Abstract
We examined whether smoking prior to non-Hodgkin lymphoma (NHL) diagnosis was associated with overall survival (OS) and conducted a meta-analysis to assess the evidence relating pre-diagnosis cigarette smoking with OS. Among 523 NHL patients, worse OS was suggested for greater pre-diagnostic smoking habits when compared to never smokers. In the meta-analysis (n=5 patient populations), inferior OS was observed for greater number of cigarettes smoked per day, years of cigarette smoking, and pack-years of cigarette smoking. The inferior survival was more pronounced for follicular than for diffuse large B-cell lymphoma. Pre-diagnosis cigarette smoking may adversely impact the survival of NHL patients.
Keywords: cigarettes, smoking, non-Hodgkin lymphoma, pooled analysis, meta-analysis
INTRODUCTION
Non-Hodgkin lymphoma (NHL) is the seventh most commonly diagnosed cancer among men and women in the U.S., with an estimated 70,000 new cases and 19,000 deaths occurring annually (American Cancer Society, 2012).
Patient age at the time of diagnosis and clinical factors comprising the International Prognostic Index and Follicular Lymphoma Prognostic Index are well-established indicators of lymphoma prognosis. However, few lifestyle factors have been shown to influence overall survival (OS) in patients with NHL. Emerging evidence suggests that pre-diagnosis cigarette smoking may increase the risk of death in NHL patients by approximately 60%–90%, although discrepancies for specific smoking measures have been reported (Battaglioli et al, 2006; Talamini et al, 2008; Geyer et al, 2010). In addition, several studies (Battaglioli et al, 2006; Geyer et al, 2010) had limited ability to control for clinical indicators of lymphoma prognosis and initial treatment.
We examined whether pre-diagnosis cigarette smoking was associated with OS in two population-based cohorts of NHL patients and whether the associations were influenced by statistical adjustment for clinical prognostic factors and initial treatment. We also conducted a meta-analysis, which incorporated the results of the present study with those of Battaglioli et al, (2006), Talamini et al, (2008) and Geyer et al, (2010), to assess the overall evidence relating pre-diagnosis cigarette smoking with survival in NHL patients.
MATERIALS AND METHODS
Study population
We performed a pooled analysis of two cohorts of NHL patients initially participating in population-based case-control studies conducted in Nebraska using similar protocols (Study I, 1983–86; Study II, 1999–2002) (Zahm et al, 1990; Chiu et al, 2005). Patients with NHL residing in the 66 counties of eastern Nebraska at the time of diagnosis were identified through the Nebraska Lymphoma Registry and Tissue Bank. Participation rates were 87% (Study I, n=385) and 74% (Study II, n=387). All patients were subsequently followed through active patient follow-up supplemented by data linkages to the Nebraska state death certificate files. Follow-up was complete for 219 (99%) patients in Study I and 361 (95%) patients in Study II. We also excluded patients who died within six months of the date of diagnosis (n=14) because their prognostic characteristics at baseline might differ from others. After exclusion, 523 patients were available for the present analysis. Detailed treatment and clinical prognostic data were available for 122 (56%) patients from Study I and 226 (63%) patients from Study II. All diagnoses were classified according to the World Health Organization (WHO) classification of NHL (Jaffe et al 2001). The study protocols were approved by the Institutional Review Board of the University of Nebraska Medical Center.
Assessment of cigarette smoking
Information on tobacco use prior to lymphoma diagnosis was collected using a structured telephone interview (Study I) and a self-administered questionnaire (Study II). To comprehensively assess the association between cigarette smoking and OS, we evaluated five measures of cigarette smoking: smoking status (never, former, current); smoking duration (years of cigarette smoking); smoking intensity (number of cigarettes smoked per day); a composite measure of smoking intensity and duration (pack-years of smoking); and years since quitting cigarette smoking.
Statistical analysis
Proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality. Pooled HRs were first calculated from study-specific risk estimates using a random-effects model. Given the similarity in the patient populations, and that no appreciable heterogeneity in the study outcomes was detected (Q test p > 0.23; I2 < 26.8%), the cohorts were combined into an aggregated dataset. Subgroup analyses included stratification by sex and age at diagnosis (<60 years, ≥60 years), as well as analysis restricted to patients with detailed clinical prognostic data and those diagnosed with follicular lymphoma (FL) or diffuse large B-cell lymphoma (DLBCL). For continuous variable models, potential non-linear relations were first examined using restricted cubic splines.
