nicotine self-administration identified in the aforementioned test and 12 infusions per session, the rate at which the rats typically self-administered nicotine. In another group, nicotine was replaced by 5-iodo-A-85380 at a unit dose of 0.2 mg/kg/infusion. This 5-iodo-A-85380 dose was calculated based on the most effective dose (i.e., 2.5 mg/kg) obtained from the aforementioned test and our previous work showing that rats with a prior history of cocaine training readily self-administered 5-iodo-A-85380 at 0.2 mg/kg/infusion (Liu et al. 2003). The third group of rats received saline substitution of nicotine. These substitution tests lasted for 10 sessions because lever responding was previously shown to be typically extinguished within 10 daily sessions when saline replaced nicotine (e.g., Liu et al. 2008).
Test 5. Effects of dFBr, 5-iodo-A-85380, and galantamine on food self-administration
Ten rats received 20 daily sessions of food self-administration. Starting on self-administration day 21, the effects of dFBr, 5-iodo-A085380, and galantamine on food self-administration were examined. There were 3 drug test phases, each of which tested one drug. These 3 test phases were separated by 5 daily sessions, in which the rats self-administered food pellets without drug pretreatment. For each drug test phase, the test sessions were conducted in a within-subjects Latin-square design and scheduled every other day with one non-treatment session in between. The test order for the 3 drugs was dFBr, 5-iodo-A-85380, and galantamine. To eliminate redundancy, these tests were performed only with the effective doses of these 3 drugs obtained from the aforementioned nicotine self-administration tests. Thus, the drug doses used were 3 and 6 mg/kg dFBr, 2.5 and 5 mg/kg 5-iodo-A-85380, and 1 and 3 mg/kg galantamine plus saline vehicle for each drug.
Measurement of dFBr in blood and cerebrospinal fluid
To confirm that dFBr penetrated the blood-brain barrier to modulate brain α4β2 nAChRs, after completion of Test 1 (see above), four rats were reused to measure the concentration of dFBr in blood and cerebrospinal fluid after systemic administration. These rats received a subcutaneous injection of 3 mg/kg dFBr, the dose that was found to be effective in reducing nicotine self-administration (see Results). Under isoflurane anesthesia, blood samples in a volume of 0.1 ml were collected via the indwelling intravenous catheter, and cerebrospinal fluid samples in a 20 μl volume were obtained via a cistern magna catheter. The sampling occurred 30 and 90 min after the dFBr injection, which matched the start and end of the self-administration test sessions.
Measurement of the concentrations of dFBr in plasma and cerebrospinal fluid were performed by the HPLC/Mass Spectrometry Analytical Core, Department of Pharmacology and Toxicology, University of Mississippi Medical Center. A Dionex Ultimate 3000 LC system with BetaBasic 18 (5 μm), 2.1×150 mm analytical column proceeded by a BetaBasic 18 (5 μm), 2.1×10 mm guard cartridge (Thermo Fisher Scientific, Pittsburgh, PA) was used with two different mobile phases. The mobile phase A was 85% acetonitrile, 14.9% methanol and 0.1% acetic acid. The mobile phase B contained 90% aqueous 0.1% acetic acid and 10% of mobile phase A.
Data analysis
The data are expressed as the mean ± SEM number of lever responses and reinforcers earned (nicotine infusions or food pellets). The data obtained for the three drugs were analyzed separately. A one-way repeated-measures analysis of variance (ANOVA) was used to analyze the data obtained from the tests using the within-subjects Latin-square design (Tests 1, 2, 3, and 5). The data obtained from the substitution tests (Test 4) were analyzed using a two-way repeated-measures ANOVA, with group as the between-subjects factor and session as the within-subjects factor. Newman-Keuls post hoc tests were used to verify differences among individual means.
Results
Nicotine self-administration
Rats readily acquired nicotine self-administration behavior in the 20 daily 1-h self-administration training sessions. Averaged across the final three sessions, the number of responses was 92.5 ± 10.1 on the active lever and 6.4 ± 1.3 on the inactive lever. The animals self-administered 13.8 ± 2.5 infusions of nicotine at a unit dose of 0.03 mg/kg/infusion.
Effects of dFBr on nicotine self-administration
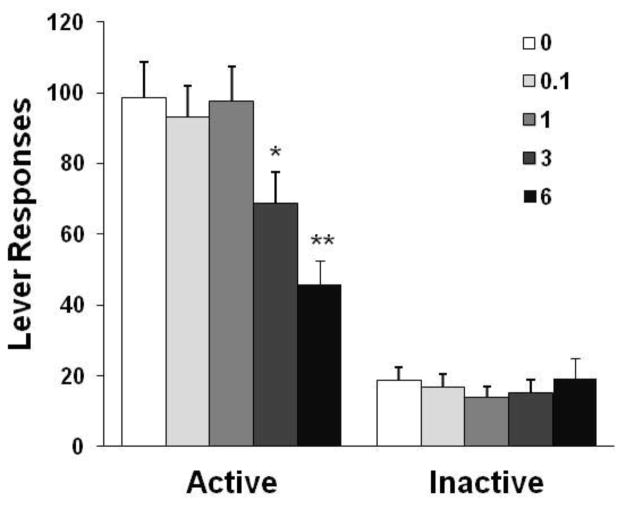
As shown in Fig. 1, dFBr pretreatment reduced nicotine self-administration behavior. The one-way ANOVA of the number of responses on the active lever yielded a significant effect of dose [F(4,44) = 8.91, p < 0.0001]. Newman-Keuls post hoc test revealed a significant difference between 6 mg/kg (p < 0.01) and 3 mg/kg (p < 0.05) vs. saline control as well as 0.1 and 1 mg/kg conditions. Correspondingly, dFBr reduced the number of nicotine infusions 48.3% at 6 mg/kg and 22.4% at 3 mg/kg (details shown in Table 1). However, responses on the inactive lever remained at very low levels and were indistinguishable among the different dose conditions [F(4,44) = 0.94, p > 0.05].
