Abstract
Background
Postmortem brain studies have shown that HDAC1—a lysine deacetylase with broad activity against histones and nonhistone proteins—is frequently expressed at increased levels in prefrontal cortex (PFC) of subjects diagnosed with schizophrenia and related disease. However, it remains unclear whether upregulated expression of Hdac1 in the PFC could affect cognition and behavior.
Methods
Using adeno-associated virus, an Hdac1 transgene was expressed in young adult mouse PFC, followed by behavioral assays for working and long-term memory, repetitive activity, and response to novelty. Prefrontal cortex transcriptomes were profiled by microarray. Antipsychotic drug effects were explored in mice treated for 21 days with haloperidol or clozapine.
Results
Hdac1 overexpression in PFC neurons and astrocytes resulted in robust impairments in working memory, increased repetitive behaviors, and abnormal locomotor response profiles in novel environments. Long-term memory remained intact. Over 300 transcripts showed subtle but significant changes in Hdac1-overexpressing PFC. Major histocompatibility complex class II (MHC II)-related transcripts, including HLA-DQA1/H2-Aa, HLA-DQB1/H2-Ab1, and HLA-DRB1/H2-Eb1, located in the chromosome 6p21.3-22.1 schizophrenia and bipolar disorder risk locus, were among the subset of genes with a more robust (>1.5-fold) downregulation in expression. Hdac1 levels declined during the course of normal PFC development. Antipsychotic drug treatment, including the atypical clozapine, did not affect Hdac1 levels in PFC but induced expression of multiple MHC II transcripts.
Conclusions
Excessive HDAC1 activity, due to developmental defects or other factors, is associated with behavioral alterations and dysregulated expression of MHC II and other gene transcripts in the PFC.
Keywords: Bipolar disorder, gene expression, major histocompatibility complex II, prefrontal cortex, protein deacetylase, schizophrenia
Postmortem brain studies implicate epigenetic alterations involving DNA methylation and histone modifications and other determinants of chromatin structure and function in the neurobiology of schizophrenia. In particular, dysregulated methylation of DNA cytosines and of histone lysine and arginine residues in the prefrontal cortex (PFC) and other corticolimbic circuitry has been reported for select gene promoters important for neurotransmission, myelination, and various other functions (1–8). Some of these epigenetic alterations were subsequently confirmed in nucleated blood cells of subjects with schizophrenia (9–11). These changes in chromatin architectures at specific loci may ultimately be driven by genetic factors (4,5,12) or may reflect aberrant epigenetic signatures in a parental germline (13,14) or adverse events during prenatal or early postnatal development (15,16). Furthermore, epigenetic dysregulation of gene expression may result from exposure to nicotine and alcohol (17,18), psychostimulants, and various other drugs of abuse (19,20).
The above findings, taken together, leave little doubt that maladaptive mechanisms in the brain's epigenetic machinery could be a critical factor in the etiology of at least some cases on the psychosis spectrum. However, with the exception of rare cases of schizophrenia caused by deleterious mutations in genes encoding chromatin regulators such as the histone H3-lysine 9 specific methyltransferase KMT1D/EHMT1(21) or the methyl-CpG-binding protein MECP2(22), the molecular pathways associated with epigenetic dysregulation leading to clinical symptoms and molecular changes in the psychotic brain, including the PFC, remain unknown.
The balance between histone acetylation and deacetylation is highly regulated in brain cells and of pivotal importance for behavioral plasticity in the brain's learning and reward circuitry (19,23–25) and could profoundly affect motivational and affective states (26,27). Sharma et al. (28) previously noticed that in a publicly accessible microarray collection from the Harvard Brain Tissue Resource Center, expression of the class I histone deacetylase, HDAC1, was significantly increased (on average 30% to 50%) in the PFC of a cohort of 19 subjects with schizophrenia compared with 25 control subjects (28) (Figure S1 in Supplement 1). Similar changes may affect a subset of patients diagnosed with bipolar disorder (28). Furthermore, microarray datasets from Narayan et al. (29), who profiled transcriptomes in PFC specimens from an Australian collection, also revealed a significant increase in HDAC1 transcript levels in 30 schizophrenia subjects compared with 29 control subjects (Figure S1 in Supplement 1). Finally, upregulated HDAC1 expression has also been reported for the neuronal layers of the hippocampus and medial temporal lobe in a third cohort of schizophrenia subjects (30). Therefore, abnormal HDAC1 expression in corticolimbic circuitry is a type of molecular pathology representative of a significant portion of cases on the mood and psychosis spectrum. However, it remains unclear whether this type of molecular alteration is detrimental to brain function, or a neutral epiphenomenon, or a medication side effect. To distinguish between these possibilities was the goal of the present study.
