Abstract
Isothermal titration microcalorimetry is combined with solution-depletion isotherm data to analyze the thermodynamics of binding of the cellulose-binding domain (CBD) from the beta-1,4-(exo)glucanase Cex of Cellulomonas fimi to insoluble bacterial microcrystalline cellulose. Analysis of isothermal titration microcalorimetry data against two putative binding models indicates that the bacterial microcrystalline cellulose surface presents two independent classes of binding sites, with the predominant high-affinity site being characterized by a Langmuir-type Ka of 6.3 (+/-1.4) x 10(7) M-1 and the low-affinity site by a Ka of 1.1 (+/-0.6) x 10(6) M-1. CBDCex binding to either site is exothermic, but is mainly driven by a large positive change in entropy. This differs from protein binding to soluble carbohydrates, which is usually driven by a relatively large exothermic standard enthalpy change for binding. Differential heat capacity changes are large and negative, indicating that sorbent and protein dehydration effects make a dominant contribution to the driving force for binding.
Full text
PDF

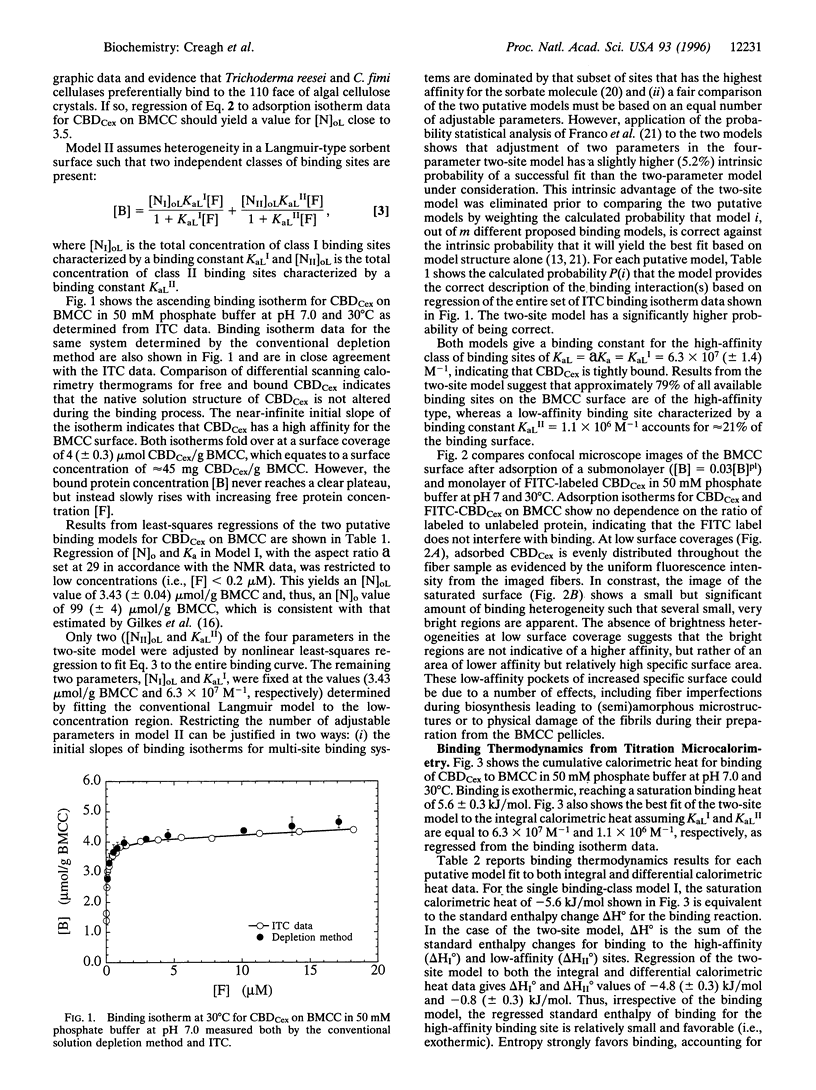
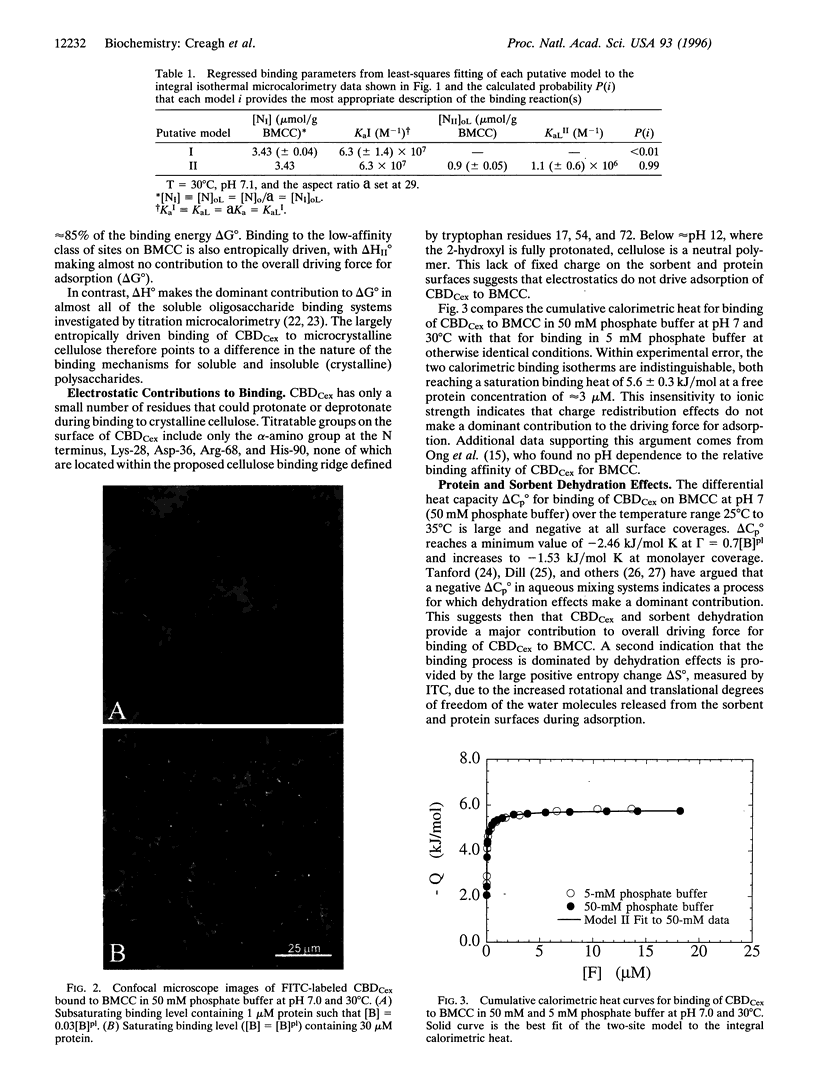


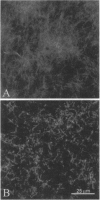
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandts J. F., Jackson W. M., Ting T. Y. A calorimetric study of the thermal transitions of three specific transfer ribonucleic acids. Biochemistry. 1974 Aug 13;13(17):3595–3600. doi: 10.1021/bi00714a030. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Eichler E., Gidney M. A., Meldal M., Ragauskas A., Sigurskjold B. W., Sinnott B., Watson D. C., Yaguchi M., Young N. M. Molecular recognition of a Salmonella trisaccharide epitope by monoclonal antibody Se155-4. Biochemistry. 1994 May 3;33(17):5172–5182. doi: 10.1021/bi00183a022. [DOI] [PubMed] [Google Scholar]