Fig. 1.
Effect of dFBr on nicotine self-administration behavior in rats (n = 12). The data are expressed as the mean ± SEM number of lever-press responses. *p < 0.05, **p < 0.01, different from saline vehicle (0), 0.1 and 1 mg/kg conditions.
Table 1.
Number of nicotine infusions rats self-administered after pretreatment with dFBr (n = 12), 5-iodo-A-58380 (n = 12), and galantamine (n = 8).
| Nicotine Infusions | |
|---|---|
| dFBr (mg/kg) | |
| 0 | 14.3± 1.5 |
| 0.1 | 14.3 ± 1.4 |
| 1 | 15.0 ± 1.3 |
| 3 | 11.1 ± 1.4a |
| 6 | 7.4 ± 1.1b,c |
| 5-iodo-A-58380 (mg/kg) | |
| 0 | 13.5 ± 1.7 |
| 1 | 13.4 ± 1.9 |
| 2.5 | 9.3 ± 1.4a |
| 5 | 7.3 ± 1.2b |
| Galantamine (mg/kg) | |
| 0 | 15.4 ± 2.2 |
| 0.3 | 13.4 ± 2.4 |
| 1 | 10.3 ± 2.1a |
| 3 | 5.0 ± 1.2b,c |
In dFBr rats:a p < 0.05 vs. 0, 0.1 and 1; b p < 0.01 vs. 0, 0.1, and 1; c p < 0.05 vs. 3.
In 5-iodo-A-58380 rats: a p < 0.05 vs. 0 and 1; b p < 0.01 vs. 0 and 1.
In galantamine rats: a p < 0.05 vs. 0; b p < 0.01 vs. 0 and 0.3; c p < 0.05 vs. 1.
Effects of 5-iodo-A-85380 on nicotine self-administration
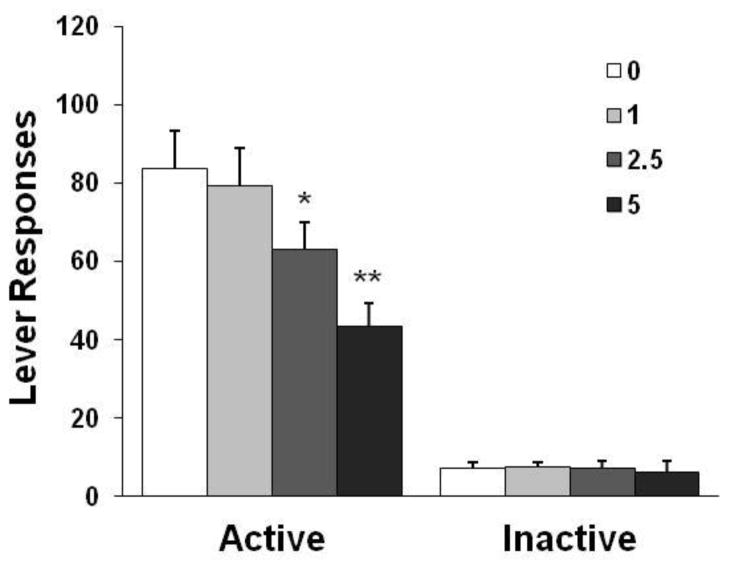
Pretreatment with 5-iodo-A-85380 reduced nicotine self-administration behavior (Fig. 2). The one-way ANOVA of the number of responses on the active lever revealed a significant effect of dose [F(3,33) = 8.51, p < 0.001]. Newman-Keuls post hoc test revealed a significant difference between 5 mg/kg (p < 0.01) and 2.5 mg/kg (p < 0.05) vs. the saline control and 1 mg/kg conditions. Similar statistical analyses showed a significant decrease in the number of nicotine infusions earned after 5-iodo-A-85380 treatment at 2.5 and 5 mg/kg (details shown in Table 1). However, 5-iodo-A-85380 did not change responses on the inactive lever [F(3,33) = 1.43, p > 0.05].
Fig. 2.
Effect of 5-iodo-A-85308 on nicotine self-administration behavior in rats (n = 12). The data are expressed as the mean ± SEM number of lever-press responses. *p < 0.05, **p < 0.01, different from saline vehicle (0) and 1 mg/kg conditions.