Methods and Materials
Analysis of human and mouse microarray data and details on adeno-associated virus vector preparation and delivery, animal surgery and antipsychotic drug treatments (APD), and behavioral studies, cell culture work, immunoblotting and immunohistochemistry procedures, quantitative reverse transcriptase-polymerase chain reaction, and statistical analysis are provided in the Supplemental Methods (in Supplement 1).
Results
Adeno-associated Virus, Serotype 9 Capsid-Mediated Expression of Hdac1 and LacZ in Adult PFC
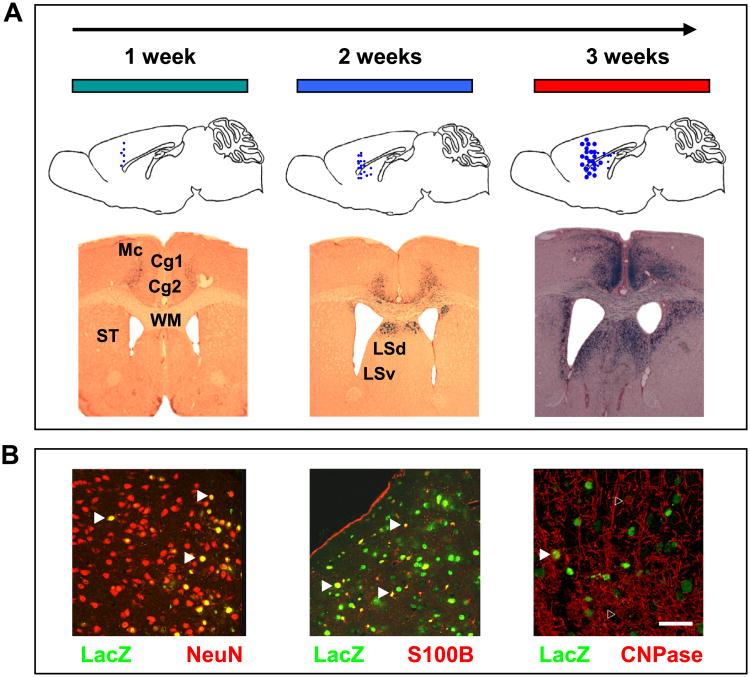
We used an adeno-associated virus, serotype 9 capsid (AAV9)-based system for long-lasting transgene expression following bilateral injections into mouse anterior medial cortex, considered the broad functional homolog to human PFC (31). Similar to the spreading pattern described for juvenile animals (32), injection of AAV9-LacZ reporter in young adult (12-week-old) C57BL/6J animals resulted, over the course of 3 weeks, in a gradual increase in numbers of cells expressing the transgene, covering at least 2.5 mm along the rostrocaudal axis of the PFC and involving all cortical layers I to VI (Figure 1A). Additional staining was found in the underlying white matter and portions of the dorsal and lateral septum (Figure 1A). A few scattered cells, positioned in dorsal hippocampus and rostral thalamus, were also labeled (Figure S2 in Supplement 1).
Figure 1.
Localized spreading and cellular expression patterns of adeno-associated virus-LacZ reporter in adult mouse prefrontal cortex (PFC). (A) (Top): Graphical representation (sagittal plane) of dorsoventral spread of virus between weeks 1 to 3 postinjection. (Bottom): Coronal sections, stained with β-galactosidase enzyme histochemistry, from adeno-associated virus-LacZ injected brains. Notice regional spreading and increased numbers of transduced cells during the time periods tested. (B) Cellular specificity of adeno-associated virus, serotype 9 capsid transductions in PFC (LacZ transgene with nuclear localization signal). Photomicrographs from PFC sections double-stained for anti-β-galactosidase (green) with (red, from left to right) anti-NeuN neuronal marker, anti-S100B for astrocytes, and anti-CNPase for oligodendrocytes. Notice double-labeled (yellow, marked by filled arrows) neurons and astrocytes but no double-labeled (unfilled arrows) oligodendroglia. Scale bar, 75 μm. Cg1, cingulate cortex (area 1); Cg2, cingulate cortex (area 2); LSd, lateral septum (dorsal part); LSv, lateral septum (ventral part); Mc, motor cortex; ST, striatum; WM, white matter.
