Abstract
Sumoylation is a recently described post-translational modification and only a few sumoylated neurotransmitter receptors are known. Through the present studies, we discovered that serotonin1A receptors (5-HT1A-Rs) can be sumoylated by SUMO1 (Small-Ubiquitin-related modifier 1) protein. The SUMO1-5-HT1A-R is ∼ 55kD, is located in the membrane fraction, but not the cytosol, and is distributed in all of the brain regions expressing 5-HT1A-Rs examined. Acute stimulation of 5-HT1A-Rs significantly increased SUMO1-5-HT1A-R in rat hypothalamus. Pre-treatment with estradiol for 2 days, which causes a partial desensitization of 5-HT1A-R signaling, potentiated agonistinduced increases in SUMO1-5-HT1A-Rs in the hypothalamus of ovariectomized rats. Using discontinuous gradient centrifugation followed by digitonin treatment, we found that the majority of SUMO1-5-HT1A-Rs is co-localized with endoplasmic-reticulum and trans-Golgi-network markers. Although a small proportion of SUMO1-5-HT1A-Rs are located in the detergent resistant microdomain (DRM) that contain active G-protein coupled receptors, their distribution was different from that of the Gαz protein that couples to the receptors. These data suggest that the SUMO1-5-HT1A-Rs are an inactive form of 5-HT1A-Rs, a finding further supported by results showing minimal 5-HT1A-R agonist binding to SUMO1-5-HT1A-Rs. Furthermore, SUMO1-5-HT1A-Rs in the DRM were increased by treatment with a 5-HT1A-R agonist, 8-OH-DPAT ((+)8-hydroxy-2-dipropylaminotetralin). Together, these data suggest that sumoylation of 5-HT1A-Rs may be related to 5-HT1A-R trafficking and internalization, which may contribute to 5-HT1A-R desensitization. Since 5-HT1A-Rs play an important role in mood regulation, the present results significantly impact on the understanding of the pathogenesis of affective disorders and development of better therapeutic approaches for these diseases.
Keywords: SUMO1, endoplasmic reticulum, lipid raft, estradiol, fluoxetine, hypothalamus
Introduction
Sumoylation is a relatively novel post-translational protein modification in which a small-ubiquitin-related modifier (SUMO) protein covalently binds to target proteins. SUMO proteins, including SUMO1, SUMO2 and SUMO 3, are small proteins with 95–101 amino acids (Wilkinson and Henley, 2010). Sumoylation alters the function of the target proteins depending on the target proteins and cell type in which it occurs (Gareau and Lima, 2010; Geiss-Friedlander and Melchior, 2007; Wilkinson and Henley, 2010). The majority of sumoylated proteins identified so far are transcription factors, in which sumoylation either facilitates their translocation to the nucleus or regulates their function in the nucleus. However, increasing evidence demonstrates that sumoylation of extranuclear proteins play important roles in the modulation of protein-protein interactions and consequently regulates the stability and function of these proteins (Andreou and Tavernarakis, 2009; Martin et al., 2007; Palmer et al., 2011; Wilkinson et al., 2010). As a result, protein sumoylation is involved in the regulation of several cellular processes, such as cell signaling, mitochondrial function and regulation of cell morphology . Furthermore, growing evidence demonstrates that the sumoylation of extra-nuclear proteins is related to the regulation of neuronal development and neuronal function (Anderson et al., 2009; Martin et al., 2007; Scheschonka et al., 2007). Studies have revealed that sumoylation is involved in the regulation of synaptic activity, such as altering neurotransmitter release, and the functioning of post-synaptic receptors, ion channels and transporters (Craig and Henley, 2012; Rusakov et al., 2011). Although this evidence suggests that sumoylation may play an important role in the regulation of neuronal function, the effects of sumoylation in central nervous system are not well understood. To date, relatively few proteins in neurons have been identified as substrates for sumoylation, especially extra-nuclear proteins. For example, only two G-protein-coupled receptors (GPCR), cannabinoid receptor 1 (CB1) and metabotropic glutamate receptor 8 (mGluR)8, have been identified as substrates for sumoylation (Enz, 2012; Gowran et al., 2009). Gowran et al found that agonist treatment decreased sumoylation of CB1 (Gowran et al., 2009). Although studies have demonstrated the sumoylation of mGluR8 (Dutting et al., 2011; Mao et al., 2011), no data are available regarding the effects of sumoylation on the function of the receptors. On the other hand, several studies on ionotropic kainate receptors demonstrate that agonist-stimulation induces sumoylation of kainate receptor subunits GluK2 (GluR6), resulting in reduced function of the receptors and increased receptor endocytosis (Chamberlain et al., 2012; Konopacki et al., 2011). Here, we report, for the first time, that another GPCR, serotonin1A receptor (5-HT1A-R) can be sumoylated.
5-HT1A-Rs play important roles in numerous neurological and behavioral processes, including mood regulation and the pathogenesis of anxiety and depression (Akimova et al., 2009). Mice constitutively lacking 5-HT1A-Rs are more anxious (Gross et al., 2000) and 5-HT1A-R agonists are used as anxiolytic drugs. Furthermore, desensitization of 5-HT1A-Rs is critical for the therapeutic effects of antidepressants (Artigas et al., 2001; Blier and Abbott, 2001). Our recent studies demonstrated that estradiol facilitates the desensitization of 5-HT1A-Rs in conjunction with the prototypical antidepressant, fluoxetine (Li et al., 2012). Thus, understanding the regulation of 5-HT1A-Rs will have a significant impact on our understanding of the pathogenesis of anxiety disorders and depression and on the development of better therapeutic approaches for affective disorders.
5-HT1A-Rs are GPCRs that are coupled to the Gαi/o-protein family (Valdizan et al., 2010). Our previous studies demonstrated that 5-HT1A-Rs in the hypothalamus mediating ACTH and oxytocin release are coupled with Gαz protein, a member of the Gαi/o protein family (Serres et al., 2000). Gαz protein and active 5-HT1A-Rs (Li et al., 2012; Renner et al., 2007), like other GPCRs, are located in the detergent resistant microdomain (DRM). The DRM contains GPCRs, G-proteins and other proteins associated with GPCR signaling, physically bringing together these components for GPCR signaling. On the other hand, proteins dynamically move in and out of the DRM based on the activity of GPCR signaling (Allen et al., 2007; Chini and Parenti, 2004). Previous studies reported regulation of 5-HT1A-Rs by post-translation modifications, such as phosphorylation, glycosylation and palmitoylation (Papoucheva et al., 2004). In the present study, we characterized the sumoylation of 5-HT1A-Rs and the possible role of sumoylation in receptor trafficking by identifying the effects of sumoylation on subcellular location of 5-HT1A-Rs and the responses to agonist stimulation and treatment with estradiol.
Methods
Animals
Adult female Sprague-Dawley rats (225- 250g) purchased from Harlan Laboratories (Indianapolis, IN) were housed two per cage in a temperature-, humidity-, and light-controlled room (12 h light/dark cycles). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and as approved by the University of Kansas Institutional Animal Care and Use Committees.
Effect of treatment with estradiol and/or fluoxetine for 2 days on the 8-OH-DPAT-induced increase in sumoylation of 5-HT1A-Rs in the hypothalamus
As we previously described (Li et al., 2012), ovariectomized female rats were injected with estradiol benzoate (EB) (10 µg/kg, 0.4ml/kg, sc) and/or fluoxetine (10 mg/kg, sc) for 2 days. Eighteen hours after the last injection, rats were injected with saline or 8-OH-DPAT, 200µg/kg, sc and were then decapitated 15 min after the injection. The brains were collected and stored at −80°C until use. The hypothalamus was dissected and prepared as described below. Three hundred micrograms of membrane protein were used for immunoprecipitation with the SUMO1 antibody followed by immunoblotting with 5-HT1A-R antibody.
Brain tissue preparation
was conducted as previously described (Li et al., 2012): Briefly, brain tissue was homogenized in 10 volume (v/w) of homogenate buffer (50 mM Tris, 150 mM NaCl and 10% sucrose, pH 7.4, containing 20 mM N-Ethylmaleimide (NEM), 1:100 diluted protease inhibitor cocktail, phosphatase inhibitor cocktail I and II (Sigma) using a homogenizer. After centrifugation at 25,000xg, 4°C for 1 hour, the supernatant was collected as the cytosolic fraction. The pellets were sonicated in solubilization buffer (20 mM Tris, pH 8, 1 mM EDTA, 100 mM NaCl, 1% sodium cholate containing 20 mM NEM, 1:100 diluted protease inhibitor cocktail, phosphatase inhibitor cocktail I and II) and then were shaken vigorously for 1 hr at 4°C. After a centrifugation at 25,000xg, 4°C for 1 hour, the supernatant was collected as the membrane fraction. Protein concentration of the samples was measured using BCA assay (Thermo Scientific Inc, Rockford IL).