Effects of galantamine on nicotine self-administration
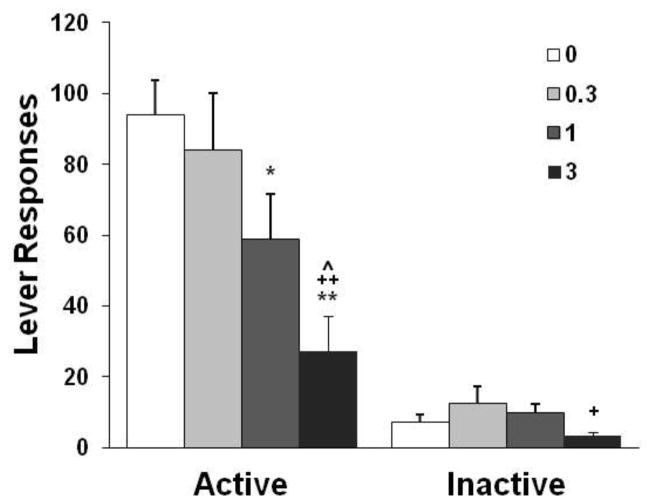
Galantamine treatment before the test sessions reduced nicotine self-administration behavior (Fig. 3). A significant effect of dose [F(3,21) = 7.38, p < 0.01] was found on the number of responses on the active lever. The number of responses was significantly suppressed by galantamine at 3 mg/kg (p < 0.01) compared with the saline control and 0.3 mg/kg conditions as well as at 1 mg/kg (p < 0.05) compared with the saline control condition. Similar statistical analyses revealed a significant decrease in the number of nicotine infusions after pretreatment with galantamine at 1 and 3 mg/kg (details shown in Table 1). A similar analysis of the responses on the inactive lever revealed a significant effect of dose, with a significant difference between 3 mg/kg and 0.3 mg/kg (p < 0.05) but not between 3 mg/kg and the saline control condition.
Fig. 3.
Effect of galantamine on nicotine self-administration behavior in rats (n = 8). The data are expressed as the mean ± SEM number of lever-press responses. *p < 0.05, **p < 0.01, different from saline vehicle condition (0); +p < 0.05, ++p < 0.01, different from 0.3 mg/kg condition; ^p < 0.05, different from 1 mg/kg condition.
Substitution of nicotine by dFBr and 5-iodo-A-85380
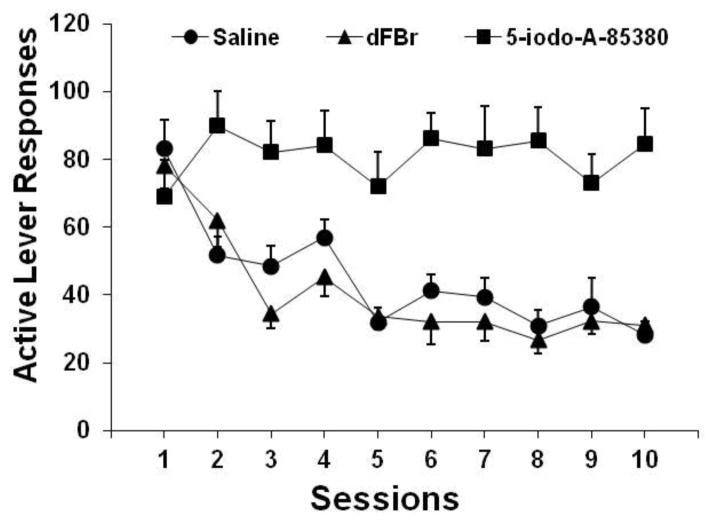
As shown in Fig. 4, the substitution of nicotine by dFBr, similar to saline substitution, failed to support self-administration behavior. However, the replacement of nicotine by 5-iodo-A-58380 sustained self-administration responding. An overall two-way repeated-measures ANOVA of the number of active lever responses obtained from these three substitution groups revealed significant effects of group [F(2,21) = 20.10, p < 0.0001] and session [F(9,189) = 9.47, p < 0.0001] and a significant group × session interaction [F(18,189) = 3.72, p < 0.0001]. Newman-Keuls post hoc tests revealed significant differences (p < 0.01) between the 5-iodo-A-58380 and saline as well as between the 5-iodo-A-58380 and dFBr groups, with no significant difference (p > 0.05) between the dFBr and saline groups. Moreover, a significant effect of session was found in both the dFBr [F(9,63) = 11.62, p < 0.0001] and saline [F(9,63) = 10.76, p < 0.0001] groups, indicating similar extinction of lever-press responding in these two groups, but no effect of session [F(9,63) = 0.52, p > 0.05] was found for the 5-iodo-A-58380 group.
Fig. 4.
Lever-press responses after substitution of nicotine by dFBr, 5-iodo-A-58380, and saline in rats (n = 8/group). The data are expressed as the mean ± SEM number of responses on the active lever.