For the meta-analysis, a systematic search of the PubMed database was conducted for studies published between 1 January 1966 and 31 August 2012 (Supplemental Figure 1). A search of SCOPUS and ISI Web of Knowledge did not produce additional relevant studies. Citations from relevant studies were searched for additional publications. The data extraction was carried out independently by two reviewers using a standardized form. The extractions were concordant. The summary risk estimates were calculated using a random-effects model (DerSimonian and Laird, 1986). Heterogeneity was tested using the Cochran Q test (DerSimonian and Laird, 1986; Cochran, 1954) and described by the I2 statistic (Higgins and Thompson, 2002). Small-study effects were assessed graphically by funnel plots of effect sizes plotted against the precision of the estimates and by Egger’s linear regression test (Egger et al, 1997). The “trim and fill” method (Duval and Tweedie, 2000) was used as a sensitivity analysis when potential small-study effects were detected. Data analyses were performed using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC, USA) and STATA version 12.1 (Stata Corp., College Station, Texas, USA). A p-value of 0.10 was chosen for the Q test and Egger’s linear regression test given the low power to detect heterogeneity and publication bias when pooling a small number of studies. All other tests were two-sided with p < 0.05 considered statistically significant unless otherwise noted
RESULTS
Pooled analysis of cigarette smoking and the risk of all-cause mortality
The patient cohorts were similar with respect to sex, education level, presence of B-symptom, and stage at diagnosis (Supplemental Table 1). Compared to never smokers, there was suggestive poorer OS for current smokers, as well as for patients in the highest category of cigarettes smoked per day, smoking duration, and pack-years of smoking (Table I). Findings were similar when examined as continuous variables or when estimated by random-effects models (data not shown). Among former smokers, a greater interval from quitting cigarette smoking to diagnosis was associated with better OS (HR=0.87 [0.80–0.93], ptrend=0.0001, per 5 years of quitting cigarette smoking) (Supplemental Figure 2). Adjustment for stage, B-symptoms, or initial treatment did not materially impact the associations nor were the results found to differ by sex or age (data not shown). Worse prognosis for pre-diagnosis smoking was suggested for FL than for DLBCL patients (Supplemental Table 2). Among former smokers, a greater interval from quitting smoking to diagnosis was associated with better OS among FL, but not DLBCL patients (data not shown).
Table I.
Hazard ratios (HRs) and 95% confidence intervals (95% CI) for all-cause mortality according to pre-diagnosis cigarette smoking
