Abstract
The late positive potential (LPP) may be a useful measure of individual differences in emotional processing across development, but little is known about the stability of the LPP across time. We assessed the LPP and behavioral measures of emotional interference following pleasant, unpleasant, and neutral images in 8- to 13-year-old youth. Approximately two years later, the same participants completed the task again (N=34). Results indicated that the LPP is moderately-to-highly reliable across development. Stability was lower and more inconsistent for behavioral measures. In addition, consistent with previous cross-sectional analyses, a decrease in occipital activity was observed at the second assessment. Results indicate that the LPP appears to be a stable measure of emotional processing, even across a fairly large period of development.
Keywords: Event-related potentials, late positive potential, emotion, reliability, development
The ability to focus attention towards the most salient, motivationally-relevant information in the environment is essential to human survival. As a result, emotional stimuli, such as those conveying threat, are often preferentially attended to and processed (for reviews, Weinberg, Ferri, & Hajcak, in press; Yiend, 2010). For example, there is evidence that emotional stimuli are detected more quickly (Frischen, Eastwood, & Smilek, 2008; Öhman, Flykt, & Esteves, 2001), viewed for longer durations (Lang, Greenwald, Bradley, & Hamm, 1993), and more likely to be recalled than neutral stimuli (Hamann, Ely, Grafton, & Kilts, 1999; Phelps, LaBar, & Spencer, 1997).
There has been growing interest in using event-related potential (ERP) and functional magnetic resonance imaging (fMRI) methods to study emotional processing. The late positive potential (LPP) is a sustained ERP component characterized by a relative positivity to emotional compared to neutral stimuli over centroparietal sites beginning around 300 ms after stimulus onset (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000). The LPP appears to measure sustained engagement and elaborative processing of motivationally-salient stimuli (Hajcak, Weinberg, MacNamara, & Foti, 2011; Olofsson, Nordin, Sequeira, & Polich, 2008; Weinberg et al., in press). There is evidence that the LPP can be elicited across development, as it has been observed in children as young as five years old (Hajcak & Dennis, 2009; Kujawa, Hajcak, Torpey, Kim, & Klein, 2012). We previously found that the LPP in children is less protracted in time than in adults (Kujawa, Weinberg, Hajcak, & Klein, 2013). In addition, cross-sectional data suggest that between middle childhood and early adolescence, the scalp distribution of the LPP shifts from more occipital sites to primarily parietal regions (Kujawa, Klein, & Hajcak, 2012).
Numerous studies have utilized the LPP as an individual difference measure, with recent work linking variability in the LPP to psychopathology. For example, there is evidence that depressed adults and children at risk for depression show reduced LPPs to emotional faces (Foti, Olvet, Klein, & Hajcak, 2010; Kujawa, Hajcak, et al., 2012). In addition, enhanced LPPs to feared stimuli have been observed in both children and adults with phobias (Leutgeb, Schäfer, Köchel, Scharmüller, & Schienle, 2010; Leutgeb, Schäfer, & Schienle, 2009; Michalowski et al., 2009), and social anxiety has been associated with increased LPPs for threatening faces and to faces overall, regardless of valence (Moser, Huppert, Duval, & Simons, 2008; Mühlberger et al., 2009). More recently, researchers have begun to examine the extent to which the LPP relates to stable individual differences in temperament. For example, observational measures of children’s fearful behavior predict enhanced LPPs to unpleasant images (DeCicco, Solomon, & Dennis, 2012; Solomon, DeCicco, & Dennis, 2012). Little is known, however, about the psychometric properties of the LPP as a measure of emotional processing.
In order to relate the LPP to trait-like individual differences and psychopathology, it is important to evaluate the extent to which the LPP is a reliable measure across time. There is evidence that at least some behavioral measures of attention towards emotional stimuli have low reliability in adult populations. For example, two studies found low test-retest reliability of attention bias scores using the dot probe (Schmukle, 2005; Staugaard, 2009). To our knowledge, no previous studies have assessed the test-retest reliability of the LPP, which may be particularly relevant for studies of children and adolescents. Child and adolescent development is associated with numerous changes in the neural circuitry related to emotion (Monk, 2008; Pfeifer & Blakemore, 2012), hence it is important to determine the extent to which measures of emotional processing remain stable across this period.
In order to evaluate the stability of the LPP across time, we recorded behavioral and electrocortical responses during an emotional-interrupt task administered to children and adolescents. Each trial of the emotional-interrupt task begins with the presentation of a neutral, pleasant, or unpleasant image, followed by a target to which the participant responds, and a second presentation of the same image. Thus, the task assesses both electrocortical reactivity to images and behavioral interference (i.e., reaction time and accuracy). Approximately two years later, the same participants completed the task for a second time. As there have been reports of developmental changes in the scalp distribution of the LPP (Hajcak & Dennis, 2009; Kujawa, Klein, et al., 2012; Kujawa et al., 2013), one major goal of the study was to evaluate whether the distribution of the LPP shifted from more occipital to parietal sites across development using longitudinal data. A second major goal of the study was to evaluate whether the LPP is a stable measure of individual differences in emotional reactivity across development, despite predicted changes in the topography of the LPP. In addition, we evaluated the stability of behavioral measures of emotional reactivity in the same task.