Effects of dFBr, 5-iodo-A-85380, and galantamine on food self-administration
As shown in Table 2, neither dFBr nor 5-iodo-A-58380 affected food pellet self-administration. The one-way repeated-measures ANOVA of the number of active lever responses obtained in both the dFBr [F(2,18) = 0.90, p > 0.05] and 5-iodo-A-58380 [F(2,18) = 0.89, p > 0.05] tests did not reveal a significant effect of dose. However, a significant effect of galantamine pretreatment dose was found on both active [F(2,18) = 7.25, p < 0.01] and inactive [F(2,18) = 7.61, p < 0.05] lever responses. Galantamine at 3 mg/kg significantly (p < 0.05) decreased the number of responses on the two levers compared with the saline control condition.
Table 2.
Food self-administration profiles after pretreatment with dFBr, 5-iodo-A-58380, and galantamine in rats (n = 10).
| Active lever responses | Food pellets | Inactive lever responses | |
|---|---|---|---|
| dFBr (mg/kg) | |||
| 0 | 235 ± 32 | 45 ± 6 | 15 ± 5 |
| 3 | 265 ± 36 | 48± 7 | 16 ± 7 |
| 6 | 221 ± 27 | 42 ± 5 | 15 ± 3 |
| 5-iodo-A-58380 (mg/kg) | |||
| 0 | 256 ± 40 | 48 ± 8 | 17 ± 7 |
| 2.5 | 234 ± 35 | 46 ± 6 | 12 ± 4 |
| 5 | 249 ± 39 | 43 ± 7 | 15 ± 6 |
| Galantamine (mg/kg) | |||
| 0 | 248 ± 38 | 43 ± 7 | 14 ± 6 |
| 1 | 178 ± 20 | 31 ± 5 | 9 ± 3 |
| 3 | 81 ± 15* | 15 ± 4 | 5 ± 2* |
p < 0.05, compared with vehicle control (0).
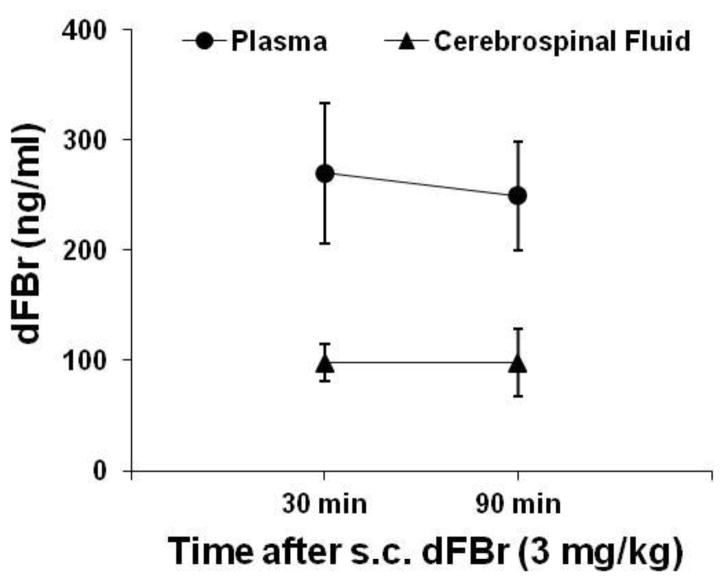
Presence of dFBr in cerebrospinal fluid
After subcutaneous administration, dFBr was present in cerebrospinal fluid during the time window that corresponded to the self-administration test sessions (Fig. 5), indicating that dFBr penetrated the blood-brain barrier. The ratio of dFBr concentrations in cerebrospinal fluid and blood samples was 36% at 30 min and 39% at 90 min after systemic injection. Moreover, based on the dFBr concentrations in blood samples (270 ng/ml at 30 min and 249 ng/ml at 90 min after administration), the half-life of dFBr was estimated to be 8.6 hours.
Fig. 5.
Concentrations of dFBr in blood and cerebrospinal fluid after subcutaneous administration in rats (n = 4). The time points for collecting blood and cerebrospinal fluid samples corresponded to the start and end times of the self-administration test sessions.
Discussion
In the rats that had already engaged in daily nicotine self-administration, dFBr (a PAM selective at α4β2 nAChRs) effectively reduced the self-administration of nicotine at 30 μg/kg/infusion, the peak dose on its dose-response curve. Pretreatment with 5-iodo-A-58380, an α4β2-selective agonist, and galantamine, a PAM at α7 nAChRs and acetylcholinesterase inhibitor also decreased nicotine self-administration while galantamine at high doses suppressed food self-administration. In contrast to 5-iodo-A-58380, dFBr failed to substitute for nicotine in supporting self-administration behavior. These results suggest that positive allosteric modulation of α4β2 nAChRs may have clinical potential to reduce nicotine consumption in habitual smokers. Moreover, α4β2-selective PAMs may prove to be free of abuse liability, an advantage over agonist medications.