| Patient Cohort I (1983–2010) |
Patient Cohort II (1999–2011) |
Pooled Analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| At Risk (n) |
Deaths (n) |
HR (95% CI)* | At Risk (n) |
Deaths (n) |
HR (95% CI)* | HR (95% CI)† | Pheterogeneity§ | |
| Smoking status | ||||||||
| Never smoker | 101 | 87 | 1.0 | 159 | 41 | 1.0 | 1.0 | |
| Former smoker | 61 | 52 | 1.0 (0.7–1.5) | 101 | 36 | 1.1 (0.7–1.7) | 1.1 (0.8–1.4) | 0.75 |
| Current smoker | 53 | 43 | 1.2 (0.8–1.7) | 48 | 19 | 1.8 (1.0–3.1) | 1.4 (1.0–1.9) | 0.24 |
| Ptrend‡ | 0.43 | 0.07 | 0.08 | |||||
| Cigarettes smoked/day (n) | ||||||||
| Never smoker | 101 | 87 | 1.0 | 159 | 41 | 1.0 | 1.0 | |
| <20 | 43 | 37 | 1.1 (0.8–1.7) | 86 | 32 | 1.3 (0.8–2.1) | 1.2 (0.9–1.6) | 0.59 |
| 20+ | 69 | 56 | 1.1 (0.8–1.6) | 63 | 23 | 1.2 (0.7–2.0) | 1.1 (0.8–1.6) | 0.79 |
| Ptrend‡ | 0.59 | 0.42 | 0.30 | |||||
| Years of cigarette smoking (n) | ||||||||
| Never smoker | 101 | 87 | 1.0 | 159 | 41 | 1.0 | 1.0 | |
| <30 years | 41 | 28 | 0.8 (0.5–1.3) | 80 | 22 | 1.0 (0.6–1.8) | 0.9 (0.7–1.3) | 0.55 |
| 30+ years | 71 | 65 | 1.2 (0.9–1.7) | 69 | 33 | 1.5 (0.9–2.3) | 1.3 (1.0–1.7) | 0.44 |
| Ptrend‡ | 0.25 | 0.13 | 0.06 | |||||
| Pack-years of cigarette smoking (n) | ||||||||
| Never smoker | 101 | 87 | 1.0 | 159 | 41 | 1.0 | 1.0 | |
| <30 years | 51 | 38 | 1.1 (0.7–1.6) | 82 | 25 | 1.1 (0.6–1.8) | 1.1 (0.8–1.5) | 0.99 |
| 30+ years | 60 | 54 | 1.1 (0.8–1.6) | 67 | 30 | 1.5 (0.9–2.4) | 1.2 (0.9–1.7) | 0.31 |
| Ptrend‡ | 0.54 | 0.12 | 0.15 | |||||
| Years since quitting cigarette smoking (n) | ||||||||
| Never smoker | 101 | 87 | 1.0 | 159 | 41 | 1.0 | 1.0 | |
| 25+ years | 34 | 28 | 0.8 (0.5–1.3) | 51 | 17 | 0.8 (0.5–1.5) | 0.8 (0.6–1.2) | 0.99 |
| <25 years | 27 | 24 | 1.4 (0.9–2.3) | 50 | 19 | 1.3 (0.8–2.3) | 1.4 (0.9–2.0) | 0.84 |
| Ptrend‡ | 0.37 | 0.46 | 0.24 | |||||
Note: Hazard ratios calculated using log-linear (Cox) proportional hazard models.
Adjusted for age (continuous), sex, and education (<12 years, 12–15 years, 16+ years) in the log-linear model component.
Adjusted for age (continuous), sex, and education (<12 years, 12–15 years, 16+ years) in the log-linear model component and study (cohort I, cohort II) as a strata variable.
P-value for the Wald chi-square test of Ho: β = 0 when modeling categories as an ordinal variable.
P-value for the Q statistic assessing heterogeneity in the study-specific hazard ratios.
Meta-analysis
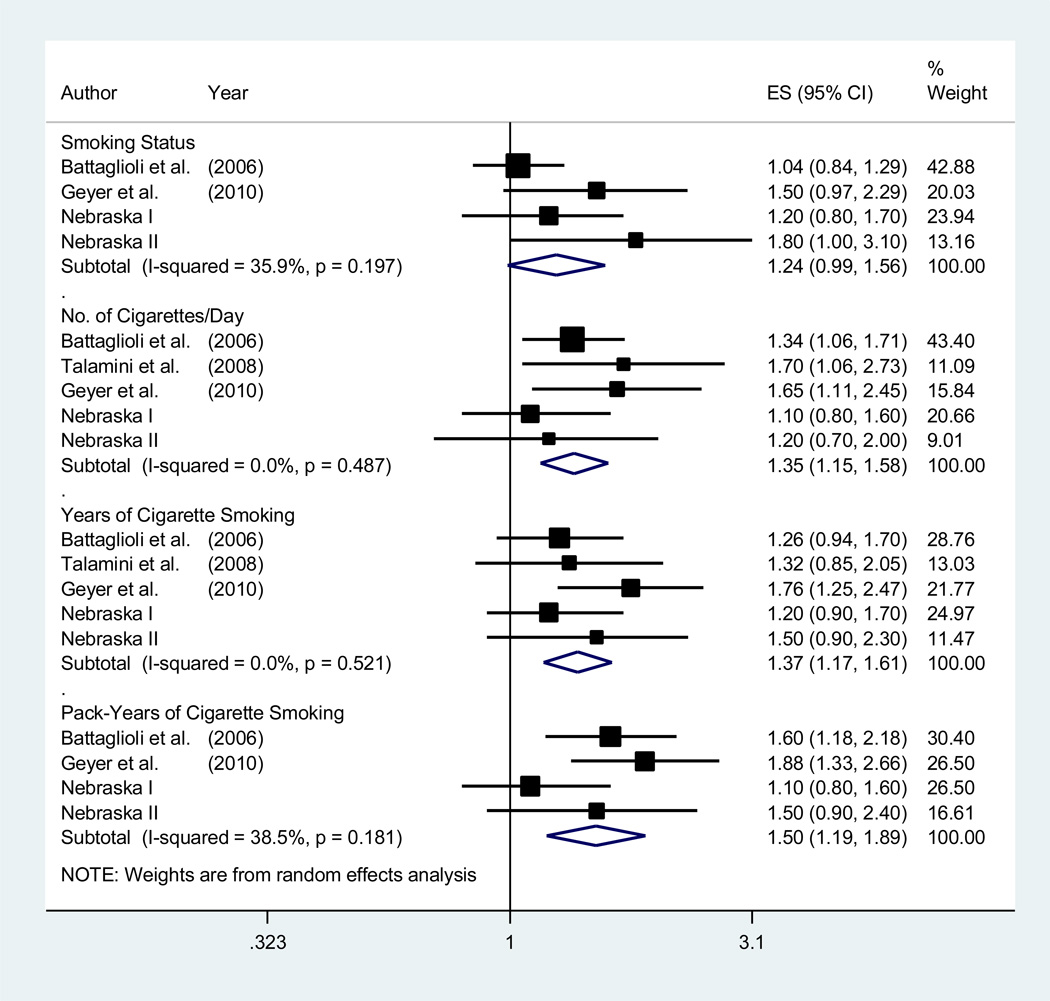