Method
Participants
Participants were recruited from Stony Brook, NY and the surrounding community using a commercial mailing list. Sixty-seven youth between the ages of 8 and 13 participated in the initial assessment. Of those participants, nine parents declined participation in the emotional-interrupt paradigm due to the nature of the images. In addition, data were excluded for one participant due to poor EEG data quality, three participants due to low accuracy, and one participant due to a technical error. Thus, data for 53 children were available for the initial assessment (results of the initial assessment are presented in Kujawa, Klein, et al., 2012). Approximately two years later, families were contacted and invited to return for a follow-up assessment, of which 49 youth participated (time between assessments, M = 1.99 years, SD = 0.08). Of these youth, five families declined the emotional interrupt task, data for two participants were excluded for low accuracy, and data for two participants were excluded for poor EEG quality. Data for 40 participants were available for the second assessment, and a total of 34 children had useable data for both assessments and were included in the current analyses. The included sample was 38% female, and the mean age at the first assessment was 11.09 (SD = 1.44). Mean age at the second assessment was 13.12 (SD = 1.39).
Measures
Emotional-interrupt task
The emotional-interrupt task was similar to that used in previous studies of children and adults (Kujawa et al., 2013; Mitchell, Richell, Leonard, & Blair, 2006; Weinberg & Hajcak, 2011), and identical versions were completed at each assessment. The task was administered using Presentation software (Neurobehavioral Systems, Inc.). A total of 60 developmentally appropriate pictures were selected from the IAPS (Lang, Bradley, & Cuthbert, 2008). Of these, 20 depicted pleasant scenes (e.g., children playing, cute animals, babies), 20 depicted neutral scenes (e.g., people in neutral situations, neutral outdoor scenes, household objects), and 20 depicted unpleasant scenes (e.g., sad or angry people, weapons, aggressive animals).1 According to normative adult ratings of valence and arousal (Lang et al., 2008), selected pleasant images were rated as more positive in valence (M = 7.51, SD = .51) than neutral (M = 5.27, SD = .35), which were rated as more positive than unpleasant (M = 3.09, SD = .76). In addition, unpleasant (M = 6.12, SD = .58) and pleasant images (M = 5.03, SD = .77) were rated as more arousing than neutral than neutral (M = 2.99, SD = .68), though unpleasant images were also rated as more arousing than pleasant. Each image was randomly presented twice (once in each of two blocks) for a total of 120 trials. Each trial began with an 800 ms fixation (+), then a picture was presented for 1,000 ms followed by a target (< or >) presented for 150 ms and the same picture presented for an additional 400 ms. The target was an arrow that pointed to the left or to the right, and participants were required to press either the left or right button on a mouse to indicate the direction of the arrow. The intertrial interval varied randomly between 1,500 and 2,000 ms.
Psychophysiological recording, data reduction and analysis
Continuous electroencephalogram (EEG) was recorded using a 34-channel Biosemi system based on the 10/20 system (32 channel cap with the addition of Iz and FCz). Two electrodes were placed on the left and right mastoids, and the electrooculogram (EOG) generated from eye blinks and movements was recorded from two facial electrodes approximately one cm above and below the participant’s left eye, one electrode approximately one cm to the left of the left eye and one approximately one cm to the right of the right eye. The ground electrode during acquisition was formed by the Common Mode Sense active electrode and the Driven Right Leg passive electrode. The data were digitized using ActiView software at 24-bit resolution with a LSB value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. Off-line analysis was performed using Brain Vision Analyzer software (Brain Products). All data were converted to a mastoid reference and band-pass filtered with cutoffs of 0.1 and 30 Hz. The EEG was segmented for each trial, beginning 200 ms before each picture onset and continuing for 1000 ms after the initial image presentation. The EEG was corrected for eye blinks (Gratton, Coles, & Donchin, 1983), and semi-automated artifact rejection was used to remove artifacts with a voltage step of more than 50 µV between sample points, a voltage difference of 300 µV within a trial, or a maximum voltage difference of less than 0.5 µV within 100 ms intervals. Visual inspection was then used to reject trials in which additional artifacts were observed.
ERPs were constructed by separately averaging the responses to pleasant, neutral, and unpleasant images. Only correct trials with responses between 150–2,100 ms after target onset were included in averages. ERPs were baseline corrected to the 200 ms interval prior to stimulus onset. The LPP was scored as the mean activity 400–1,000 ms after the onset of the pre-target image averaged at parietal (P3, P4, and Pz) and occipital sites (O1, O2, and Oz).