Before discussing the behavioral data, one critical issue with regard to the pharmacokinetics of dFBr should be addressed. Because α4β2 nAChRs located in the brain reward circuit, namely the mesolimbic dopamine system, play a pivotal role in mediating the reinforcing properties of nicotine (Corrigall et al. 1994; Exley and Cragg 2008; Mineur and Picciotto 2008; Tapper et al. 2004; Vieyra-Reyes et al. 2008; Wonnacott et al. 2005), dFBr must be able to penetrate the blood-brain barrier so that it can act at brain α4β2 nAChRs. Given that dFBr has not yet been studied in vivo since its isolation from North Sea bryozoan Flustra foliacea (Lysek et al. 2002) and identification as a PAM at α4β2 nAChRs (Kim et al. 2007; Sala et al. 2005; Weltzin and Schulte 2010), the present study reused some of the rats after completion of the behavioral tests to obtain such pharmacokinetic information. The results showed that dFBr was present in cerebrospinal fluid after subcutaneous injection within a time window that corresponded to the self-administration test sessions. The data demonstrated the ability of dFBr to penetrate the blood-brain barrier. As such, dFBr was able to reach and bind brain α4β2 nAChRs and thus potentiated the activity of these receptors in response to nicotine and endogenous acetylcholine. From a pharmacokinetic perspective, only approximately one-third of systemically injected dFBr crossed the blood-brain barrier (see Fig. 5). With dFBr as a prototype, future research should develop new PAMs with improved brain α4β2 nAChR bioavailability. Moreover, based on the changes of dFBr blood concentrations over the 1-h period (from 30 to 90 min after administration), the half-life of dFBr was estimated to be 8.6 hours. The elimination rate of dFBr from rat body allowed to perform the drug tests on an every other day schedule. Moreover, self-administration behavior fully recovered on the following day non-treatment sessions in this study and also immediately after termination of chronic (10 days) dFBr administration in our ongoing experiments (unpublished). Together, interpretation of current results should be free of carry-over effect of dFBr due to its accumulation, if any.
In the test sessions conducted after the establishment of stable nicotine self-administration, dFBr dose-dependently reduced lever-press responses and consequently the number of nicotine infusions earned. The dose range of dFBr (0.1–6 mg/kg) was determined based on the effective in vitro concentrations of dFBr to enhance nicotine actions (Kim et al. 2007; Weltzin and Schulte 2010). After pre-session administration of dFBr at its highest dose (6 mg/kg), nicotine intake was reduced by 48.3%. Although a greater suppressant effect of dFBr may have been expected if larger doses were used, the present study did not go beyond 6 mg/kg because of drug supply limitations. Responses on the inactive lever during the test sessions remained unchanged after dFBr pretreatment, and dFBr did not change lever-press responses during the food self-administration tests under both the FR5 (Table 2) and a progressive-ratio schedule (data not shown). These results exclude the possible nonspecific effects of dFBr on general locomotor activity, goal-directed operant behavior, and motivation for reward. Therefore, dFBr produced a selective suppressant effect on nicotine self-administration behavior under the current nicotine unit dose of 0.03 mg/kg/infusion, which is the peak dose on its dose-response curve. These results support our hypothesis that a PAM at α4β2 nAChRs reduces nicotine intake by enhancing the actions of both nicotine and endogenous acetylcholine in rats that were already engaged in routine nicotine self-administration. In principle, however, the α4β2 PAMs are predicted to increase self-administration if nicotine doses are on the ascending limb of the dose-response curve. The prediction seems to gain support from ongoing work (unpublished) showing that dFBr increases lever responses for 15 μg/kg/infusion nicotine. Nevertheless, the results may be readily generalized to the human condition since the treatment-seeking smokers usually represent the heavy smoking population. Treatment with PAMs may reduce the amount of nicotine self-administered through smoking because smokers treated with a PAM at α4β2 nAChRs may experience the same level of the subjective reinforcing effects of nicotine while consuming less nicotine. Therefore, smokers who receive PAM treatment may reduce their tobacco intake because of their decreased need for nicotine. As such, PAMs at α4β2 nAChRs may be particularly useful in reducing the risks related to the inhalation of the hazardous chemicals contained in tobacco smoke in regular smokers with obstinate smoking habits.
Similar to dFBr, 5-iod-A-85380 also effectively decreased nicotine self-administration. 5-iod-A-85380 is a synthetic α4β2 nAChR agonist with approximately 150-fold greater selectivity for α4β2 nAChRs than nicotine (Mukhin et al. 2000). In a magnetic resonance imaging study (Gozzi et al. 2006) performed in drug-naive rats, 5-iodo-A-85380 (5 mg/kg) and nicotine induced almost the same pattern of neural activation in the brain, indicating similar pharmacological actions on brain α4β2 nAChRs. In fact, the suppressant effect of 5-iodo-A-85380 observed in the present study is consistent with our unpublished data that showed that pretreatment with 0.8 mg/kg nicotine significantly reduced nicotine self-administration behavior and a previous study that demonstrated the inhibitory effect of pre-session nicotine treatment on ongoing nicotine self-administration, with an ID50 of approximately 1 mg/kg (Mansbach et al. 2000). However, in contrast to the inhibitory effect of nicotine on food intake reported previously (Baettig et al. 1980), 5-iodo-A-85380 did not alter lever-press responses for food self-administration. The suppressant effect of nicotine on food intake, therefore, may involve other subtypes of nAChRs rather than α4β2 nAChRs (Mineur et al. 2011).