- Carver J. P., Michnick S. W., Imberty A., Cumming D. A. Oligosaccharide-protein interactions: a three-dimensional view. Ciba Found Symp. 1989;145:6–26. doi: 10.1002/9780470513828.ch2. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Din N., Forsythe I. J., Burtnick L. D., Gilkes N. R., Miller R. C., Jr, Warren R. A., Kilburn D. G. The cellulose-binding domain of endoglucanase A (CenA) from Cellulomonas fimi: evidence for the involvement of tryptophan residues in binding. Mol Microbiol. 1994 Feb;11(4):747–755. doi: 10.1111/j.1365-2958.1994.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Franco R., Gavaldà M. T., Canela E. I. A computer program for enzyme kinetics that combines model discrimination, parameter refinement and sequential experimental design. Biochem J. 1986 Sep 15;238(3):855–862. doi: 10.1042/bj2380855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Jervis E., Henrissat B., Tekant B., Miller R. C., Jr, Warren R. A., Kilburn D. G. The adsorption of a bacterial cellulase and its two isolated domains to crystalline cellulose. J Biol Chem. 1992 Apr 5;267(10):6743–6749. [PubMed] [Google Scholar]
- Gilkes N. R., Warren R. A., Miller R. C., Jr, Kilburn D. G. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J Biol Chem. 1988 Jul 25;263(21):10401–10407. [PubMed] [Google Scholar]
- Greenwood J. M., Gilkes N. R., Kilburn D. G., Miller R. C., Jr, Warren R. A. Fusion to an endoglucanase allows alkaline phosphatase to bind to cellulose. FEBS Lett. 1989 Feb 13;244(1):127–131. doi: 10.1016/0014-5793(89)81177-9. [DOI] [PubMed] [Google Scholar]
- Greenwood J. M., Ong E., Gilkes N. R., Warren R. A., Miller R. C., Jr, Kilburn D. G. Cellulose-binding domains: potential for purification of complex proteins. Protein Eng. 1992 Jun;5(4):361–365. doi: 10.1093/protein/5.4.361. [DOI] [PubMed] [Google Scholar]
- Kennedy W. F., Jr, Everett G. B., Cobb L. A., Allen G. D. Simultaneous systemic and hepatic hemodynamic measurements during high peridural anesthesia in normal man. Anesth Analg. 1971 Nov-Dec;50(6):1069–1077. doi: 10.1213/00000539-197150060-00029. [DOI] [PubMed] [Google Scholar]
- Majlof L., Forsgren P. O. Confocal microscopy: important considerations for accurate imaging. Methods Cell Biol. 1993;38:79–95. doi: 10.1016/s0091-679x(08)61000-6. [DOI] [PubMed] [Google Scholar]