Procedure
At each assessment, assent was obtained from participants and written informed consent was obtained from parents. Next, EEG sensors were attached and participants completed a series of tasks. The results of other tasks administered during the initial experimental session are presented elsewhere (Bress, Smith, Foti, Klein, & Hajcak, 2012; Glenn et al., 2012; Meyer, Weinberg, Klein, & Hajcak, 2012). All tasks were counterbalanced. The entire assessment took approximately 2 hours each time, and participants were paid $20 per hour for their participation. In addition, children received additional prizes, snacks, and a bonus for participating in a gambling task (see Bress et al., 2012). At the start of the emotional-interrupt task, the participant was instructed to press the left or right button on a mouse to indicate the direction of the arrow on the screen. Prior to the start of the task, participants completed 10 practice trials. Participants were contacted approximately 2 years following the initial assessment to return to the laboratory to again complete the emotional-interrupt task.
Results
Developmental Change over Two Years
LPP
Given previous cross-sectional evidence of developmental shifts in the scalp distribution of the LPP (Hajcak & Dennis, 2009; Kujawa, Klein, et al., 2012; Kujawa et al., 2013), analyses were first computed to evaluate whether there are mean-level changes in the LPP using longitudinal data. ERPs and scalp distributions at the first and second assessments are presented in Figure 1. A 2 (Assessment: time 1 and time 2) X 3 (Emotion: neutral, pleasant and unpleasant images) X 2 (Electrode site: parietal, occipital) within-subjects ANOVA was computed to examine changes in the LPP over time. As expected, the LPP was significantly modulated by emotion, F(2, 66) = 32.78, p < .001, η2p = .50. Both pleasant, F(1, 33) = 15.85, p < .001, η2p = .32, and unpleasant images, F(1, 33) = 57.87, p < .001, η2p = .64, were associated with an increased positivity compared to neutral images. Unpleasant images were also associated with an increased positivity compared to pleasant images, F(1, 33) = 18.96, p < .001, η2p = .37. Overall, the LPP was larger at occipital compared to parietal sites, F(1, 33) = 227.71, p < .001, η2p = .87, and at the first assessment compared to the second assessment, F(1, 33) = 35.05, p < .001, η2p = .52. The emotion by electrode site, F(2, 66) = 6.74, p < .01, η2p = .17, and electrode site by time, F(1, 33) = 25.87, p < .001, η2p = .44, interactions were also significant. The emotion by time, F(2, 66) = 1.69, p = .19, η2p = .05, and three-way interactions were not significant, F(2, 66) = 0.10, p = .90, η2p = .00.
Figure 1.
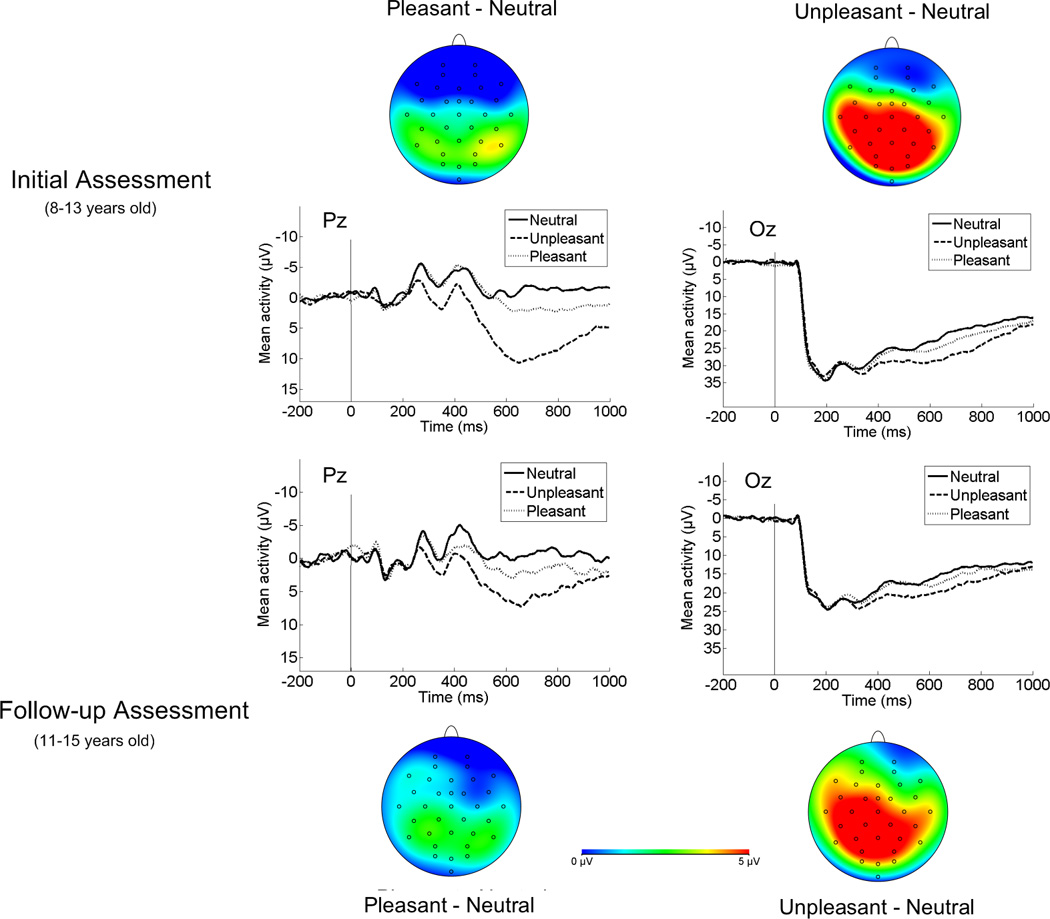
Emotion-neutral scalp distributions and ERPs (negative up) at the initial assessment and the follow-up assessment.