Given that PAMs do not directly activate α4β2 nAChRs, PAMs are hypothesized to produce no reinforcing actions on their own. This hypothesis was tested using the self-administration paradigm, which is the most direct approach to assessing the reinforcing properties of a drug (Ator and Griffiths 2003; Moser et al. 2011). In the rats that were trained to stably self-administer nicotine in daily sessions, dFBr failed to support self-administration behavior when it was substituted for nicotine. In these substitution sessions, responses on the active lever quickly decreased in a manner very similar to the saline substitution condition (i.e., extinction). The inability of dFBr to maintain lever-press responding indicates that dFBr on its own did not produce reinforcing actions. In contrast, the α4β2 nAChR agonist 5-iodo-A-85380 readily sustained lever-press responding when it replaced nicotine in the infusion solution. This finding is consistent with our previous work that showed that rats with a prior history of cocaine training acquired 5-iodo-A-85380 self-administration at 0.2 mg/kg/infusion (Liu et al. 2003). Altogether, these data demonstrate the reinforcing actions of 5-iodo-A-85380. Thus, PAMs at α4β2 nAChRs, such as dFBr, may be free of abuse and dependence liability and be a unique alternative medication with advantages over the current FDA-approved mediations for smoking cessation (i.e., nicotine replacement, bupropion, and varenicline). Varenicline is a partial agonist at α4β2 nAChRs. Bupropion acts as an antagonist at nAChRs, in addition to its inhibitory effect on monoamine transporters. Both agonist and antagonist medications are well known to have significant drawbacks (Marx et al. 2000; McKee et al. 2009; Nemeth-Coslett et al. 1986; Rose et al. 2001). For example, agonists usually exhibit liability for abuse or dependence and result in receptor desensitization or altered receptor protein expression that lead to tolerance and subsequent loss of activity. Smokers treated with antagonists may increase the intensity at which they inhale cigarette smoke to compensate for the decreased reinforcing actions of nicotine, which greatly increases the intake of the hazardous chemicals contained in cigarette smoke. It is noted, however, that more studies that use other models of nicotine addiction are needed to fully examine the potential of abuse. For example, a drug discrimination paradigm can be used to assess whether dFBr on its own produces subjective effects or creates interoceptive states that are similar to the effects of nicotine. Above all, the present results, in which dFBr was shown to reduce nicotine intake and lack reinforcing actions in the current self-administration paradigm, may inspire further investigations of PAMs at α4β2 nAChRs as smoking cessation medications.
The present study showed that galantamine reduced nicotine self-administration at 1–3 mg/kg and suppressed food self-administration at 3 mg/kg. This finding can be interpreted within the framework of the multiple pharmacological actions of this agent. First, galantamine can produce an allosteric modulating effects on α4β2 receptors (Maelicke et al. 2001), which may contribute to the observed suppressant effect of galantamine on nicotine self-administration. Second, galantamine acts as an inhibitor of acetylcholinesterase (Thomsen et al. 1991; Woodruff-Pak et al. 2001), which is an enzyme responsible for the degradation of the neurotransmitter acetylcholine. Thus, the increased level of endogenous acetylcholine after galantamine treatment may have partially substituted for nicotine to produce reinforcing actions, thereby leading to a reduction of nicotine self-administration. Third, although galantamine acts as a PAM at α7 receptors (Bertrand and Gopalakrishnan 2007; Santos et al. 2002), such an action may not be involved in the observed effect because of the fact that α7 nAChRs have been shown to not play a role in mediating nicotine reward in most studies (Grottick et al. 2000; Pons et al. 2008; Stolerman et al. 2004; van Haaren et al. 1999; Walters et al. 2006). Regardless of these proposed underlying mechanisms, the suppressant effect on galantamine on nicotine self-administration is consistent with a recent study that used a similar paradigm (Hopkins et al. 2012). The suppressant effect of 3 mg/kg galantamine on food self-administration was similar to the inhibitory effect of a different acetylcholinesterase inhibitor, tacrine (Grasing et al. 2008). However, Hopkins et al (2012) reported no effect of galantamine (up to 5 mg/kg) on sucrose self-administration. This discrepancy may be attributable to difference in the reinforcers and durg exposure history. The rats of this study had a history of dFBr and 5-iodo-A-85380 administration prior to galantamine tests. The repeated exposure to these compounds might have sensitized the rats to galantamine.
In summary, the present study explored the potential of PAMs at α4β2 nAChRs to reduce nicotine intake using a rat model of drug self-administration. The results showed that a recently developed PAM, dFBr, selectively suppressed nicotine self-administration at a nicotine unit dose maintaining the most robust self-administration behavior. However, dFBr on its own failed to support self-administration behavior, indicating its lack of reinforcing actions. By comparison, the α4β2 nAChR-selective agonist 5-iodo-A-85380, in addition to its inhibitory effect on nicotine self-administration, was readily self-administered by rats, demonstrating the reinforcing actions of this nicotinic agonist. Galantamine, a PAM at α7 and also α4β2 nAChRs and inhibitor of acetylcholinesterase, exerted a suppressant effect on the self-administration of both nicotine and food, with close effective doses. Collectively, these results suggest that positive allosteric modulation of α4β2 nAChRs may prove to be a novel approach to smoking reduction with little liability for abuse and dependence compared with agonist medications.