Isolation of DRM from cortex tissue
To determine whether sumoylated 5-HT1A-Rs are located in the DRM, we isolated DRM from the cortex using a protocol described by Kumari and Francesconi (Kumari and Francesconi, 2011) with minor modification. Briefly, rat cortex was homogenized with a homogenizer driven by an overhead-motor at 500 rpm for 25 strokes in 10 volume of homogenate buffer (10 mM Tris–HCl, pH 7.4, 5 mM EDTA, 320 mM sucrose, 20mM NEM, 1:100 dilution of protease inhibitor cocktail (Sigma) and phosphatase inhibitor II & III cocktails (Sigma)). The homogenate was centrifuged at 800xg for 15 min at 4°C to remove the nucleus and cell debris. The supernatant was then centrifuged at 30,000xg for 30 min at 4°C. The pellet was resuspended in 2.2 ml of extracting buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100 (v/v), 20mM NEM, 1:100 dilution of protease and phosphatase inhibitor cocktails) and was then incubated for 10 min in ice. Two ml of the Triton X-100-treated homogenate was adjusted to a final concentration of 40% sucrose with an 80% sucrose solution in extracting buffer. The 40% sucrose Triton X-100-treated homogenate was placed on the bottom of a 13 ml centrifugation tube and was overlaid with 6 ml of 30% sucrose on the top. Finally, 2 ml of 5% sucrose buffer was placed on the top of 30% sucrose. The sucrose gradient was then centrifuged at 230,000x g, 4°C for 16 hours. After the centrifugation, 1 ml fractions were collected from top to bottom. To solubilize the membrane protein, 0.1 ml of 10% sodium cholate was added to the fractions, which were then sonicated and shaken for one hour at 4°C. The fractions were aliquoted and stored in −80°C until use. The protein concentration of the fractions was measured using Pierce BCA protein assay kit (Thermo Scientific Inc, Rockford IL). Ten and 300 µg of protein were used for immunoblot and immunoprecipitation assays, respectively.
Subcellular fractionation of brain samples
Subcellular organelles were separated using a discontinuous OptiPrep gradient centrifugation based on the manufacture’s protocol (http://www.axis-shield-density-gradient-media.com/organelleindexes.htm, Application 05 and 22) with modification. The rat brain regions were dissected and homogenized with 4 volumes of homogenate buffer (0.32M sucrose, 1mM EDTA 10mM Tris-HCl pH 7.4, 20 mM NEM, 1/100 dilution of protease and phosphatase inhibitors) using a Powergen 1000 (Fisher Scientific) homogenizer at speed 5, 4°C for ∼10 sec. After centrifugation at 1500x g, 4°C for 15 min, the supernatant was used for discontinuous gradient centrifugation. Iodixanol (Optiprep, Sigma) was diluted into 7.5, 10, 12.5, 15, 20, 25 and 30% with Diluent (0.25mM sucrose, 6mM EDTA 60mM Tris-HCl pH 7.4, 20 mM NEM, 1/100 dilution of protease and phosphatase inhibitors). 1 ml of each solution was under-layered from 7.5% to 30% sequentially in a 13 ml SW41 centrifuge tube. 1 ml of brain supernatant was layered on the 7.5% iodixanol solution and centrifuged at 200,000xg (40,300 rpm) at 4°C for 3 hours in a SW41 rotor. After the centrifugation, 0.5 ml fractions were collected from top to bottom of the tube. To solubilize the membrane proteins, 50 µl 10% sodium cholate was added into each fraction. The solution was sonicated at 25 Amp 3 times for 5 sec each with 5 sec intervals in ice and was then shaken vigorously at 4°C for 1 hour. The solution was then aliquoted and stored in −80°C until used for immunoblotting and immunoprecipitation of sumoylated 5-HT1A-Rs.
Digitonin treatment of the plasma membrane and the endoplasmic reticulum (ER) fractions
Digitonin binds to cholesterol, which shift the membranes containing high levels of cholesterol, such as the plasma membrane and endosomes, to a higher density, whereas the membrane compartments containing low cholesterol concentrations, such as ER and lysosomes, display little or no shift by digitonin treatment. To confirm that the SUMO1-5-HT1A-Rs are located in the trans-Golgi network (TGN), ER-Golgi intermediate compartment (ERGIC) and ER, we treated hippocampal subcellular fractions containing the ER and TGN with digitonin followed by a second discontinuous gradient centrifugation. The subcellular fractions containing plasma membrane were used as a control for digitonin treatment. The digitonin treatment protocol was modified from Castle JD (Castle, 2004).
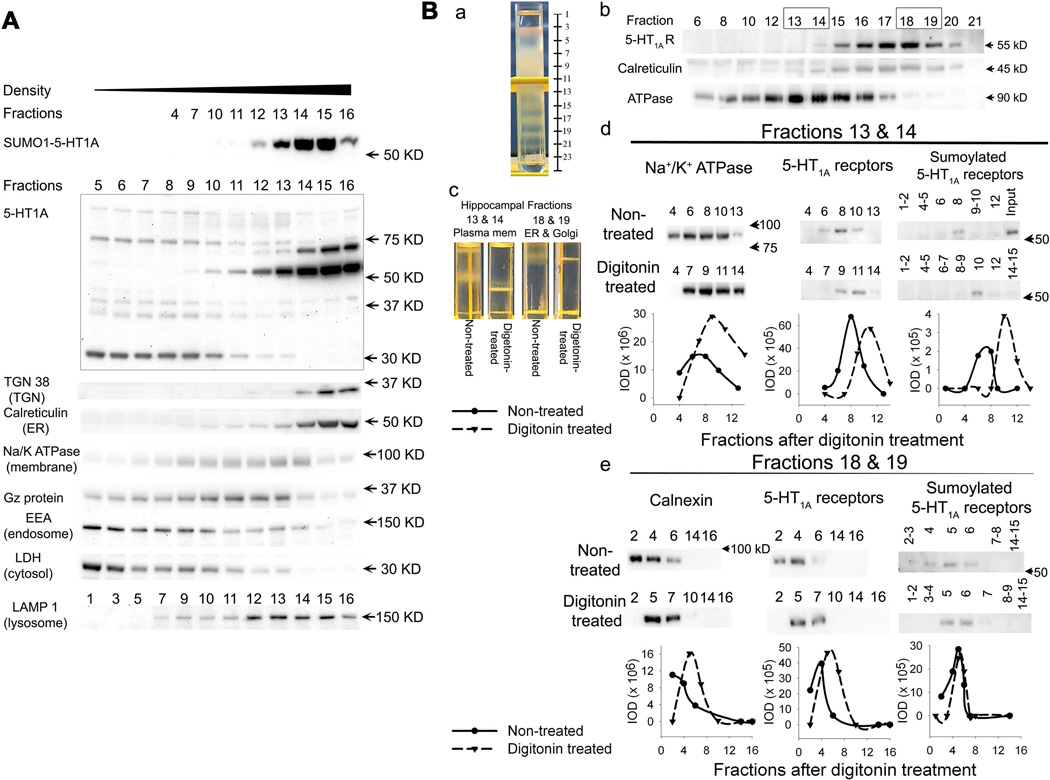
Hippocampal subcellular fractions (0.5 ml each) were obtained using gradient centrifugation as described above. The distributions of 5-HT1A-R, Na+/K+ ATPase and calnexin were identified using immunoblot assay (Fig.4). The fractions (#13 and #14) containing high levels of Na+/K+ ATPase, a marker of plasma membrane and the fractions (#18 and #19) containing high levels of calnexin, a ER marker, were pooled, respectively, and divided into two samples (∼0.5 ml/vial), which were treated with vehicle or digitonin (1.7 mg/ml) for 15 min at 4°C. The digitonin and vehicle- treated fractions were then subjected to a second discontinue gradient centrifugation. Fraction 13 & 14 were loaded on the top of a gradient with 17.5 –35% iodixanal (0.5 ml each of 17.5, 20, 22.5, 25, 27.5, 30, 32.5 and 35% iodixanol), whereas the fractions 18 & 19 were loaded on a discontinue gradient with 25–50% iodixanol (0.5 ml each of 25, 27.5, 30, 32.5, 35, 37.5,40, 45 and 50% iodixanol). After centrifugation at 230,000xg, at 4°C for 3 hours in a MLS 50 rotor, the fractions (0.25 ml each) were collected from the top to the bottom of the centrifuge tubes. The proteins in the fractions were solubilized by adding 25µl of 10% sodium cholate and shaken vigorously for 1 hour. The fractions were then aliquoted and stored in −80°C until use for immunoblot and immunoprecipitation.
Figure 4. Distribution of SUMO1-5-HT1A-Rs in the subcellular fractions from discontinuous iodixanol gradient centrifugation.
A. Comparison of the distribution of SUMO1-5-HT1A-Rs with the distribution of 5-HT1A-Rs, Gαz proteins and markers of subcellular organelles in the rat cortex. SUMO1-5-HT1A-R levels were determined using immunoprecipitation with a SUMO1 antibody followed by immunoblotting with a 5-HT1A-R antibody. The distribution of 5-HT1A-Rs, Gαz proteins and markers of organelles were determined using immunoblots with 10 µg of protein. B. Digitonin-induced shift of subcellular fractions in the hippocampus. a. Picture of discontinuous iodixanol gradient centrifugation showing the protein bands relative to the fractions collected. b. subcellular fractions of the hippocampus separated by discontinuous iodixanol gradient centrifugation. The boxed fractions (13 & 14) and (18 & 19) were pooled and further used for the digitonin-induced shift study. c. Example of discontinuous iodixanol gradient centrifugation of digitonin-treated hippocampal subcellular fractions, showing that digitonin treatment induced a shift of protein bands to the higher density in subcellular fractions 13 & 14 (plasma membrane), but not in fractions 18 & 19. d and e. Effects of digitonin treatment on the distribution of SUMO1-5-HT1A-Rs, 5-HT1A-Rs and subcellular markers, Na+/K+ ATPase (plasma membrane marker) and calnexin (ER marker) in digitonin-treated subcellular fractions of plasma membrane (fractions 13 & 14) and ER fractions (fraction 18 & 19), respectively. SUMO1-5-HT1A-R was determined using immunoprecipitation with a SUMO1 antibody followed by immunoblotting with a 5-HT1A receptor antibody. The distribution of 5-HT1A-R, Na+/K+ ATPase and calnexin were determined using immunoblots with 5 µg protein. The IOD of the bands of SUMO1-5-HT1A-R, 5-HT1A-R, Na+/K+ ATPase and calnexin was measured and presented as IOD.