To interpret the emotion by electrode site interaction, the effect of electrode site was examined separately for unpleasant minus neutral and pleasant minus neutral difference scores. The relative increase for unpleasant compared to neutral images was greater at parietal than occipital sites, F(1, 33) = 11.54, p < .01, η2p = .26, whereas the increased LPP for pleasant compared to neutral images was similar at parietal and occipital sites, F(1, 33) = 1.01, p = .32, η2p = .03.
To interpret the electrode site by time interaction, the effect of time was examined at both parietal and occipital sites. At occipital sites, the effect of time was significant, F(1, 33) = 73.67, p < .001, η2p = .69, with reduced activation over occipital sites across development (see Figure 2). At parietal sites, the effect of time was not significant, F(1, 33) = 1.68, p = .21, η2p = .05.
Figure 2.
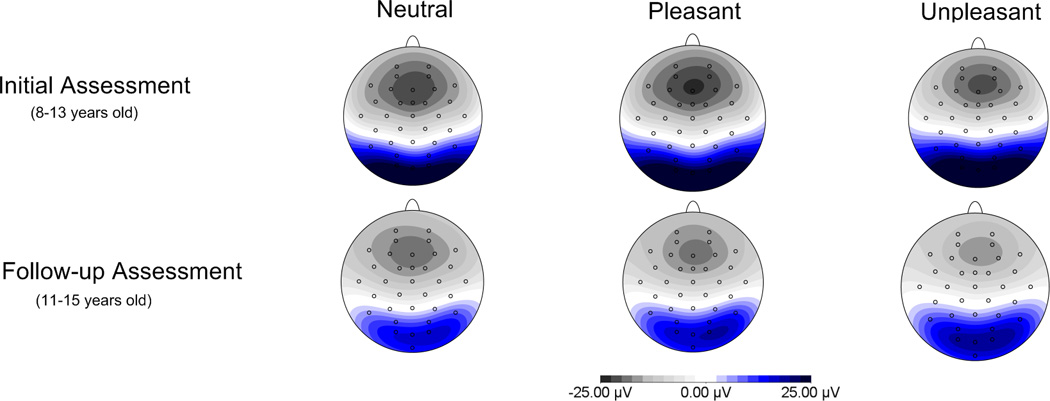
Scalp distributions depicting overall activation at the first and second assessment. Less overall activation was observed over occipital sites at the second assessment relative to the first assessment
Behavioral measures
Additional analyses were computed to evaluate developmental changes in behavioral measures of responses to emotional stimuli. First, a 2(Time) X 3(Emotion) ANOVA was computed to examine the effects of the assessment and emotional stimuli on reaction time (RT). RT was significantly faster at the second assessment, F(1, 33) = 28.04, p < .001, η2p = .46. The main effect of emotion was also significant, F(2, 66) = 3.28, p < .05, η2p = .09, with pleasant images associated with slower RTs compared to neutral images, F(1, 33) = 9.74, p < .01, η2p = .23. The difference between unpleasant and neutral on RT did not reach significance, F(1, 33) = 2.69, p = .11, η2p = .08. The time by emotion interaction was not significant, F(2, 66) = .05, p = .95, η2p = .00.
Next, A 2(Time) X 3(Emotion) ANOVA was computed to examine the effects on accuracy. The effect of emotion was significant, F(2, 66) = 4.79, p < .05, η2p = .13, with lower accuracy for unpleasant compared to neutral images, F(1, 33) = 9.62, p < .01, η2p = .23. The difference between accuracy on pleasant compared to neutral trials was not significant, F(1, 33) = 0.80, p = .38, η2p = .02. The main effect of time, F(1, 33) = 1.17, p = .29, η2p = .03, and time by emotion interaction, F(2, 66) = 1.43, p = .25, η2p = .04, were not significant.
Two-Year Stability of LPP and Behavioral Measures
Next, analyses were computed to evaluate the extent to which LPP and behavioral measures are stable across two years, despite the developmental changes previously observed. In order to evaluate stability of the LPP at occipital and parietal sites and behavioral (i.e., RT and accuracy) measures, Pearson’s r and intraclass correlations (ICC) were calculated across time points. Though Pearson’s r and ICC are both measures of stability, r assesses rank-order stability, whereas ICC reflects both rank-order and mean-level stability. Both measures have the potential to provide useful information about stability and change in the LPP across development. Two-way mixed single measures ICCs (absolute agreement) were calculated, corresponding to Shrout and Fleiss (1979) ICC(3, 1).