Three previous studies reporting on the association between pre-diagnosis cigarette smoking and OS in patients with NHL were identified (Battaglioli et al, 2006; Talamini et al, 2008; Geyer et al, 2010). The OS was worse for patients smoking the greatest number of cigarettes/day, years of cigarette smoking, and pack-years of cigarette smoking when compared to never smokers or patients in the lowest tertile of exposure (Figure 1). No appreciable heterogeneity in the study outcomes was detected (p > 0.18; I2 < 38.6%), nor was there evidence of small-study effects for any measure of smoking intensity or duration (p > 0.63). For smoking status, potential small-study effects were detected and re-analysis of the association using the “trim and fill” method imputed two potentially missing studies and produced an adjusted summary HR of 1.07 (0.93–1.25). In analyses restricted to FL and DLBCL patients (Supplemental Figure 3), the inferior OS was more pronounced for FL patients.
Figure 1.
Forest plot for the random-effects meta-analysis examining the associations between pre-diagnosis cigarette smoking and all-cause mortality. The black squares and horizontal lines represent the study-specific hazard ratios and 95% confidence intervals (CIs) for all-cause mortality. The size of the square is relative to the weight contributed to the summary estimate (inversely proportional to the variance). The diamonds represent the summary estimates and associated 95% CIs. The Q and I2 and statistics were used to assess heterogeneity in the study outcomes. The hazard ratios for the number of cigarettes smoked per day, years of cigarette smoking, and pack-years of cigarette smoking contrast patients in the highest tertile of exposure to those in the lowest, or to never smokers. The hazard ratios for smoking status contrast current smokers at diagnosis to never smokers.
ES, effect size
DISCUSSION
In our patient cohorts, worse OS was suggested for those reporting current smoking at diagnosis, or greater cumulative exposure to cigarette smoking prior to NHL diagnosis, when compared to never smokers. Among former smokers, a greater interval from smoking cessation to diagnosis was associated with better OS. In a meta-analysis, incorporating our findings with those of previously published reports, worse OS was observed for NHL patients who reported greater smoking habits prior to lymphoma diagnosis. The inferior survival associated with smoking was most pronounced for pack-years of cigarette smoking and for FL patients.
Our finding of worse OS associated with cigarette smoking prior to NHL diagnosis is consistent with previous reports (Battaglioli et al, 2006; Talamini et al, 2008; Geyer et al, 2010). In the meta-analysis, the inferior OS was most pronounced for pack-years of smoking. This suggests that cumulative exposure to cigarette smoke may have a greater impact on OS than intensity or duration alone. Interestingly, after accounting for potentially missing studies, OS did not differ from unity for smoking status. However, this measure does not account for cumulative exposure and the summary estimate was driven largely by the results for the Italian patients for whom the intensity of smoking may have differed from those in the USA.