- Ong E., Gilkes N. R., Miller R. C., Jr, Warren A. J., Kilburn D. G. Enzyme immobilization using a cellulose-binding domain: properties of a beta-glucosidase fusion protein. Enzyme Microb Technol. 1991 Jan;13(1):59–65. doi: 10.1016/0141-0229(91)90189-h. [DOI] [PubMed] [Google Scholar]
- Sigurskjold B. W., Svensson B., Williamson G., Driguez H. Thermodynamics of ligand binding to the starch-binding domain of glucoamylase from Aspergillus niger. Eur J Biochem. 1994 Oct 1;225(1):133–141. doi: 10.1111/j.1432-1033.1994.00133.x. [DOI] [PubMed] [Google Scholar]
- Spurlino J. C., Rodseth L. E., Quiocho F. A. Atomic interactions in protein-carbohydrate complexes. Tryptophan residues in the periplasmic maltodextrin receptor for active transport and chemotaxis. J Mol Biol. 1992 Jul 5;226(1):15–22. doi: 10.1016/0022-2836(92)90119-5. [DOI] [PubMed] [Google Scholar]
- Svensson B., Jespersen H., Sierks M. R., MacGregor E. A. Sequence homology between putative raw-starch binding domains from different starch-degrading enzymes. Biochem J. 1989 Nov 15;264(1):309–311. doi: 10.1042/bj2640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomme P., Warren R. A., Gilkes N. R. Cellulose hydrolysis by bacteria and fungi. Adv Microb Physiol. 1995;37:1–81. doi: 10.1016/s0065-2911(08)60143-5. [DOI] [PubMed] [Google Scholar]
- Wiseman T., Williston S., Brandts J. F., Lin L. N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989 May 15;179(1):131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- Wishnia A. Substrate specificity at the alkane binding sites of hemoglobin and myoglobin. Biochemistry. 1969 Dec;8(12):5064–5070. doi: 10.1021/bi00840a058. [DOI] [PubMed] [Google Scholar]
- Xu G. Y., Ong E., Gilkes N. R., Kilburn D. G., Muhandiram D. R., Harris-Brandts M., Carver J. P., Kay L. E., Harvey T. S. Solution structure of a cellulose-binding domain from Cellulomonas fimi by nuclear magnetic resonance spectroscopy. Biochemistry. 1995 May 30;34(21):6993–7009. [PubMed] [Google Scholar]