Means, standard deviations, correlations (r), and ICCs between measures at Time 1 and Time 2 are presented in Table 1. According to Pearson’s r values, the LPP was highly stable across assessment periods, and RT was associated with small-to-moderate rank-order stability. Accuracy following unpleasant images was moderately stable, but accuracy after neutral and pleasant images was not. For LPPs, ICC measures of stability were good (Cicchetti, 1994) and comparable to Pearson’s r. For behavioral measures, only the ICC for accuracy following unpleasant images reached significance. Figure 3 depicts the correlations (Pearson’s r) between Time 1 and Time 2 LPP (i.e., mean activity 400–1,000 ms after stimulus onset) at each electrode for pleasant (top), unpleasant (middle), and neutral (bottom) images. Correlations were highest where the LPP was maximal, over centro-parietal and occipital regions.
Table 1.
Means (SDs) and test-retest reliability of behavioral and ERP measures
| Time 1 Mean (SD) |
Time 2 Mean (SD) |
Time 1 to Time 2 r |
Time 1 to Time 2 ICC |
|
|---|---|---|---|---|
| Neutral RT (ms) | 600.42(214.41) | 422.98(56.36) | .41* | .13 |
| Pleasant RT (ms) | 610.96(214.75) | 432.05(66.13) | .26 | .09 |
| Unpleasant RT (ms) | 609.34(214.04) | 428.97(75.98) | .44** | .17† |
| Neutral Accuracy (% correct) | 96.91(2.76) | 94.85(6.31) | .14 | .09 |
| Pleasant Accuracy (% correct) | 95.44(4.01) | 95.15(4.39) | −.07 | −.07 |
| Unpleasant Accuracy (% correct) | 94.12(4.68) | 93.90(5.85) | .38* | .38* |
| Neutral parietal LPP (µV) | 1.66(9.57) | 1.05(7.69) | .62*** | .61*** |
| Pleasant parietal LPP (µV) | 4.43(10.33) | 3.37(8.50) | .74*** | .73*** |
| Unpleasant parietal LPP (µV) | 9.10(9.59) | 6.62(7.73) | .70*** | .66*** |
| Neutral occipital LPP (µV) | 22.50(10.14) | 15.50(8.40) | .72*** | .55*** |
| Pleasant occipital LPP (µV) | 24.82(10.75) | 17.28(9.05) | .83*** | .64*** |
| Unpleasant occipital LPP (µV) | 27.89(9.63) | 19.32(9.92) | .83*** | .60*** |
p = .05
p < .05
p < .01
p < .001
Figure 3.
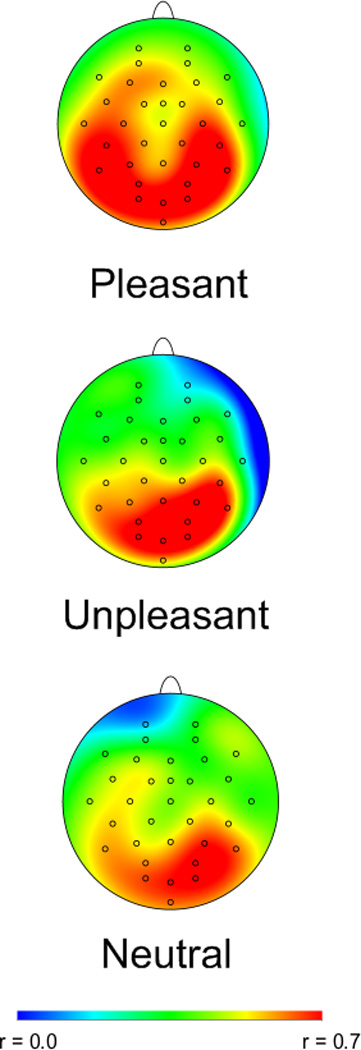
Scalp distributions depicting the correlation (Pearson’s r) between mean activity at the first and second assessments 400–1000 ms after picture onset. Correlations were greatest over centro-parietal and occipital sites, where the LPP is observed.
In order to evaluate the stability of reactivity to emotional relative to neutral images, difference scores were calculated by subtracting neutral from both pleasant and unpleasant values for RT, accuracy, and the LPP at occipital and parietal sites. Pearson’s r and ICCs between Time 1 and 2 for these difference scores are presented in Table 2. The unpleasant LPP over parietal sites was moderately stable across two years (Figure 4). A negative r was found between the RT for pleasant images at Time 1 and Time 2; however, the ICC was not significant. Pearson’s r and ICC did not reach significance for any of the remaining difference scores.
Table 2.
Means (SDs) and test-retest reliability of behavioral and ERP difference scores (emotion-neutral)
| Time 1 Mean (SD) |
Time 2 Mean (SD) |
Time 1 to Time 2 r |
Time 1 to Time 2 ICC |
|
|---|---|---|---|---|
| Pleasant-Neutral RT (ms) | 10.55(33.82) | 9.08(29.84) | −.34* | −.35 |
| Unpleasant-Neutral RT (ms) | 8.92(43.57) | 5.99(37.77) | −.16 | −.16 |
| Pleasant-Neutral Accuracy (% correct) | −1.47(5.26) | 0.29(4.95) | .13 | .13 |
| Unpleasant-Neutral Accuracy (% correct) | −2.79(4.91) | −0.96(5.40) | −.07 | −.07 |
| Pleasant-Neutral parietal LPP (µV) | 2.77(5.27) | 2.32(5.13) | .11 | .11 |
| Unpleasant-Neutral parietal LPP (µV) | 7.44(5.56) | 5.57(5.45) | .48** | .46** |
| Pleasant-Neutral occipital LPP (µV) | 2.32(4.52) | 1.77(4.53) | .15 | .15 |
| Unpleasant-Neutral occipital LPP (µV) | 5.39(5.82) | 3.81(5.19) | .26 | .26 |
p < .05
p < .01
Figure 4.