3H-8-OH-DPAT binding of cortex subcellular fractions
To determine whether the sumoylated 5-HT1A-R are active, we determined the agonist binding activity of 5-HT1A-Rs in the subcellular fractions of cortical tissue. Cortex subcellular fractions were produced by discontinuous centrifugation as described above with slight modification. The tissue was homogenized and centrifuged without adding inhibitors because inhibitors disrupt the receptor binding activity. The discontinuous gradient centrifugation was performed as described in the subcellular fraction section except 10, 15, 17.5, 20, 22.5, 25, 27.5, 30, 35 and 40% Optiprep was used. After centrifugation, 1 ml fractions were collected from top to bottom. To determine the correlation between sumoylated 5-HT1A-R and binding activity, 0.5 ml aliquots of each fraction were added to 50µl 10% sodium cholate containing 200 mM NEM, 1/10 dilution of protease and phosphatase inhibitors immediately to prevent protein degradation. The aliquot was then sonicated and shaken to solubilize membrane proteins as described above and stored in −80°C until used for immunoprecipitation and immunoblotting. The remaining 0.5 ml aliquot of each fraction was used for a 3H-8-OH-DPAT binding assay immediately after collection of the fractions.
3H-8-OH-DPAT binding was conducted as previously described (Li et al., 1994). Subcellular fractions containing 150µg of protein were incubated with assay buffer (50 mM Tris-HCl, pH 7.4 10 mM MgSO4 and 0.5 mM EDTA) containing 3 nM 3H-8-OH-DPAT in a total volume of 0.5ml at room temperature for one hour. The non-specific binding was defined in the presence of 10µM 5-HT. The total and non-specific binding of each fraction were measured in triplicate and duplicate, respectively. The reaction solution was then filtered with GF/B filter paper and washed three times with 5 ml of 50 mM Tris-HCl, pH7.4. The radioactivity of the filter paper was counted in 5 ml of scintillation solution. The specific binding was calculated by subtracting the mean of non-specific binding from the mean of total binding.
Immunoprecipitation
Immunoprecipitation was conducted using 200–500 µg protein of the brain tissue preparation or fractions in total volume of 500 µl IP buffer (50 mM Tris, pH 7.4, 10 mM EGTA, 100 mM NaCl, 0.5% Triton X-100, containing 20 mM NEM, 1:100 dilution of protease inhibitor cocktail and phosphatase inhibitor cocktails). After preabsorption of endogenous IgG with 25µl pre-washed agarose-protein G beads (Invitrogen, CA). The protein was incubated with antibody, as listed in Table 1, at 4°C, overnight with nuitating. As a control, the same amount of IgG made in the same animal as the primary antibody was added into another tube containing same amount tissue. The solution was then incubated with 50µl pre-washed agarose-protein G beads for 2 hours at 4°C with nuitating. After centrifugation at 1000 × g, 4°C for 3 min, the supernatant was decanted. The beads were then washed with 0.5 ml IP buffer followed by centrifugation at 1000xg, 4°C for 3 min. After washing three times, 25 µl PAGE sample buffer (1.3X) was added and incubated at 95°C for 5 min to elute bound proteins. The eluate (∼35–40µl) from each immunoprecipitation reaction was resolved in SDS-PAGE gels to identify the proteins.
Table 1.
Antibodies used for immunoprecipitation and immunoblots
| Antibodies | IP | Immunoblot | Source |
|---|---|---|---|
| Mouse-anti-SUMO-1 (D-11) | 4 µg | Santa Cruz #sc-5308 | |
| Rabbit-anti-SUMO-1 | 1:1,000 | Enzo #BML-PW0505 | |
| Rabbit-anti-Gαz (1–20) | 4 µg | 1:2,000 | Santa Cruz #sc-388 |
| Rabbit-anti-5HT1A receptor | 15 µg | 1:500 | Gift from Dr. Scrogin |
| Mouse-anti-Flotillin-1 | 1:2,000 | BD #610820 | |
| Rabbit-anti-Ubiquitin | 1:1,000 | DAKO#Z0458 | |
| Goat-anti-ubc9 | 1:200 | Santa Cruz #sc-5231 | |
| Mouse-anti-Na+/K+ ATPase α1 | 1:2,000 | Santa Cruz #sc-21712 | |
| Rabbit-anti-calreticulin | 1:2,000 | Abcam #Ab4 | |
| Rabbit-anti-calnexin | 1:10,000 (1 hour) | GenScript #A01240 | |
| Rabbit-anti-LMAN1 | 1:500 | Epitomic #T3357 | |
| Rabbit-anti-TGN38 (M290)* | 1:1,000 | Santa Cruz #sc-33784 | |
| HRP conjugated LDH | 1:4,000 | Abcam #Ab7639-1 | |
| Goat-anti-EEA1 (C-15) | 1:1,000 | Santa Cruz #sc-6414 | |
| Goat-anti-Lamp1 (C20) | 1:200 | Santa Cruz #sc-8098 | |
| Mouse-anti-β-actin (C4) | 1:20,000 (30–60 min) | MP #08691001 |
Abcam: Abcam Inc. Cambridge, MA; BD: BD Biosciences San Jose, CA; DAKO: DAKO A/S, Denmark; Enzo: Enzo Life Sciences Inc. Farmingdale, NY; Epitomics: Epitomics Inc. Burlingame, CA; MP: MP Biomedicals, LLC, Solon, OH; Novus: Novus Biologicals Inc., Littleton, Co; Dr. Scrogin: Karie E Scrogin at Loyola University Chicago; Santa Cruz: Santa Cruz Biotechnology Inc., Santa Cruz, CA.
Wash with 2% milk in 1% Tween 20 TBS.
Immunoblot assay
was conducted as described in our previous publication(Creech et al., 2012). Briefly, samples were resolved in the SDS-PAGE gel (12% Acrylamide, Acrylamide: bis-acrylamide = 30: 0.2). After transferring the protein to PVDF membrane, the membrane was then incubated with 5% non-fat dry milk for one hour, followed by incubating with primary antibody (Table 1) overnight at 4°C. After washing with TBS containing 1% Tween 20 three times, the membranes were incubated with HRP-conjugated secondary antibody for 1 hour at room temperature. The protein bands were then detected using chemiluminescent detection. The image was captured using the Chemidoc XRS+ Image System and analyzed using the Image Lab program (Bio-Rad laboratories, Hercules, CA).
Data analysis and statistics
All data were analyzed by analysis of variance (ANOVA) using StatView (Abacus Concepts Inc., Berkeley, CA), unless otherwise mentioned. If a significant difference was detected, a Student-Newman-Keuls post-hoc test was used to evaluate differences between individual groups.