Acknowledgments
This work was supported by NIH grant DA017288 from the National Institute on Drug Abuse and startup funds from the Department of Psychiatry and Human Behavior, University of Mississippi Medical Center. The author would like to thank Courtney Jernigan, Laura Beloate, Ramachandram Avusula, Treniea Tolliver, and Trisha Patel for their excellent technical assistance and Dr. Rodney Baker and Mrs. Christine Purser at the HPLC/Mass Spectrometry Analytical Core, Department of Pharmacology and Toxicology, University of Mississippi Medical Center for their great effort for the measurement of dFBr in blood and cerebrospinal fluid samples.
References
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, Williams KE, Reeves KR. Varenicline versus transdermal nicotine patch for smoking cessation: Results from a randomised, open-label trial. Thorax. 2008;63:717–24. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baettig K, Martin JR, Classen W. Nicotine and amphetamine: differential tolerance and no cross-tolerance for ingestive effects. Pharmacol Biochem Behav. 1980;12:107–11. doi: 10.1016/0091-3057(80)90423-2. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–63. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Besson M, David V, Baudonnat M, Cazala P, Guilloux JP, Reperant C, Cloez-Tayarani I, Changeux JP, Gardier AM, Granon S. Alpha7-nicotinic receptors modulate nicotine-induced reinforcement and extracellular dopamine outflow in the mesolimbic system in mice. Psychopharmacology (Berl) 2012;220:1–14. doi: 10.1007/s00213-011-2422-1. [DOI] [PubMed] [Google Scholar]
- Brunzell DH. Preclinical evidence that activation of mesolimbic alpha 6 subunit containing nicotinic acetylcholine receptors supports nicotine addiction phenotype. Nicotine Tob Res. 2012;14:1258–69. doi: 10.1093/ntr/nts089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–43. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011;2:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- CDC. Cigarette smoking in adults-United State, 2007. MMWR Weekly. 2008;57(45):1121–6. [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–84. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ. Analysis of allosterism in functional assays. J Pharmacol Exp Ther. 2005;315:740–54. doi: 10.1124/jpet.105.090886. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant RV, Buchhalter AR, Buchman AC, Henningfield JE. Pharmacotherapy for tobacco dependence. Handb Exp Pharmacol. 2009:487–510. doi: 10.1007/978-3-540-69248-5_17. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- Goh CW, Aw CC, Lee JH, Chen CP, Browne ER. Pharmacokinetic and pharmacodynamic properties of cholinesterase inhibitors donepezil, tacrine, and galantamine in aged and young Lister hooded rats. Drug Metab Dispos. 2011;39:402–11. doi: 10.1124/dmd.110.035964. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. Region-specific effects of nicotine on brain activity: a pharmacological MRI study in the drug-naive rat. Neuropsychopharmacology. 2006;31:1690–703. doi: 10.1038/sj.npp.1300955. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–63. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Grasing K, He S, Yang Y. Dose-related effects of the acetylcholinesterase inhibitor tacrine on cocaine and food self-administration in rats. Psychopharmacology (Berl) 2008;196:133–42. doi: 10.1007/s00213-007-0944-3. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–9. [PubMed] [Google Scholar]
- Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD. Galantamine, an Acetylcholinesterase Inhibitor and Positive Allosteric Modulator of Nicotinic Acetylcholine Receptors, Attenuates Nicotine Taking and Seeking in Rats. Neuropsychopharmacology. 2012;37:2310–21. doi: 10.1038/npp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002;72:559–68. doi: 10.1016/s0091-3057(02)00723-2. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Dependence potential and abuse liability of nicotine replacement therapies. Biomed Pharmacother. 1989;43:11–7. doi: 10.1016/0753-3322(89)90185-6. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kim JS, Padnya A, Weltzin M, Edmonds BW, Schulte MK, Glennon RA. Synthesis of desformylflustrabromine and its evaluation as an alpha4beta2 and alpha7 nACh receptor modulator. Bioorg Med Chem Lett. 2007;17:4855–60. doi: 10.1016/j.bmcl.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Perry KW, Wong DT. Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats. Neuropharmacology. 2002;42:181–90. doi: 10.1016/s0028-3908(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196:365–75. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Koren AO, Yee SK, Pechnick RN, Poland RE, London ED. Self-administration of 5-iodo-A-85380, a beta2-selective nicotinic receptor ligand, by operantly trained rats. Neuroreport. 2003;14:1503–5. doi: 10.1097/00001756-200308060-00020. [DOI] [PubMed] [Google Scholar]