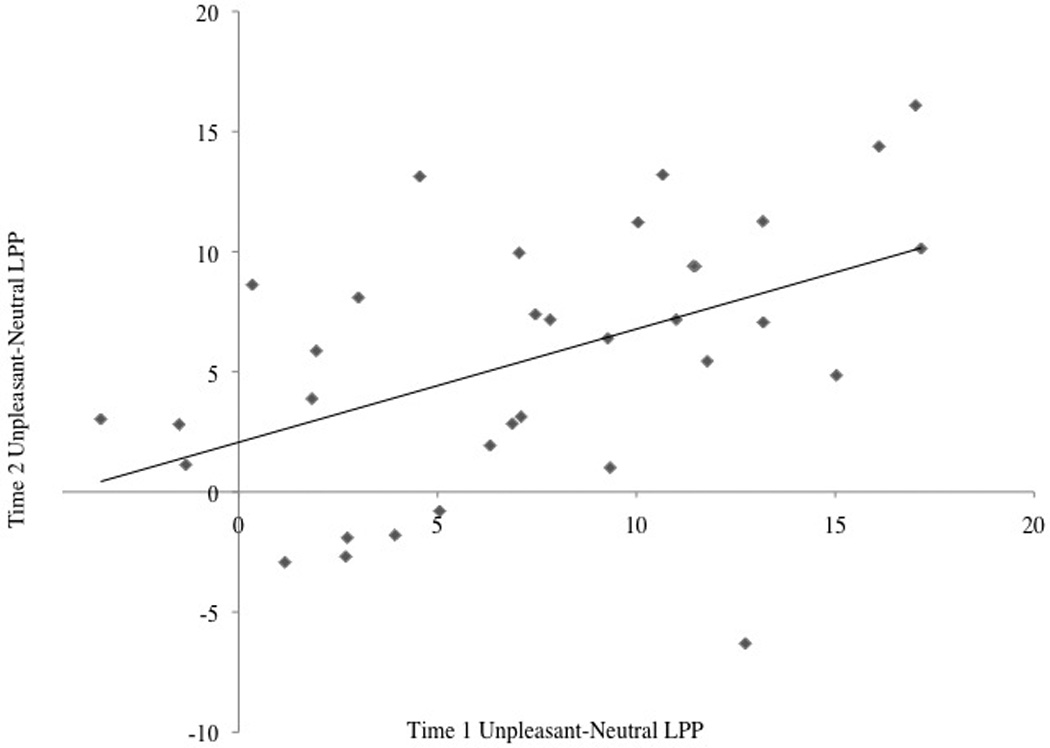
Scatter plot depicting the moderate correlation between the first and second assessments for the unpleasant-neutral difference LPP at parietal sites.
Discussion
The current study examined test-retest stability of the LPP and behavioral measures of emotional processing across development. With regard to rank-order stability, results indicated that across a period of two years, overall LPPs to emotional and neutral images were highly stable. In addition, behavioral measures were moderately, but inconsistently, correlated over time. Accounting for both mean level and rank-order stability, LPPs show good test-retest reliability (see Cicchetti, 1994 for guidelines for interpreting ICCs), while behavioral measures show poor to fair reliability. Given the significant developmental changes occurring in late childhood and early adolescence, as well as the relatively long period of time between assessments, these results are encouraging and suggest that the LPP may be a reliable trait-like measure of emotional processing.
The relative increase in LPP for emotional compared to neutral images may be particularly interesting for evaluating individual differences in emotional processing. The unpleasant minus neutral LPP difference score was moderately reliable and in the fair range according to Cicchetti (1994). On the other hand, stability coefficients were not significant for the pleasant minus neutral difference score. This may have to do with the nature of the images used in the pleasant category, as there is evidence that there is substantial variability in response to specific types of stimuli within the broad categories of pleasant and unpleasant (Briggs & Martin, 2009; Weinberg & Hajcak, 2010). The images selected for the current study were chosen for younger children and included a number of positive images of recreational and exciting sports activities, a category of images that has been shown to elicit a smaller LPP in adults (Briggs & Martin, 2009; Weinberg & Hajcak, 2010). Thus, it is possible that the pleasant images used in this study were particularly low in motivational salience for older adolescents, and this may contribute to the lack of reliability for pleasant-neutral difference scores. The pleasant images in the current study also included a number of affiliative images (e.g., cute babies and animals), which have been associated with a comparable LPP to threat images in adults (Weinberg & Hajcak, 2010). It is possible that the reliability for pleasant images may be comparable to that of unpleasant images if more affilitative rather than sports images were used. However, relatively little work has focused on the ways that children respond to specific classes of stimuli, and future research should continue to develop emotional stimuli that are both salient and developmentally appropriate.