Results
Characterization of sumoylated 5-HT1A-Rs
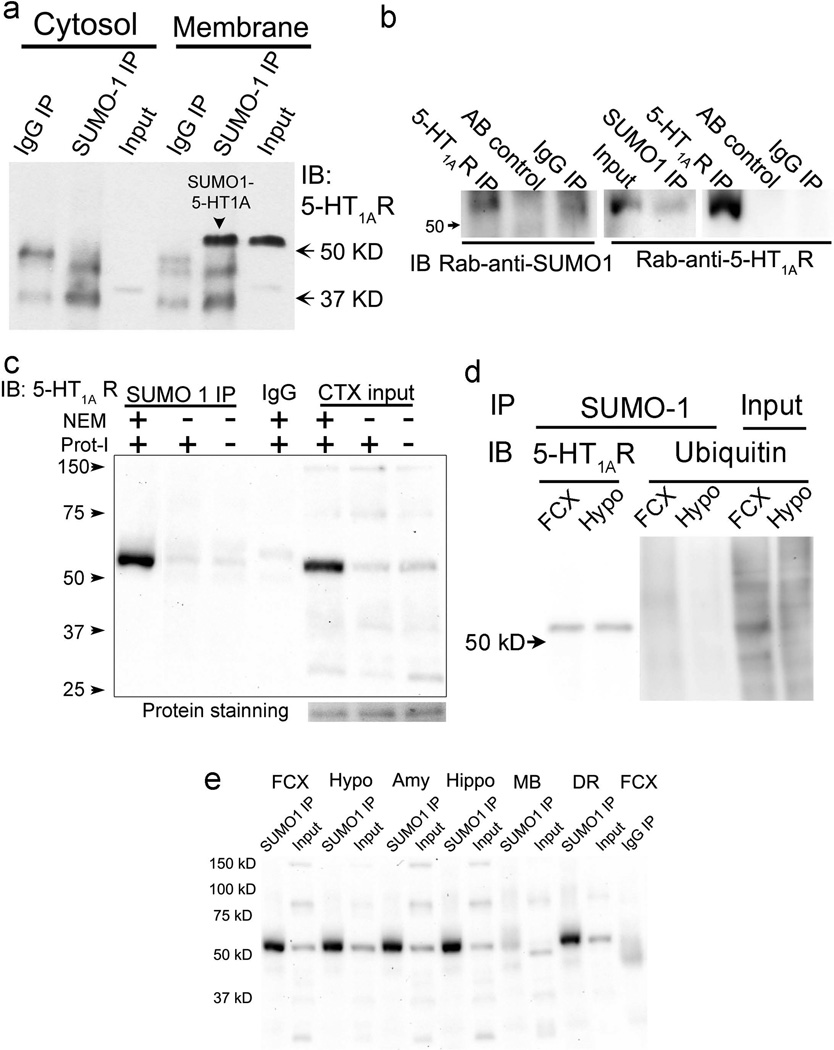
Using immunoprecipitation with a SUMO1 antibody followed by immunoblotting with a 5-HT1A-R antibody (characterized previously(Li et al., 2012)), we detected a ∼55 KD band in the membrane fraction, but not in the cytosolic fraction of rat cortex, suggesting SUMO1 conjugation to 5-HT1A-Rs in the membrane (Fig.1a). To verify the sumoylation of 5-HT1A-Rs, we immunoprecipitated 5-HT1A-Rs from a cortical membrane preparation with a rabbit-anti-5-HT1A-R antibody. A SUMO1-5-HT1A-R band at 55 kD was detected by a rabbit-anti-SUMO1 antibody; a 5-HT1A-R antibody was used to further confirm the identity of the 55kD band (Fig.1b). In another experiment, we compared the sumoylated 5-HT1A-Rs from cortical tissue in the presence or absence of NEM, an irreversible cysteine peptidase inhibitor that inhibits sentrin/SUMO-specific proteases (SENPs), the protease responsible for de-sumoylation of proteins. Without NEM, the amount of 5-HT1A-R precipitated by the SUMO1 antibody was significantly reduced (Fig.1c), confirming that the SUMO1 antibody-precipitated 5-HT1A-Rs are sumoylated. On the other hand, protease inhibitors did not prevent the de-sumoylation of SUMO1from 5-HT1A-Rs . Instead, without protease inhibitor, a ∼30 kD mass band of 5-HT1A-R was increased (Fig.1c), suggesting that the 30 kD band detected by the 5-HT1A-R antibody could be degraded 5-HT1A-R. To rule out the possibility that the SUMO1 antibody cross-reacted with ubiquitin, we blotted the samples immunoprecipitated with the SUMO-1 antibody with an ubiquitin antibody. No band was detected in either the frontal cortex or hypothalamic samples (Fig.1d), although the SUMO1-5HT1A-R and several ubiquitin proteins were detected in the SUMO1-immunoprecipitated and input samples, respectively.
Figure 1. Characterization of SUMO1-5-HT1A-Rs in rat brain.
a. SUMO1-5-HT1A-Rs (55 kD) are observed in the membrane, but not the cytosolic fractions of rat cortex. Immunoprecipitation (IP) with mouse-anti-SUMO1 followed by immunoblotting (IB) with rabbit-anti-5-HT1A-R. b. Confirming the SUMO1-5-HT1A-Rs using 5-HT1A-R antibody for immunoprecipitation followed by immunoblotting with rabbit-anti-SUMO1 antibody in the rat cortex homogenates. Left blot: immunoprecipitation with a rabbit-anti-5-HT1A antibody followed by immunoblotting with a rabbit anti-SUMO1 antibody (detected with Clean-blot (Thermo Scientific Inc, Rockford IL)); Middle blot: immunoprecipitation with a mouse-anti-SUMO1 antibody followed by immunoblotting with a rabbit-anti-5-HT1A-R antibody; and right blot: re-immunoblotting the blot in the left panel with a rabbit-anti-5-HT1A receptor antibody. AB control: immunoprecipitation without sample as a bead control; IgG IP: immunoprecipitation with same concentration of IgG (same species as primary antibody) as an IP control. c. SUMO1-5-HT1A-Rs are reduced in the absence of NEM, an inhibitor of SENPs, the peptidase for de-sumoylation of proteins. SUMO1-5-HT1A-Rs were compared in the presence and absence of NEM and protease inhibitors (Prot-I). d. The SUMO1 antibody does not pull-down ubiquitin-conjugated proteins. Hypothalamus and frontal cortex preparations were immunoprecipitated with SUMO1 antibody followed by immunoblotting with an anti-ubiquitin antibody. As a control, left two lanes contain samples immunoprecipitated with the SUMO1 antibody followed by immunoblotting for 5-HT1A-R. e. SUMO1-5-HT1A-Rs are distributed in the brain regions that express 5-HT1A-Rs. Input: sample without immunoprecipitation; Amy: amygdala; DR: dorsal raphe; FCX: Frontal cortex; Hippo: hippocampus; Hypo: hypothalamus; MB: midbrain.
To determine which brain regions contain sumoylated 5-HT1A-R, we examined numerous brain regions that express 5-HT1A-Rs, including the frontal cortex, cortex, hippocampus, hypothalamus and midbrain. Since 5-HT1A-Rs in the dorsal raphe are autoreceptors that are located in the 5-HT neurons, we also measured sumoylated 5-HT1A-Rs in the dorsal raphe. Using immunoprecipitation with a SUMO1 antibody followed by immunoblotting with a 5-HT1A-R antibody, we found that the sumoylated 5-HT1A-Rs existed in all of the brain regions examined (Fig.1e). Most of brain regions contained comparable amounts of sumoylated 5-HT1A-Rs, except the midbrain in which sumoylated 5-HT1A-Rs were less abundant than in other brain regions (Fig.1e).
Pre-treatment with estradiol (EB) for 2 days potentiates the (+)8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT)-induced increase in sumoylation of 5-HT1A-R in rat hypothalamus
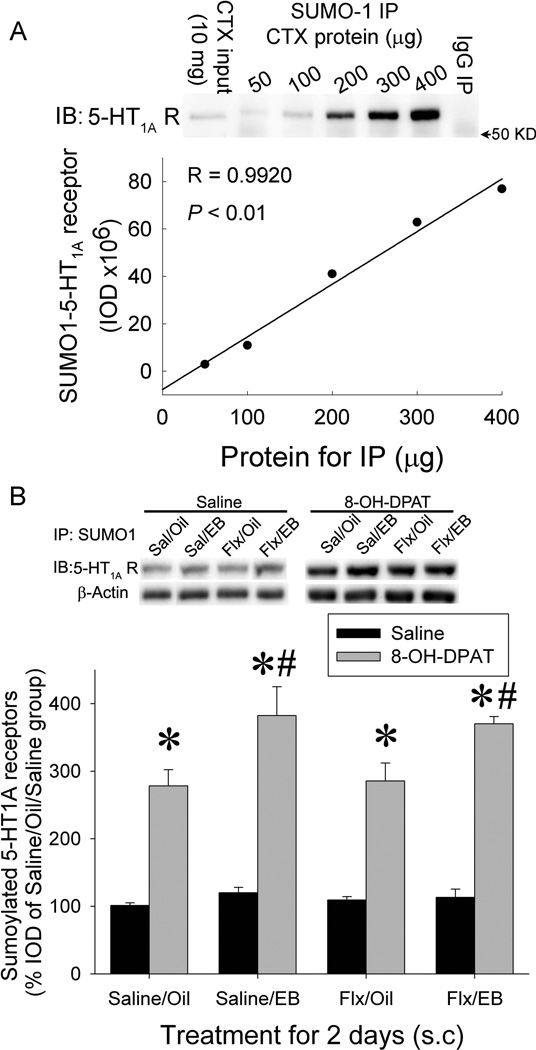
To determine whether the sumoylation of 5-HT1A-R is related to the activity of 5-HT1A-Rs, we determined the effects of repeated treatment with EB and/or fluoxetine and acute injection of 8-OH-DPAT on sumoylation of 5-HT1A-R. Our previous studies demonstrated that a combined injection of EB and fluoxetine for 2 days produced a rapid desensitization of 5-HT1A-Rs relative to fluoxetine alone as measured by hormonal responses to 5-HT1A-R agonist, 8-OH-DPAT(Li et al., 2012). In the present study, we determined whether sumoylation of 5-HT1A-R is related to EB facilitation of fluoxetineinduced desensitization of 5-HT1A-Rs. To quantify the SUMO1-5-HT1A-Rs, we first produced a protein concentration curve to determine the linear range of immunoprecipitation of SUMO1-5-HT1A-Rs. Cortex membrane preparations containing 50, 100, 200, 300 and 400 µg protein were used for immunoprecipitation with SUMO1 antibody followed by immunoblotting with 5-HT1A-R antibody. As Fig 2a shows, the SUMO1-5-HT1A-R levels detected were linearly correlated to the protein concentration in the range between 100–400 µg of protein. Based on this study, we used 200–300 µg of protein in subsequent experiments.