To determine which of the major cell populations express the transgene (which was driven by a generic cytomegalovirus promoter) when packaged into the AAV9 capsid, we employed co-localization studies on brain sections of mice injected with the AAV9-LacZ reporter by double staining for β-galactosidase in combination with the neuronal marker NeuN (Millipore, Schwalbach, Germany), the astrocytic marker S-100B (Abcam, Cambridge UK), and the oligodendrocyte marker CNPase (Sigma Aldrich, Munich, Germany). We consistently found co-localization for NeuN and S-100B with β-galactosidase in approximately 50% of neurons and a slightly lower percentage of astrocytes. However, for CNPase and β-galactosidase, the overlapping signal was observed in less than 5% of CNPase positive cells (Figure 1B; Figure S3 in Supplement 1). We concluded that AAV9 primarily transduces neurons and astrocytes but only a very small portion of the oligodendrocyte population.
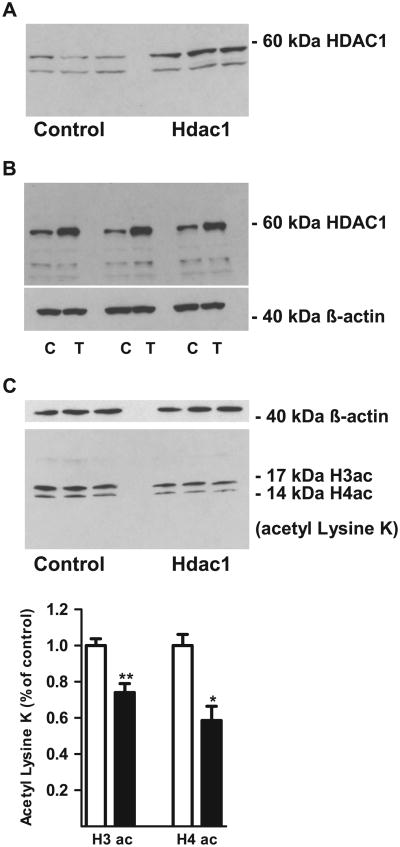
Using the above system, a full-length Hdac1 complementary DNA transfected into adult mouse PFC and N1E-115 mouse neuroblastoma cells resulted in robust upregulation of HDAC1 protein levels (Figure 2A,B; Figure S4 in Supplement 1). This increase in HDAC1 protein was associated with a 20% to 25% reduction of bulk histone H3 and H4 acetylation levels in the cell lysate, suggesting that the transgene indeed conveys a histone deacetylase activity (Figure 2C). Expression and protein levels of neuronal housekeeping genes, including NeuN and SynI, remained unaffected in adeno-associated virus (AAV)-Hdac1 cortex even several weeks after the injection (Figure S5 in Supplement 1), which is consistent with previous reports (33).
Figure 2.
Adeno-associated virus, serotype 9 capsid-mediated HDAC1 expression and activity. (A, C) Western blots against HDAC1 on (A) prefrontal cortex lysates from (left) control and (right) adeno-associated virus-Hdac1 mice; notice increased 60 kDa immunoreactivity corresponding to full-length HDAC1 but not for lower molecular weight (background) band (B) mouse N1E-115 neuroblastoma cells, showing (top image) increased 60 kDa HDAC1 in transfected (T) compared to control (C) cells. Lower image shows 42 kDa β-actin as loading control. (C) (Top) β-actin loading control and (bottom) bulk histone acetylation in anti-acetyl-lysine K immunoblots from N1E-115 cells, 24 hours posttransfection with Hdac1 (right) and control (left side). Lower band, histone H4ac, and upper band, H3ac. Notice decreased H3 and H4 acetylation in the Hdac1 transfected cells (black), relative to control cells (white bars), as summarized in the bar graph for quantification of acetyl-histones. Optical densities from immunoblots were normalized with β-actin levels and expressed as percentage of the control values. n = 3 per group. *p < .05, **p < .01, paired t test.
Working Memory Performance, Repetitive Behaviors, and Response to Novelty Are Altered after AAV-Mediated Hdac1 Expression in the PFC
To test whether Hdac1 overexpression would model the working memory deficits and other alterations attributed to a dysfunctional PFC in schizophrenia, we injected AAV-Hdac1 and AAV-LacZ as a control bilaterally into the PFC of young adult (12-week-old) C57BL/6J mice, then tested the animals' behavior, starting at the end of the third week postinjection. We probed 1) working memory in two different maze systems, each with a different batch of animals; 2) response to novelty in locomotor reactivity and object investigation assays; 3) compulsive behavior measured by marble burying; and 4) general reactivity as assessed by vocalization. To explore the specificity of any changes observed in the working memory paradigm, we also measured long-term memory function with standard passive-avoidance and novel object recognition tests.