It is important to note that difference scores are expected to be less reliable than overall scores, as they include the error associated with measuring both the reactivity to emotional and neutral images. In addition, changes in the scalp distribution of the LPP across development may reduce stability. It is also possible, however, that there are developmental changes in the psychological processing of emotional images that contribute to the lower stability of difference scores. We are unaware of previous studies that have examined the long-term stability of the LPP in adults; thus, we cannot determine the extent to which the relatively low stability of difference scores is driven by developmental changes or by error in the measurement of the LPP across time. Future research evaluating the stability of behavioral and LPP measures of emotional reactivity in adults is needed to evaluate this question.
Stability of behavioral measures (i.e., reaction time and accuracy) did not consistently reach significance. Overall reaction times following neutral and unpleasant images were moderately stable in terms of rank-order stability, but reaction times for pleasant images were not. Relatively low rank-order stability suggests that there may be nonsystematic developmental changes in behavioral responses to emotional images. ICCs for reaction times were lower than Pearson’s r, likely because overall reaction times decreased across assessments. In terms of accuracy, only accuracy following unpleasant images was stable across time. Taken together, these results suggest that reaction time and accuracy change considerably across time and may not be the most reliable measures of individual differences in emotional processing in children. In addition, the emotion minus neutral difference scores for reaction time and accuracy were unreliable across time. Though a significant correlation was observed for the pleasant minus neutral difference in reaction time, this was a negative rather than positive association. It is unclear why children who show greater behavioral interference from pleasant images at the initial assessment would show less interference at the follow up assessment. This finding may further highlight inconsistencies in behavioral measures across development.
Importantly, similar patterns of LPP modulation by emotion were observed at each assessment and few developmental changes in the LPP were observed. This suggests that psychological processes involved in emotional reactivity may remain relatively stable across time. The major developmental change observed was an overall decrease in the amplitude of the LPP, particularly at occipital sites. There are several possible explanations for this change. First, there is evidence that development is associated with a decrease in the latency and amplitude of the P300, which has been proposed to be linked to synaptic pruning and myelination (Berman, Friedman, & Cramer, 1990; Berman et al., 2006; Courchesne, 1978; Stauder, Molenaar, & Van der Molen, 1999). Though the P300 seems to overlap with the LPP, there is also evidence that it may be a somewhat distinct component (Foti, Hajcak, & Dien, 2009; Kujawa et al., 2013). It is possible that similar developmental processes contribute to the reductions in both the LPP and P300. However, the decrease in the LPP in the current study appeared somewhat specific to occipital sites, a finding that is consistent with our previous observations based on cross-sectional analyses (Kujawa, Klein, et al., 2012; Kujawa, Weinberg, et al., 2012). We have previously suggested (Kujawa, Weinberg, et al., 2012) that there may be a developmental shift in emotional processing from a reliance on brain regions associated with visual processing areas to more connectivity with the frontal attention networks that may sustain the LPP in adults (Moratti, Saugar, & Strange, 2011). The current findings remain consistent with this possibility; however, future research could further explore this possibility by linking ERP and fMRI measures of emotional reactivity across development.
A few limitations of the current study should be noted. First, though we have used the same paradigm in previous studies of ERPs to emotional images in children (Kujawa, Klein et al., 2012; Kujawa, Weinberg et al., 2012), emotional IAPS images were not matched on valence and arousal. Lang et al. (2008) provide normative ratings of valence and arousal; however, child ratings are only available for a subset of images in the current study. As we did not collect valence and arousal ratings from the current participants, we are unable to evaluate the extent to which differences in valence and arousal may contribute to the results. Consistent with normative adult ratings, it is possible that the selected unpleasant images were more arousing than pleasant images, which may contribute to the weaker effects for the pleasant minus neutral difference score. In addition, there may be developmental changes in the valence and arousal of the images that we cannot account for here. Future research could examine how valence and arousal relate to the stability of the LPP.
Taken together, the current findings suggest that the LPP is a relatively reliable measure of emotional reactivity across childhood and adolescence. In addition, the LPP appears to be more reliable than some behavioral measures of emotional interference, suggesting ERP methods may be particularly useful for developmental research and individual differences studies.
Highlight.