Figure 2. Treatment with estradiol (EB) for 2 days potentiates the 8-OH-DPAT-induced increase in sumoylation of 5-HT1A-Rs.
a. Protein concentration curve for quantification of SUMO1-5-HT1A-Rs. 50–400 µg protein of cortex preparation was immunoprecipitated with a mouse-anti-SUMO1antibody followed by immunoblotting with a rabbit-anti-5-HT1A-R antibody. IgG: immunoprecipitation with the same amount of mouse IgG instead of a SUMO1 antibody. b. Effect of EB and/or fluoxetine treatment for 2 days on 8-OH-DPAT-induced increase in SUMO1-5-HT1A-Rs. SUMO1-5-HT1A-Rs were measured in the hypothalamic membrane preparation of rats pre-treated with EB and /or fluoxetine for 2 days and challenged with saline or 8-OH-DPAT 15 min before decapitation. The SUMO1-5-HT1A-R levels were normalized to SUMO1-β-actin and then calculated as percent of the oil/saline/saline-treated samples in the same blot. The data are presented as the mean ± SEM of 4–6 rats. * indicates significant difference from saline-challenged rats with same pre-treatment; # indicates significantly different from saline/oil pre-treated group with same challenge.
To determine the effects of treatment with EB and /or fluoxetine for 2 days and acute injection of 8-OH-DPAT on the sumoylation of 5-HT1A-R, the hypothalamic membrane preparations from ovariectomized female rats treated with EB and /or fluoxetine for 2 days and then challenged with 8-OH-DPAT were used to determine SUMO1-5-HT1A-R. Our results showed that acute treatment with 8-OH-DPAT significantly increased SUMO1-5-HT1A-Rs in the hypothalamus (Fig.2b). Although no significant difference was detected among groups treated with EB and/or fluoxetine in saline-challenged rats, treatment with EB and EB+fluoxetine, but not fluoxetine alone, produced a significant increase in the sumoylated 5-HT1A-Rs in 8-OH-DPAT-challenged rats (Fig.2b, three-way ANOVA: main effect of fluoxetine F(1,39)= 0.004, P=0.95; main effect of EB: F(1,39)= 12.64, P=0.001; main effect of 8-OH-DPAT: F(1,39)= 214.76, P<0.001; interaction between fluoxetine X EB: F(1,39)=0.335, P=0.56; interaction between fluoxetine × 8-OH-DPAT: F(1,39)= 0.01, P=0.91; interaction between EB × 8-OH-DPAT F(1,39)= 7.77 P=0.0082; and interaction of fluoxetine X EB X 8-OH-DPAT: F(1,39)=0.005, P=0.94). These data suggest that pre-treatment with EB potentiates the 8-OH-DPAT-induced increase in the sumoylation of 5-HT1A-Rs.
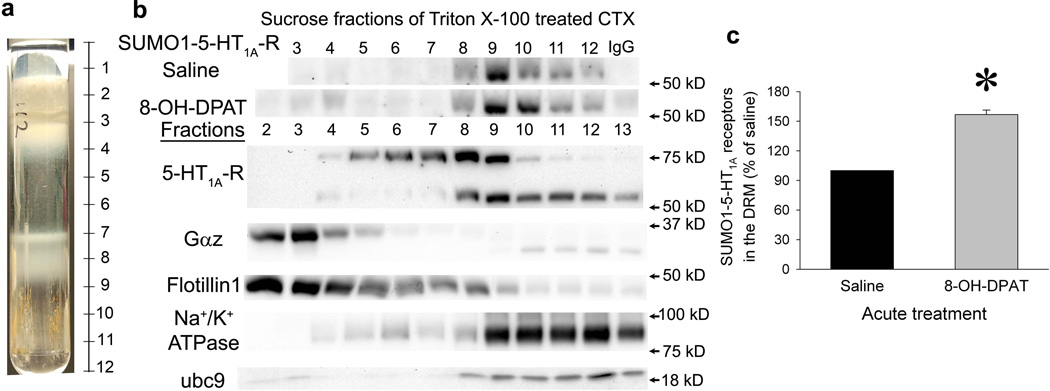
Small proportion of sumoylated 5-HT1A-Rs exists in the DRM, but is not co-localize with Gαz proteins and increases following stimulation with a 5-HT1A-R agonist
Like other GPCRs, active 5-HT1A-Rs are located in the DRM. To determine whether the sumoylated 5-HT1A-Rs are located in the DRM, we isolated the DRM using a standard protocol (Kumari and Francesconi, 2011), cold Triton X-100 treatment followed by sucrose gradient centrifugation. As shown in figure 3a, an opaque DRM band was observed at intersection of the 5% and 30% sucrose solutions, while another cloudy band was observed at the intersection between the 30% and 40% sucrose solutions. The DRM was located in fractions 2–4 as verified by flotillin-1, a DRM marker. Similar to our previous results, we demonstrated that Gαz protein (∼35 kd) was mainly located in the DRM (Li et al., 2012). The majority of sumoylated 5-HT1A-Rs was not located in the DRM but in the Triton X-100 soluble fractions. On the other hand, a small proportion of SUMO1-5-HT1A-Rs were detected in the DRM fractions (Fig.3b, fractions 2–4). However, compared to flotillin-1 and Gαz proteins, the distribution of the SUMO1-5-HT1A-Rs was shifted to the right, suggesting that sumoylated 5-HT1A-Rs do not couple to G proteins. More importantly, acute treatment with 8-OH-DPAT increased the SUMO1-5-HT1A-Rs in the DRM fractions (Fig.3b and 3c, Paired t test between saline and 8–OH-DPAT treatment in each experiment: t= −12.22, P=0.0066, n=4). Together, these data suggest that sumoylation may be related to the internalization of 5-HT1A-R.
Figure 3. Distribution of SUMO1-5-HT1A-Rs in sucrose gradient fractions of Triton X-100-treated cortex preparation.
DRM of cortex was isolated as described in the online methods. a. A picture of sucrose gradient centrifugation sample showing bands at the intersection between 5% and 30% sucrose and the intersection between 30% and 40% sucrose. b. The distribution of SUMO1-5-HT1A-R, 5-HT1A-Rs, Gαz protein, ubc 9 and plasma membrane markers in the sucrose fractions of Triton X-100-treated cortex. SUMO1-5-HT1A-R was determined using immunoprecipitation with a SUMO1 antibody followed by immunoblotting with a 5-HT1A-R antibody using cortex homogenates from rats injected with saline or 8-OH-DPAT 15 min before decapitation. The distribution of 5-HT1A-Rs, Gαz proteins, ubc9 (key enzyme for sumoylation), flotillin 1(DRM marker) and Na+/K+ ATPase (plasma membrane marker) were determined using immunoblots with 10 µg protein/fraction. The fractions in the blot correlate to the fractions indicated in Fig 3a. IgG: mouse IgG was used for immunoprecipitation instead of a SUMO1 antibody. c. Acute treatment with 8-OH-DPAT significantly increases SUMO1-5-HT1A-Rs in the DRM. The integrated optic density (IOD) of the band of SUMO1-5-HT1A-R (total IOD – background IOD) was measured in fractions 3–7. Then, the area under the curve (AUC) of the IOD of fractions 3–7 was determined using Sigma Plot (Systat Software Inc, San Jose, CA). The percent of ACU for saline-treated rats in same experiment was then calculated. The data were presented as mean ± SEM of % ACU of saline-treated rats from 4 separate experiments. * indicates significantly different from saline-treated rats, P< 0.05 by a paired t-test between saline and 8-OH-DPAT-treated samples in each experiment.
Subcellular distribution of sumoylated 5-HT1A-Rs
To further determine the subcellular location of sumoylated 5-HT1A-Rs, we conducted a discontinuous gradient centrifugation with 7.5–30% iodixanol in the cortex preparation. The subcellular organelles located in the fractions collected from the gradient centrifugation were verified by immunoblotting with several organelle markers as shown in Figure 4A. Several bands of 5-HT1A-Rs were detected on immunoblots. The distribution of these different molecular mass 5-HT1A-Rs varied. In the plasma membrane, the 5-HT1A-Rs with ∼37–40 kD molecular mass were detected, which is the predicted size of 5-HT1A-Rs based on the amino acid sequence. On the other hand, the antibody detected a small size band in the cytosolic fraction, which could be degraded 5-HT1A-R. The most abundant 5-HT1A-R detected by the immunoblot was a 55 kD mass band. The distribution of this band was similar to the sumoylated 5-HT1A-Rs and was predominantly co-localized with TGN 38, a marker for the TGN, and calreticulin, a marker for the ER (Fig.4A). Similar to the observation from the DRM preparation, a small proportion of SUMO1-5-HT1A-Rs were distributed in the plasma membrane fractions that were identified by Na+/K+ ATPase, flotillin-1 and Gαz protein. On the other hand, no SUMO1-5HT1A-R was detected in the fractions containing endosomal protein (as marked by early endosome antigen 1 (EEA 1)) and cytosol (as marked by lactate dehydroxylase (LDH)). Although the distribution of the lysosome marker, LAMP 1, was close to that of SUMO1-5-HT1A-Rs, they did not overlap.