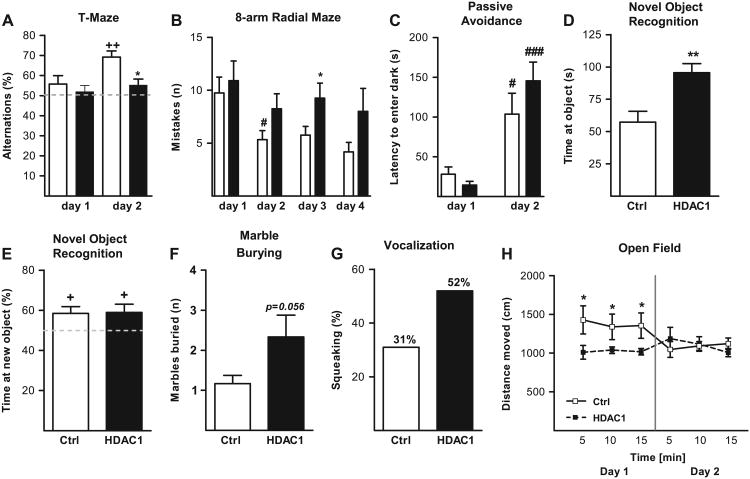
Working memory was probed using a T-maze and an eight-arm radial maze in two independent batches of mice (both tests are dependent on normal PFC function [34,35]). For both tests, there was a significant impairment in AAV-Hdac1 mice, compared with AAV-LacZ mice. In the T-maze paradigm in which the AAV-LacZ control animals showed the expected increase in number of alternations from 50% (chance) on the first day to above 70% on the second day, performance in the Hdac1 overexpressing mice remained at chance levels (F1,36 = 7.62; p < .01; two-way mixed analysis of variance [ANOVA], effect of treatment [Hdac1 or LacZ] on % of alternations) (Figure 3A). Likewise, in the eight-arm radial maze (applied over the course of 4 days), AAV-Hdac1 injected mice made more mistakes (defined as repeat entries into an arm the animal has already visited during the same test session) before completing the session than control animals (F1,22 = 8.87; p < .01; two-way mixed ANOVA, effect of transgene type [Hdac1 or LacZ] on number of mistakes), a phenotype that was prominent on days 2, 3, and 4 of this paradigm (Figure 3B). Yet, we did not observe a consistent trend for perseverations in the Hdac1 injected mice, as alternations in the T-maze were not significantly lower than 50% either. Furthermore, long-term memory functions as probed with the passive-avoidance test (36,37) and the novel object recognition paradigm (38,39) remained indistinguishable from control animals after Hdac1 overexpression in the PFC (Figure 3C,E). No effect of transgene on passive avoidance was detected by two-way mixed ANOVA (F1,26 = .22; p = ns) and both groups exhibited increased latencies to enter into the conditioned compartment (Figure 3C). Furthermore, Wilcoxon signed rank test revealed that both groups had a preference for the novel object (above chance level of 50%) in the novel object recognition test (Figure 3E). Therefore, the Hdac1-induced impairments in working memory are specific and not due to a generalized alteration in other types of memory in these animals.
Figure 3.
Behavioral performance of adeno-associated virus (AAV)-Hdac1 and AAV-LacZ mice. (A) Spontaneous alternations in the T-maze, indicative for working memory performance, are reduced in mice injected with AAV-Hdac1 compared with AAV-LacZ control mice. Notice that only AAV-LacZ mice show alternations above chance on day 2, as expected from (normal) wild-type mice. (B) Increased number of mistakes (repeat entries) in the 8-arm radial maze in mice injected with AAV-Hdac1. (C) Unchanged long-term memory performance in a passive avoidance learning paradigm and (D, E) in the novel object recognition test. Preference for the new object (on day 2) was significantly increased in both groups. (D) Yet, the time exploring the novel object on day 1 was higher in AAV-Hdac1 mice. (F) Marble burying test and (G) vocalizations (% squeaked when handled). (H) Locomotor activity in the open field recorded over 15 minutes on 2 consecutive days. Notice lack of adaptive changes in AAV-Hdac1 mice. +p < .05, + +p < .01, difference from chance level (Wilcoxon signed-rank test). #p < .05, ###p < .001, difference within the same treatment group between consecutive days. *p < .05, **p < .01, and ***p < .001, difference between different treatment groups, t test or Newman-Keuls post hoc test, when appropriate.