Of note, we found that cigarette smoking adversely impacted the OS of FL patients to a greater extent than DLBCL patients. Smoking has been associated with a higher frequency of the chromosomal translocation t(14;18) that occurs commonly in FL and is associated with BCL2 overexpression and inhibited apoptosis. Smoking may also contribute to the creation of a microenvironment that promotes tumour growth and it has been shown that the microenvironment plays an important role in FL prognosis (Dave et al, 2004). Although our subtype-specific analyses were hampered by small numbers, our findings suggest that the association between smoking and prognosis may differ for disease subtypes.
Strengths of the current study include the confirmation of NHL diagnoses by an expert hematopathologist, the relatively long patient follow-up, and the assessment of the overall evidence relating pre-diagnosis cigarette smoking with OS in NHL patients. Limitations include the inability to examine associations for cigarette smoking occurring after diagnosis or lymphoma-specific survival, limited control for the potential confounding effects of co-morbid conditions or health behaviours associated with both smoking and OS. While generally considered a valid measure, smoking was self-reported and may result in a modest underestimation of smoking habits as compared to biological assessment (Gorber et al, 2009).
In conclusion, our results provide further support for the hypothesis that cigarette smoking prior to a diagnosis of NHL adversely impacts OS. Future studies should assess the impact of smoking habits after lymphoma diagnosis and whether pre-diagnosis smoking adversely influences tumour molecular characteristics related to prognosis.
Supplementary Material
Acknowledgments
FUNDING: This research was supported by research grant 99B083 from the American Institute for Cancer Research and, in part, by grants CA94770 and CA100555 from the National Cancer Institute.
Footnotes
Author conributions:
N.J.O. performed the statistical analysis and drafted the manuscript. A.M.E., B.A-K., D.D.C., and S.M.S. contributed to the analysis and interpretation of the data. D.D.W. conducted the pathology review and contributed to the analysis and interpretation of the data. B.C-H.C. planned the study, procured the funding, and contributed to the analysis and interpretation of the data. All authors contributed to the writing of the manuscript and approved the final version.
REFERENCES
- American Cancer Society. Cancer Facts and Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- Battaglioli T, Gorini G, Costantini AS, Crosignani P, Miligi L, Nanni O, Stagnaro E, Tumino R, Vineis P. Cigarette smoking and alcohol consumption as determinants of survival in non-Hodgkin's lymphoma: a population-based study. Annuals of Oncology. 2006;17:1283–1289. doi: 10.1093/annonc/mdl096. [DOI] [PubMed] [Google Scholar]
- Chiu BC, Kolar C, Gapstur SM, Lawson T, Anderson JR, Weisenburger DD. Association of NAT and GST polymorphisms with non-Hodgkin's lymphoma: a population-based case-control study. Britsh Journal of Haematology. 2005;128:610–615. doi: 10.1111/j.1365-2141.2004.05358.x. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, Miller TP, LeBlanc M, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Connors JM, Lansdorp PM, Ouyang Q, Lister TA, Davies AJ, Norton AJ, Muller-Hermelink HK, Ott G, Campo E, Montserrat E, Wilson WH, Jaffe ES, Simon R, Yang L, Powell J, Zhao H, Goldschmidt N, Chiorazzi M, Staudt LM. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. New England Journal of Medicine. 2004;35:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinincal Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer SM, Morton LM, Habermann TM, Allmer C, Davis S, Cozen W, Severson RK, Lynch CF, Wang SS, Maurer MJ, Hartge P, Cerhan JR. Smoking, alcohol use, obesity, and overall survival from non-Hodgkin lymphoma: a population-based study. Cancer. 2010;11:2993–3000. doi: 10.1002/cncr.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine and Tobacco Reseach. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medince. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H. Pathology and genetics of tumors of hematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. World health organization classification of tumors. [Google Scholar]
- Talamini R, Polesel J, Spina M, Chimienti E, Serraino D, Zucchetto A, Zanet E, Franceschi S, Tirelli U. The impact of tobacco smoking and alcohol drinking on survival of patients with non-Hodgkin lymphoma. International Journal of Cancer. 2008;122:1624–1629. doi: 10.1002/ijc.23205. [DOI] [PubMed] [Google Scholar]
- Zahm SH, Weisenburger DD, Babbitt PA, Saal RC, Vaught JB, Cantor KP, Blair A. A case-control study of non-Hodgkin's lymphoma and the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) in eastern Nebraska. Epidemiology. 1990;1:349–356. doi: 10.1097/00001648-199009000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.