Examined two-year stability of the LPP to emotional images in youth
The LPP is a moderately reliable measure of emotional reactivity across development
Behavioral measures of emotional interference are less consistently reliable
Reductions in activation over occipital sites were observed across development
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
IAPS pictures used were: pleasant (1463, 1710, 1750, 1811, 2070, 2091, 2092, 2224, 2340, 2345, 2347, 7325, 8031, 8200, 8461, 8496, 8497, 8370, 7400, 7330); neutral (5395, 7026, 7130, 7190, 7175, 2514, 7038, 2580, 5390, 7090, 5500, 5731, 5740, 7100, 5900, 7000, 7002, 7009, 7010, 7039); unpleasant (1050, 1052, 6571, 1205, 1200, 1300, 1304, 1930, 2458, 9600, 2691, 2703, 2800, 2811, 2900, 3022, 6190, 6213, 6231, 6510)
References
- Berman S, Noble EP, Antolin T, Sheen C, Conner BT, Ritchie T. P300 development during adolescence: Effects of DRD2 genotype. Clinical Neurophysiology. 2006;117(3):649–659. doi: 10.1016/j.clinph.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Berman S, Noble EP, Antolin T, Sheen C, Conner BT, Ritchie T. P300 development during adolescence: Effects of DRD2 genotype. Clinical Neurophysiology. 2006;117(3):649–659. doi: 10.1016/j.clinph.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology. 2012;89:156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs KE, Martin FH. Affective picture processing and motivational relevance: Arousal and valence effects on ERPs in an oddball task. International Journal of Psychophysiology. 2009;72(3):299–306. doi: 10.1016/j.ijpsycho.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6(4):284–290. [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: Changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography & Clinical Neurophysiology. 1978;45(4):468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52(2):95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- DeCicco JM, Solomon B, Dennis TA. Neural correlates of cognitive reappraisal in children: An ERP study. Developmental Cognitive Neuroscience. 2012;2(1):70–80. doi: 10.1016/j.dcn.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46(3):521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Frischen A, Eastwood JD, Smilek D. Visual search for faces with emotional expressions. Psychological Bulletin. 2008;134(5):662. doi: 10.1037/0033-2909.134.5.662. [DOI] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. The development of fear learning and generalization in 8-13 year-olds. Developmental Psychobiology. 2012;54(7):675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80(3):333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman E, editors. Handbook of event-related potential components. New York: Oxford University Press; 2011. [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry. 2012;53(2):207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Hajcak G. Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience. 2012;2(4):458–467. doi: 10.1016/j.dcn.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Hajcak G, Klein DN. Differentiating event?related potential components sensitive to emotion in middle childhood: Evidence from temporal-spatial PCA. Developmental Psychobiology. 2013;55:539–550. doi: 10.1002/dev.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, FL: University of Florida; 2008. International Affective Picture System (IAPS): Affective ratings of pictures and instructional manual. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Köchel A, Scharmüller W, Schienle A. Psychophysiology of spider phobia in 8- to 12-year-old girls. Biological Psychology. 2010;85(3):424–431. doi: 10.1016/j.biopsycho.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology. 2009;82(3):293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowski JM, Melzig CA, Weike AI, Stockburger J, Schupp HT, Hamm AO. Brain dynamics in spider-phobic individuals exposed to phobia-relevant and other emotional stimuli. Emotion. 2009;9(3):306–315. doi: 10.1037/a0015550. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Richell RA, Leonard A, Blair RJR. Emotion at the expense of cognition: Psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology. 2006;115(3):559–566. doi: 10.1037/0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology; Development and Psychopathology; doi: doi:10.1017/S095457940800059X; 2008. pp. 1231–1250. [DOI] [PubMed] [Google Scholar]
- Moratti S, Saugar C, Strange BA. Prefrontal-occipitoparietal coupling underlies late latency human neuronal responses to emotion. Journal of Neuroscience. 2011;31(47):17278–17286. doi: 10.1523/JNEUROSCI.2917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, Simons RF. Face processing biases in social anxiety: An electrophysiological study. Biological Psychology. 2008;78(1):93–103. doi: 10.1016/j.biopsycho.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Herrmann MJ, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. Journal of Neural Transmission. 2009;116(6):735–746. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, present, and future. Social cognitive and affective neuroscience. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LaBar KS, Spencer DD. Memory for emotional words following unilateral temporal lobectomy. Brain and Cognition. 1997;35(1):85–109. doi: 10.1006/brcg.1997.0929. [DOI] [PubMed] [Google Scholar]
- Schmukle SC. Unreliability of the dot probe task. European Journal of Personality. 2005;19(7):595–605. [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Solomon B, DeCicco JM, Dennis TA. Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience. 2012;2(1):110–119. doi: 10.1016/j.dcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder JEA, Molenaar PCM, Van der Molen MW. Brain activity and cognitive transition during childhood: A longitudinal event-related brain potential study. Child Neuropsychology. 1999;5(1):41–59. [Google Scholar]
- Staugaard SR. Reliability of two versions of the dot-probe task using photographic faces. Psychology Science Quarterly. 2009;51(3):339–350. [Google Scholar]
- Weinberg A, Ferri J, Hajcak G. Interactions between Attention and Emotion: Insights from the Late Positive Potential. In: Robinson MD, Watkins ER, Harmon-Jones E, editors. Handbook of cognition and emotion. New York, NY: Guilford Press; pp. 35–54. (in pross) [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion. 2010;10(6):767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. 2011;23(10):2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention: A review of attentional processing of emotional information. Cognition and Emotion. 2010;24(1):3–47. [Google Scholar]