To further confirm that the location of sumoylated 5-HT1A-Rs in the TGN and ER was not due to contamination with the plasma membrane, we conducted a digitonininduced density shift study. Treatment with digitonin shifts membrane compartments containing high cholesterol, such as the plasma membrane and endosome, to a higher density. On the other hand, the compartments containing low concentrations of cholesterol, such as Golgi and ER, do not shift or only minimally shift with digitonin treatment. Thus, we hypothesized that if SUMO1-5-HT1A-Rs were located in the TGN and/or ER, the digitonin treatment would not shift or minimally shift the SUMO1-5-HT1A-R to a higher density. However, if the SUMO1-5-HT1A-R are in the plasma membrane or endosome, digitonin treatment should shift it to higher density. Subcellular fractions of the hippocampus were obtained by discontinuous gradient centrifugation with 7.5–30% iodixanol (Fig.4Ba and 4Bb). The subcellular fractions containing plasma membrane (fraction 13 and 14, marked by Na+/K+ ATPase) and ER (fraction 18 and 19, marked by the peak of the calnexin fractions) were treated with digitonin or vehicle followed by a second discontinuous gradient centrifugation with 17.5 −35% (for plasma membrane fractions) and 25–50% (for ER fractions) iodixanal, respectively (Fig.4Bc). The results showed that treatment with digitonin shifted the distributions of SUMO1-5-HT1A-Rs and Na+/K+ ATPase in the plasma membrane fractions to a higher density (Fig.4Bc and 4Bd). On the other hand, only a very small shift was observed in the distributions of SUMO1-5-HT1A-R and calnexin in the ER fraction (Fig. 4Bc and 4Be). These results suggest that sumoylated 5-HT1A-Rs are mainly located in the TGN and ER, whereas relatively low amounts are located in the plasma membrane.
SUMO1-5-HT1A-R has low affinity for 5-HT1A-R agonist
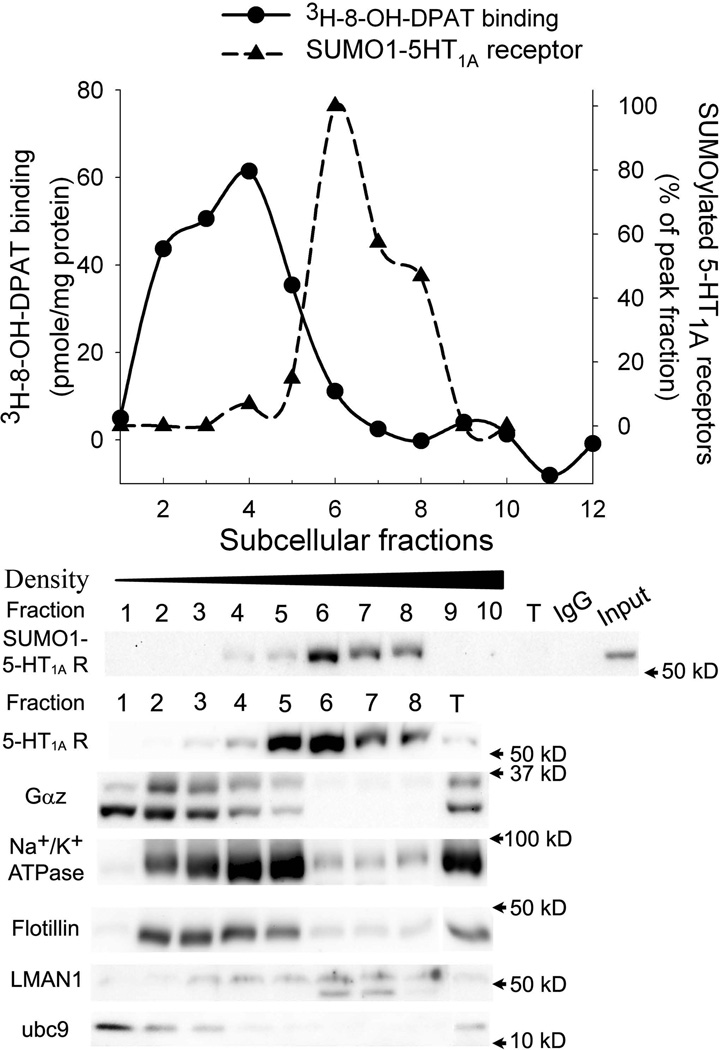
We next conducted a 3H-8-OH-DPAT binding assay in the subcellular fractions to determine whether the sumoylated 5-HT1A-Rs are functional. Subcellular fractions of the cortex were obtained from discontinuous gradient centrifugation with 10–40% iodixanal and were collected as 1 ml per fraction. Each fraction was divided into two. One half was used for 3H-8-OH-DPAT binding and another half was used for immunoprecipitation of SUMO1-5-HT1A-Rs. The results showed that 3H-8-OH-DPAT binding distributed in fractions 2 to 6 with a peak at fraction 4, which correlated with the plasma membrane makers, Na+/K+ ATPase and flotillin, and the 35 kD Gαz proteins (Fig.5). On the other hand, SUMO1-5-HT1A-Rs are distributed from fraction 4 to 9 with a peak at fraction 6, which is similar to LMAN1 (lectin mannose-binding 1, ERGIC 53), the marker for the ERGIC (Fig.5). Although the distribution curve of 3H-8-OH-DPAT binding overlapped with that of SUMO1-5-HT1A-R, fractions 4 and 5 have high 3H-8-OH-DPAT binding but very low levels of SUMO1-5-HT1A-Rs. On the other hand, fraction 6 has high levels of SUMO1-5-HT1A-Rs, but little 3H-8-OH-DPAT binding (Fig.5). These data suggest that SUMO1-5-HT1A-Rs have low affinity to 3H-8-OH-DPAT and thus, are not active receptors.
Figure 5. Comparison of the distribution of 3H-8-OH-DPAT binding with that of SUMO1-5-HT1A-R.
Subcellular fractions (1ml/fraction) were generated by discontinuous gradient centrifugation with 10%–40% iodixanol. Each fraction was divided into two parts, one for the 3H-8-OH-DPAT binding assay and one for immunoprecipitation for SUMO1 and immunoblot for 5-HT1A-R, Gαz protein, ubc9 and markers of subcellular organelles. Top graph compares the profiles of the distribution of 3H-8-OH-DPAT binding (solid line) with that of SUMO1-5-HT1A-R (dashed line), in which IOD of the blot was measured and calculated as % of the peak fraction. Bottom panel shows immunoblots of 5-HT1A-R, Gαz protein, ubc 9 and markers of subcellular organelles. The SUMO1-5-HT1A-Rs were determined by immunoprecipitation with SUMO1 antibody followed by 5-HT1A-R.
It is interesting that SUMO-conjugating enzyme (ubc 9), a key enzyme involved in sumoylation, was co-localized with plasma membrane markers, Na+/K+ ATPase and flotillin, in the subcellular fractions (Fig.5). Similarly, a small portion of ubc 9 was detected in the DRM in the Triton X-100 treated sucrose fractions (Fig.3B). These data suggest that sumoylation can occur in the DRM, however, only a small fraction of sumoylated receptor is actually retained in this region of the plasma membrane.
Discussion
Protein sumoylation is an important post-translational modification that has recently gained attention. Although the vast majority of studies on sumoylation focus on the regulation of nuclear proteins, recent studies have revealed that sumoylation can also play an important role in regulating the function of extra-nuclear proteins (Scheschonka et al., 2007). To date, only a few sumoylated GPCRs have been identified and the effect of sumoylation varies among these GPCRs (Scheschonka et al., 2007; Wilkinson et al., 2010). In the present study, we report, for the first time, that 5-HT1A-Rs can be sumoylated by SUMO-1 proteins. Acute treatment with the 5-HT1A-R agonist, 8-OH-DPAT increases the sumoylated 5-HT1A-Rs in the hypothalamus of rats. This increase is potentiated by 2 days of treatment with estradiol, which produces a partial desensitization of 5-HT1A-Rs (Li et al., 2012). The majority of sumoylated 5-HT1A-R is Triton X-100 soluble and co-localizes with TGN, ERGIC and the ER markers. On the other hand, a small proportion of SUMO1-5-HT1A-Rs was detected in the DRM. However, they are not co-localized with Gαz proteins that are known to couple to active 5-HT1A-Rs. The SUMO1-5-HT1A-Rs have minimal 5-HT1A-R agonist binding, further supporting that they are inactive receptors. Furthermore, acute stimulation of 5-HT1A-Rs increases the SUMO1-5-HT1A-Rs in DRM. These data suggest that sumoylation of 5-HT1A-Rs inactivates 5-HT1A-R, which may be related to the desensitization of 5-HT1A-Rs.
5-HT1A-R is one of the most well studied serotonin receptors. Multiple lines of evidence demonstrate that 5-HT1A-Rs play an important role in mood regulation and may be involved in the etiology of affective disorders. SSRI-induced desensitization of 5-HT1A-Rs may be necessary for SSRI-mediated therapeutic effects on affective disorders. Studies have demonstrated that treatment with estradiol produces a partial desensitization of 5-HT1A-Rs several brain regions (Bethea et al., 2000; Creech et al., 2012; Mize et al., 2003). Furthermore, estradiol-treatment for 2 days facilitates the SSRI-induced desensitization on 5-HT1A-Rs (Li et al., 2012). Therefore, it is important to understand the mechanisms underlying estradiol-induced desensitization of 5-HT1A-Rs. In the present study, we found that estradiol potentiates the 8-OH-DPAT-induced increase in sumoylation of 5-HT1A-Rs. Since SUMO1-5-HT1A-Rs are inactive, the increase in the SUMO1-5-HT1A-Rs may be related to the desensitization of the receptors. A G-protein coupled estrogen receptor, GPER, co-localizes with 5-HT1A-Rs in the DRM and is necessary and sufficient for estradiol-induced partial desensitization of 5-HT1A-R signaling (McAllister et al., 2012; Xu et al., 2009). Hence, it is possible that stimulation of the GPER signaling pathways by estradiol activates the sumoylation system, which in turn facilitates the sumoylation of 5-HT1A-Rs induced by 8-OH-DPAT.