To further confirm the specificity of the working memory deficits after AAV-Hdac1, we injected a third vector, AAV(9)-green fluorescent protein (AAV-GFP) into the PFC. As expected, AAV-GFP mice were indistinguishable from AAV-LacZ mice, as it pertains to working memory performance in the radial maze and T-maze tests (Figure S6A,B in Supplement 1). Furthermore, working memory performance in AAV-GFP and AAV-LacZ mice was indistinguishable from mice that underwent the same anesthesia protocol (systemic ketamine) and surgery, but instead of AAV, they received a PFC injection of one microliter of sterile, 5% sucrose solution (Figure S6C in Supplement 1). Very similar test scores were obtained from naive and hitherto untreated mice (Figure S6D in Supplement 1), which is consistent with other reports that ketamine anesthesia in adult mice does not affect spatial working memory and motor task performance (40). From this, we concluded that the working memory defect in AAV-Hdac1 mice is attributable to excess levels of histone (lysine) deacetylase 1 in PFC and not due to AAV injection or procedure-related factors.
In the novel object recognition test, we noticed that AAV-Hdac1 mice visited objects much more often, and thus spent on the first day of testing significantly more time at the unfamiliar object than control mice (t test) (Figure 3D). To check whether this reflects an enhanced disposition for repetitive and stereotyped behavior (as opposed to actual novelty seeking), we employed the marble burying test (41,42), a test that in rodents is thought to measure the correlate of obsessive-compulsive behaviors that are under some control of the PFC (43). Interestingly, there was a very strong tendency (t test, p = .056) for increased burying behaviors in Hdac1 overexpressors, compared with animals injected with LacZ (Figure 3F).
Vocalization (audible to the human ear), which for adult laboratory mice has been linked to (mildly to severe) stressful situations (44,45), was increased by 50% in the AAV-Hdac1 mice, compared with AAV-LacZ (Figure 3G). To find additional evidence for an altered response to mild stress forms, such as exposure to a novel environment, we employed a 2-day paradigm to measure the changes in locomotor activity induced by repeated exposures to a novel open field situation for 15 minutes (46). Importantly, the PFC is thought to exert some control over locomotor activity induced by a novel open field environment (47,48). Therefore, it is interesting to note while AAV-LacZ mice showed the expected adaptive response with a 30% to 40% decline in locomotor hyperactivity upon re-exposure on day 2, the locomotor activity of AAV-Hdac1 mice did not follow any adaption pattern over time (Figure 3H).
Hdac1-Mediated Gene Expression Changes in the PFC
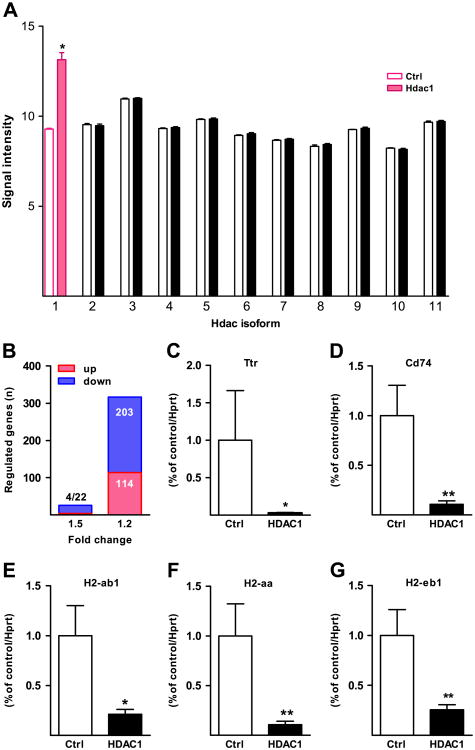
The prefrontal transcriptomes from AAV-Hdac1 and AAV-LacZ mice were profiled with the Mouse Gene 1.0 ST Array (Affymetrix, Santa Clara, California) (n = 4/group). Of note, among the 11 Hdac class I, II, and IV genes (class I Hdacs are homologues to yeast Rpd3 and class II Hdacs are homologues to yeast HdaI; Hdac11 is the sole representative of class IV; and class I and II Hdac genes are defined by a catalytic pocket with a zinc ion at the base [49,50]) that were represented on the array (Hdac1 to Hdac11), only the Hdac1 transcript showed a significant increase in the AAV-Hdac1 group, while differences in expression of the remaining 10 Hdac genes were minimal (<5%) between the groups (Figure 4A). These microarray data confirm that Hdac1 is the only class I/II Hdac that is overexpressed in PFC of AAV-Hdac1 injected mice.