- Lysek N, Rachor E, Lindel T. Isolation and structure elucidation of deformylflustrabromine from the North Sea bryozoan Flustra foliacea. Z Naturforsch C. 2002;57:1056–61. doi: 10.1515/znc-2002-11-1218. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX, Zerlin M. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol Psychiatry. 2001;49:279–88. doi: 10.1016/s0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque EX. Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Behav Brain Res. 2000;113:199–206. doi: 10.1016/s0166-4328(00)00214-x. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Chambers LK, Rovetti CC. Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: comparison with mecamylamine. Psychopharmacology (Berl) 2000;148:234–42. doi: 10.1007/s002130050047. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–73. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Marx CE, McIntosh E, Wilson WH, McEvoy JP. Mecamylamine increases cigarette smoking in psychiatric patients. J Clin Psychopharmacol. 2000;20:706–7. doi: 10.1097/00004714-200012000-00023. [DOI] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Harrison EL, Coppola S, George TP. Effects of the nicotinic receptor antagonist mecamylamine on ad-lib smoking behavior, topography, and nicotine levels in smokers with and without schizophrenia: a preliminary study. Schizophr Res. 2009;115:317–24. doi: 10.1016/j.schres.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–2. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol. 2008;75:323–33. doi: 10.1016/j.bcp.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser P, Wolinsky T, Castagne V, Duxon M. Current approaches and issues in non-clinical evaluation of abuse and dependence. J Pharmacol Toxicol Methods. 2011;63:160–7. doi: 10.1016/j.vascn.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57:642–9. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of mecamylamine on human cigarette smoking and subjective ratings. Psychopharmacology (Berl) 1986;88:420–5. doi: 10.1007/BF00178502. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology. 1989;2:273–9. doi: 10.1016/0893-133x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Kenny PJ. Molecular Mechanisms Underlying Behaviors Related to Nicotine Addiction. Cold Spring Harb Perspect Med. 2012;3(1):a01211. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC. Acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking. Pharmacol Biochem Behav. 2001;68:187–97. doi: 10.1016/s0091-3057(00)00465-2. [DOI] [PubMed] [Google Scholar]
- Sala F, Mulet J, Reddy KP, Bernal JA, Wikman P, Valor LM, Peters L, Konig GM, Criado M, Sala S. Potentiation of human alpha4beta2 neuronal nicotinic receptors by a Flustra foliacea metabolite. Neurosci Lett. 2005;373:144–9. doi: 10.1016/j.neulet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Santos MD, Alkondon M, Pereira EF, Aracava Y, Eisenberg HM, Maelicke A, Albuquerque EX. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol Pharmacol. 2002;61:1222–34. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–43. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Pereira EF, Roth U, Weber KH, Albuquerque EX, Maelicke A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol Pharmacol. 1996;49:1–6. [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–7. [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–71. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Thomsen T, Kaden B, Fischer JP, Bickel U, Barz H, Gusztony G, Cervos-Navarro J, Kewitz H. Inhibition of acetylcholinesterase activity in human brain tissue and erythrocytes by galanthamine, physostigmine and tacrine. Eur J Clin Chem Clin Biochem. 1991;29:487–92. doi: 10.1515/cclm.1991.29.8.487. [DOI] [PubMed] [Google Scholar]
- Toll L, Zaveri NT, Polgar WE, Jiang F, Khroyan TV, Zhou W, Xie XS, Stauber GB, Costello MR, Leslie FM. AT-1001: a high affinity and selective alpha3beta4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology. 2012;37:1367–76. doi: 10.1038/npp.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82:984–95. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Iida Y, Mukai T, Mamede M, Ishizu K, Ogawa M, Magata Y, Konishi J, Saji H. 5-[123I]Iodo-A-85380: assessment of pharmacological safety, radiation dosimetry and SPECT imaging of brain nicotinic receptors in healthy human subjects. Ann Nucl Med. 2004;18:337–44. doi: 10.1007/BF02984473. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt L, Geerts R, Verhaeghe T, Willems B, Bode W, Lavrijsen K, Meuldermans W. Pharmacokinetics and tissue distribution of galantamine and galantamine-related radioactivity after single intravenous and oral administration in the rat. Arzneimittelforschung. 2004;54:85–94. doi: 10.1055/s-0031-1296941. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Anderson KG, Haworth SC, Kem WR. GTS-21, a mixed nicotinic receptor agonist/antagonist, does not affect the nicotine cue. Pharmacol Biochem Behav. 1999;64:439–44. doi: 10.1016/s0091-3057(99)00054-4. [DOI] [PubMed] [Google Scholar]
- Vieyra-Reyes P, Picciotto MR, Mineur YS. Voluntary oral nicotine intake in mice down-regulates GluR2 but does not modulate depression-like behaviors. Neurosci Lett. 2008;434:18–22. doi: 10.1016/j.neulet.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–44. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Weltzin MM, Schulte MK. Pharmacological characterization of the allosteric modulator desformylflustrabromine and its interaction with alpha4beta2 neuronal nicotinic acetylcholine receptor orthosteric ligands. J Pharmacol Exp Ther. 2010;334:917–26. doi: 10.1124/jpet.110.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 2000;149:198–202. doi: 10.1007/s002130000382. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–9. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel RW, 3rd, Wenk GL. Galantamine: effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning. Proc Natl Acad Sci U S A. 2001;98:2089–94. doi: 10.1073/pnas.031584398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–72. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]