EB negatively regulates a number of other GPCRs including mu-opioid, GABAB and CB1 receptors (Kellert et al., 2009; Kelly et al., 1992; Lagrange et al., 1996; Peckham et al., 2011). Further studies are needed to determine if receptor sumoylation is the mechanism by which EB reduces signaling via these GPCRs, however sumoylation of µ-opioid and GABAB receptors has not been reported. Although CB1 receptors are sumoylated, agonist stimulation decreases sumoylation of CB1 receptors and results in internalization of the receptors (Gowran et al., 2009).. The results on CB1 receptors suggest that the effect of EB on CB1 receptors is not mediated via increased sumoylation of the receptors.
The DRM of plasma membrane, including lipid raft and caveolae, is a planar domain in the membrane containing high concentrations of cholesterol and glycosphingolipid (Allen et al., 2007; Chini and Parenti, 2004). The DRM plays a role in assembling components of the GPCR signaling pathways, physically bringing together these components for GPCR signaling. G-protein coupled 5-HT1A-Rs are located in the DRM (Pucadyil and Chattopadhyay, 2004). In the present study, we found a small proportion of SUMO1-5-HT1A-Rs is located in the DRM, which is unlikely to be contaminated by high density fractions. This is clearly demonstrated in Triton X-100-treated sucrose samples in which SUMO1-5-HT1A-Rs in the DRM are located at fractions 3 and 4 whereas the SUMO1-5-HT1A-Rs in the Triton X-100 soluble fractions appear in the fraction 8–12 (Fig.3). Furthermore, the SUMO1-5-HT1A-Rs in the plasma membrane are shifted by digitonin-treatment, while the SUMO1-5-HT1A-Rs in the ER fractions are not shifted by digitonin (Fig. 4B). Since 5-HT1A-Rs are coupled to Gαz proteins, one can expect that active 5-HT1A-Rs, i.e. G protein-coupled receptors, should be co-localized with Gαz proteins. However, the distribution of the SUMO1-5-HT1A-Rs in the DRM is shifted to the right from that of Gαz proteins, suggesting that SUMO1-5-HT1A-Rs are not coupled to Gαz proteins. This is further supported by the observation that 5-HT1A-R agonist binding to SUMO1-5-HT1A-Rs is minimal. Together with the observation that treatment with 8-OH-DPAT significantly increased sumoylated 5-HT1A-Rs in the DRM (Fig.3), these data suggest that SUMO1-5-HT1A-Rs are inactive and that may be related to agonist-induced internalization of the receptors. This observation is similar to recent findings that stimulation with kainate receptor agonist induces sumoylation of a kainate receptor subunit, GluK2, and triggers the internalization of GluK2-containing kainate receptors (Chamberlain et al., 2012; Konopacki et al., 2011).
Studies have demonstrated that palmitoylation is required for 5-HT1A-Rs to be transported to the DRM of plasma membrane, where they couple with Gαi proteins and become active (Kobe et al., 2008; Papoucheva et al., 2004; Renner et al., 2007). Our present results suggest that sumoylation of 5-HT1A-Rs may be related to the internalization of the receptors, i.e., removal of the receptors from DRM. Thus, we can speculate that palmitoylation of 5-HT1A-Rs promote their translocation to the DRM in the plasma membrane, where they couple to G proteins and thus, are able to bind with agonists and activate 5-HT1A-R signaling pathways. After stimulation by agonists, the 5-HT1A-Rs dissociate from G proteins and are sumoylated. Sumoylation may trigger the removal of 5-HT1A-Rs from the DRM. A similar case was reported by Schmidt et al (Schmidt et al., 2009). Palmitoylation of Bright, a B-cell restricted transcription factor, promotes the factor localizing into the DRM. After B-cell antigen receptor binds to ligand, Bright is sumoylated and then removed from the DRM (Schmidt et al., 2009). Our present results showed that a key enzyme in sumoylation, ubc 9, is located in the DRM. Furthermore, results from others and our studies demonstrated that several DRM proteins, such as caveolin-3, arrestin-3 and Gαz in the DRM can be sumoylated (Fuhs and Insel, 2011; Li et al., 2012; Wyatt et al., 2011). These data suggest that sumoylation can occur in DRM. Further studies are required to verify this hypothesis.
Our present results show that the majority of sumoylated 5-HT1A-Rs are Triton X-100 soluble and co-localize with TGN, ERGIC and ER markers. Recent studies showed that 5-HT1A-Rs are not only located on the plasma membrane, but also in intra-cellular organelles (Cheng et al., 2011; Xu et al., 2009) and many GPCRs accumulate in intracellular compartments, such as TGN, Golgi complex and ER (Achour et al., 2008). Currently, we do not know the function of these intracellular SUMO1-5-HT1A-Rs and since we cannot individually isolate the TGN, ERGIC and ER, we do not know which of these organelles contain sumoylated 5-HT1A-Rs. If the sumoylated 5-HT1A-Rs are located in the TGN, we could speculate that the SUMO1-5-HT1A-Rs come from DRM after agonist stimulation and are stored in TGN for either recycling or lysosomal degradation. However, as recent studies have revealed that some GPCRs are translocated to TGN through the endosome, in response to agonist stimulation, it is not likely that 5-HT1A-Rs are internalized from the DRM to the TGN since, we did not detect the SUMO1-5-HT1A-Rs in the subcellular fractions labeled with the endosomal marker. On the other hand, if the SUMO1-5-HT1A-Rs are located in the ER or ERGIC, sumoylation could be related to the regulation in maturation and export of 5-HT1A-R from the ER to the plasma membrane. GPCRs are synthesized by the ribosome in the ER and then, undergo maturation processes, including insertion in the membrane and folding to their native conformation. Matured GPCRs are then packaged into vesicles and transported to the Golgi complex, ERGIC, cis-Golgi and TGN and finally, are sorted into the plasma membrane (Achour et al., 2008). Thus, we can speculate that the SUMO1-5-HT1A-Rs could serve as “reserved” 5-HT1A-Rs that are stored in the ER. Activation of 5-HT1A-R signaling pathways trigger sumoylation of newly synthesized 5-HT1A-Rs in the ER, which prevents export of the receptors and results in retention of the receptors in the ER. If this is the case, the sumoylation of 5-HT1A-R regulates the export trafficking of the receptor. This hypothesis can explain our observation that acute administration of 5-HT1A-R agonist, 8-OH-DPAT, significantly increased sumoylated 5-HT1A-Rs. Several proteins have been reported to be sumoylated in the ER (Felberbaum et al., 2012; Majumdar et al., 2011; Yip et al., 2012). For example, sumoylation of transcription factor X box-binding protein 1(XBP1) in the ER results in accumulation of the protein in the ER, reduction of its activity and increases in ER stress responses (Jiang et al., 2012). These data suggest that sumoylation in the ER serves as a mechanism to regulate the maturation of proteins, including protein folding and transport. However, to date, no data have been reported regarding the sumoylation of GPCRs in the ER, although one study found that CB1 receptors are sumoylated tonically and desumoylated up on agonist stimulation (Gowran et al., 2009).
In conclusion, the present results demonstrate, for the first time, that 5-HT1A-Rs can be sumoylated. Our results strongly suggest that the sumoylated 5-HT1A-Rs are inactive. Sumoylation may be related to the regulation of 5-HT1A-R trafficking, such as agonist-induced internalization and/or retaining 5-HT1A-R in the ER. Similar to the effects of sumoylation of other plasma membrane proteins and GPCRs, sumoylation of 5-HT1A-Rs may serve as a negative control mechanism contributing to the desensitization of the receptors in response to agonist stimulation. Indeed, we also found that EB treatment which causes desensitization of 5-HT1A-R signaling also increased sumoylation of 5-HT1A-Rs. Since EB and 5-HT1A-Rs play an important role in mood regulation, the present results will have a significant impact on understanding the regulation of 5-HT1A-Rs and thus, the development of new approaches to treat affective disorders.
Acknowledgements
The authors thank Dr. Karie Scrogin for kindly providing 5-HT1A receptor antibody.
Role of Funding Source
Funding for this study was provided by NIMH Grant MH058448 (N.A.M.). The NIMH had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Qian Li performed assays, conceived of the direction of the biochemical experiments and wrote the manuscript. Nancy Muma contributed to the design of the project, data analysis and played a substantial role in the writing of the manuscript.
The authors declare no actual or potential financial and other conflicts of interest related to the submitted manuscript.
References
- Achour L, Labbe-Jullie C, Scott MG, Marullo S. An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci. 2008;29:528–535. doi: 10.1016/j.tips.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Anderson DB, Wilkinson KA, Henley JM. Protein SUMOylation in neuropathological conditions. Drug News Perspect. 2009;22:255–265. doi: 10.1358/dnp.2009.22.5.1378636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou AM, Tavernarakis N. SUMOylation and cell signalling. Biotechnology journal. 2009;4:1740–1752. doi: 10.1002/biot.200900219. [DOI] [PubMed] [Google Scholar]
- Artigas F, Celada P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends In Pharmacological Sciences. 2001;22:224–228. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Gundlah C, Mirkes SJ. Ovarian steroid action in the serotonin neural system of macaques. Novartis.Found.Symp. 2000;230:112–130. doi: 10.1002/0470870818.ch9. [DOI] [PubMed] [Google Scholar]
- Blier P, Abbott FV. Putative mechanisms of action of antidepressant drugs in affective and anxiety disorders and pain. J.Psychiatry Neurosci. 2001;26:37–43. [PMC free article] [PubMed] [Google Scholar]
- Castle JD. Purification of organelles from mammalian cells. Current protocols in protein science / editorial board. 2004 doi: 10.1002/0471140864.ps0402s37. John E. Coligan … [et al.] Chapter 4, Unit 4 2. [DOI] [PubMed] [Google Scholar]
- Chamberlain SE, Gonzalez-Gonzalez IM, Wilkinson KA, Konopacki FA, Kantamneni S, Henley JM, Mellor JR. SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat Neurosci. 2012;15:845–852. doi: 10.1038/nn.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SB, Quinn JA, Graeber CT, Filardo EJ. Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J Biol Chem. 2011;286:22441–22455. doi: 10.1074/jbc.M111.224071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- Craig TJ, Henley JM. Protein SUMOylation in spine structure and function. Curr Opin Neurobiol. 2012;22:480–487. doi: 10.1016/j.conb.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech RD, Li Q, Carrasco GA, Van de Kar LD, Muma NA. Estradiol induces partial desensitization of serotonin 1A receptor signaling in the paraventricular nucleus of the hypothalamus and alters expression and interaction of RGSZ1 and Galphaz. Neuropharmacology. 2012;62:2040–2049. doi: 10.1016/j.neuropharm.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutting E, Schroder-Kress N, Sticht H, Enz R. SUMO E3 ligases are expressed in the retina and regulate SUMOylation of the metabotropic glutamate receptor 8b. Biochem J. 2011;435:365–371. doi: 10.1042/BJ20101854. [DOI] [PubMed] [Google Scholar]
- Enz R. Structure of metabotropic glutamate receptor C-terminal domains in contact with interacting proteins. Front Mol Neurosci. 2012;5:52. doi: 10.3389/fnmol.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felberbaum R, Wilson NR, Cheng D, Peng J, Hochstrasser M. Desumoylation of the endoplasmic reticulum membrane VAP family protein Scs2 by Ulp1 and SUMO regulation of the inositol synthesis pathway. Mol Cell Biol. 2012;32:64–75. doi: 10.1128/MCB.05878-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhs SR, Insel PA. Caveolin-3 undergoes SUMOylation by the SUMO E3 ligase PIASy: sumoylation affects G-protein-coupled receptor desensitization. J Biol Chem. 2011;286:14830–14841. doi: 10.1074/jbc.M110.214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nature reviews. Molecular cell biology. 2010;11:861–871. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nature reviews. Molecular cell biology. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gowran A, Murphy CE, Campbell VA. Delta(9)-tetrahydrocannabinol regulates the p53 post-translational modifiers Murine double minute 2 and the Small Ubiquitin MOdifier protein in the rat brain. FEBS Lett. 2009;583:3412–3418. doi: 10.1016/j.febslet.2009.09.056. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang XX, Hen R. Altered fear circuits in 5-HT1A receptor KO mice. Biological Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Fan Q, Zhang Z, Zou Y, Cai R, Wang Q, Zuo Y, Cheng J. SENP1 deficiency promotes ER stress-induced apoptosis by increasing XBP1 SUMOylation. Cell Cycle. 2012;11:1118–1122. doi: 10.4161/cc.11.6.19529. [DOI] [PubMed] [Google Scholar]
- Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol. 2009;622:15–24. doi: 10.1016/j.ejphar.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe F, Renner U, Woehler A, Wlodarczyk J, Papusheva E, Bao G, Zeug A, Richter DW, Neher E, Ponimaskin E. Stimulation- and palmitoylationdependent changes in oligomeric conformation of serotonin 5-HT1A receptors. Biochim Biophys Acta. 2008;1783:1503–1516. doi: 10.1016/j.bbamcr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Konopacki FA, Jaafari N, Rocca DL, Wilkinson KA, Chamberlain S, Rubin P, Kantamneni S, Mellor JR, Henley JM. Agonist-induced PKC phosphorylation regulates GluK2 SUMOylation and kainate receptor endocytosis. Proc Natl Acad Sci U S A. 2011;108:19772–19777. doi: 10.1073/pnas.1111575108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Francesconi A. Identification of GPCR localization in detergent resistant membranes. Methods Mol Biol. 2011;746:411–423. doi: 10.1007/978-1-61779-126-0_24. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Wagner EJ, Ronnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64:114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- Li Q, Brownfield MS, Levy AD, Battaglia G, Cabrera TM, Van de Kar LD. Attenuation of hormone responses to the 5-HT1A agonist ipsapirone by long-term treatment with fluoxetine, but not desipramine, in male rats. Biol.Psychiatry. 1994;36:300–308. doi: 10.1016/0006-3223(94)90627-0. [DOI] [PubMed] [Google Scholar]
- Li Q, Sullivan NR, McAllister CE, Van de Kar LD, Muma NA. Estradiol accelerates the effects of fluoxetine on serotonin 1A receptor signaling. Psychoneuroendocrinology. 2013;38:1145–1157. doi: 10.1016/j.psyneuen.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Petrescu AD, Xiong Y, Noy N. Nuclear translocation of cellular retinoic acid-binding protein II is regulated by retinoic acid-controlled SUMOylation. J Biol Chem. 2011;286:42749–42757. doi: 10.1074/jbc.M111.293464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ. Posttranslational modification biology of glutamate receptors and drug addiction. Frontiers in neuroanatomy. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci. 2007;8:948–959. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CE, Creech RD, Kimball PA, Muma NA, Li Q. GPR30 is necessary for estradiol-induced desensitization of 5-HT(1A) receptor signaling in the paraventricular nucleus of the rat hypothalamus. Psychoneuroendocrinology. 2012;37:1248–1260. doi: 10.1016/j.psyneuen.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize AL, Young LJ, Alper RH. Uncoupling of 5-HT1A receptors in the brain by estrogens: regional variations in antagonism by ICI 182,780. Neuropharmacology. 2003;44:584–591. doi: 10.1016/s0028-3908(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Papoucheva E, Dumuis A, Sebben M, Richter DW, Ponimaskin EG. The 5-hydroxytryptamine(1A) receptor is stably palmitoylated, and acylation is critical for communication of receptor with Gi protein. J Biol Chem. 2004;279:3280–3291. doi: 10.1074/jbc.M308177200. [DOI] [PubMed] [Google Scholar]
- Peckham EM, Graves SM, Jutkiewicz E, Becker JB, Traynor JR. Role of gonadal hormones on mu-opioid-stimulated [(3)(5)S]GTPgammaS binding and morphine-mediated antinociception in male and female Sprague-Dawley rats. Psychopharmacology (Berl) 2011;218:483–492. doi: 10.1007/s00213-011-2335-z. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim Biophys Acta. 2004;1663:188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Renner U, Glebov K, Lang T, Papusheva E, Balakrishnan S, Keller B, Richter DW, Jahn R, Ponimaskin E. Localization of the mouse 5- hydroxytryptamine(1A) receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol Pharmacol. 2007;72:502–513. doi: 10.1124/mol.107.037085. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Savtchenko LP, Zheng K, Henley JM. Shaping the synaptic signal: molecular mobility inside and outside the cleft. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.03.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheschonka A, Tang Z, Betz H. Sumoylation in neurons: nuclear and synaptic roles? Trends Neurosci. 2007;30:85–91. doi: 10.1016/j.tins.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Kim D, Ippolito GC, Naqvi HR, Probst L, Mathur S, Rosas-Acosta G, Wilson VG, Oldham AL, Poenie M, Webb CF, Tucker PW. Signalling of the BCR is regulated by a lipid rafts-localised transcription factor, Bright. EMBO J. 2009;28:711–724. doi: 10.1038/emboj.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres F, Li Q, Garcia F, Raap DK, Battaglia G, Muma NA, Van de Kar LD. Evidence that G(z)-proteins couple to hypothalamic 5-HT(1A) receptors in vivo. J.Neurosci. 2000;20:3095–3103. doi: 10.1523/JNEUROSCI.20-09-03095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdizan EM, Castro E, Pazos A. Agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int.J.Neuropsychopharmacol. 2010;13:835–843. doi: 10.1017/S1461145709990940. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–145. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev. 2010;64:195–212. doi: 10.1016/j.brainresrev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt D, Malik R, Vesecky AC, Marchese A. Small ubiquitin-like modifier modification of arrestin-3 regulates receptor trafficking. J Biol Chem. 2011;286:3884–3893. doi: 10.1074/jbc.M110.152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, DonCarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SC, Cotteret S, Chernoff J. Sumoylated protein tyrosine phosphatase 1B localizes to the inner nuclear membrane and regulates the tyrosine phosphorylation of emerin. J Cell Sci. 2012;125:310–316. doi: 10.1242/jcs.086256. [DOI] [PMC free article] [PubMed] [Google Scholar]