Figure 4.
Hdac1-sensitive transcripts in adult prefrontal cortex. (A) Signal intensities for the 11 different Hdac(1–11) isoforms. Note selective upregulation of Hdac1 (red bars) in adeno-associated virus-Hdac1, compared with adeno-associated virus-LacZ prefrontal cortex. (B) Number of genes (blue) downregulated and (red) upregulated among the pool of significantly regulated genes with a more than 1.5-fold or 1.2-fold change; p < .05 (after false discovery rate). (C-G) Quantitative reverse transcriptase-polymerase chain reaction-based verification of Hdac1-downregulated major histocompatibility complex class II and other genes, normalized to Hprt housekeeping gene. Data are expressed relative to control group. *p < .05, **p < .01, Mann-Whitney U test. Ctrl, control.
To estimate the number of Hdac1-sensitive transcripts in the PFC, we compared PFC transcriptomes in AAV-Hdac1 and AAV-LacZ mice, using two filters: 1) >1.2-fold change; and 2) p value < .05 after correction for false discovery. There were 317 differentially expressed genes between the two groups of mice (Table S1 in Supplement 1). Stricter filtering to identify Hdac1-sensitive genes with >1.5-fold change (compared with LacZ transduced control animals) yielded 26 genes (Table 1). The large majority, or more than two thirds of differentially expressed genes, were downregulated upon Hdac1 overexpression (Figure 4B; Table S1 in Supplement 1), consistent with the notion that HDAC1 is, in the nervous system, primarily associated with repressive chromatin remodeling and negative regulation of gene expression (33,51).
Table 1. List of PFC Transcripts with >1.5-fold, Significant Difference Between AAV-Hdac1 and AAV-LacZ.
| Gene Symbol | Name | FC | p Value | FDR |
|---|---|---|---|---|
| Downregulated Genes | ||||
| M28833/igkv8-21 | Immunoglobulin kappa variable 8-21 | −25.86 | .019 | .041 |
| Ttr | Transthyretin | −8.64 | .039 | .047 |
| Cd74 | Cluster of differentiation 74 | −4.08 | .038 | .047 |
| H2-Ab1 | Histocompatibility 2, class II antigen A, beta 1 | −3.84 | .025 | .044 |
| H2-Aa | Histocompatibility 2, class II antigen A, alpha | −3.74 | .025 | .044 |
| Gbp4 | Guanylate-binding protein 4 | −2.98 | .038 | .047 |
| Cxcl9 | Chemokine (C-X-C) motif, ligand 9 | −2.23 | .047 | .049 |
| Ighg | Immunoglobulin heavy chain γ | −2.23 | .028 | .045 |
| Gm12250 | Interferon gamma inducible protein (Ifi) 47 pseudogene | −2.18 | .036 | .047 |
| Igtp | Immunity-related GTPase, family M, member 2 | −2.18 | .048 | .049 |
| LOC100046973 | −2.08 | .048 | .049 | |
| Serping1 | Serping1 | −2.07 | .045 | .048 |
| H2-Eb1 | Histocompatibility 2, class II antigen E, beta | −2.07 | .028 | .045 |
| Ifi203 | Interferon activated gene 203 | −1.99 | .047 | .049 |
| Gm7016 | −1.85 | .048 | ||
| Art2b | ADP-ribosyltransferase | −1.67 | .047 | .049 |
| Samhd1 | SAM domain and HD domain-containing protein 1 | −1.63 | .037 | .047 |
| Tmem140 | Transmembrane protein 140 | −1.58 | .028 | .045 |
| Igfbp7 | Insulin-like growth factor-binding protein 7 | −1.55 | .039 | .047 |
| Cp | Ceruloplasmin | −1.54 | .018 | .04 |
| Tnfsf10 | Tumor necrosis factor | −1.54 | .021 | .041 |
| Irf1 | Interferon regulatory factor 1 (ligand) superfamily, member 10 | −1.52 | .044 | .048 |
| Upregulated Genes | ||||
| Hdac1/Gm4864 | Histone deacetylase 1 (pseudogene) | 10.87 | <.001 | <.001 |
| ENSMUST00000097231 | Small nucleolar RNA (SNORD) 115 | 1.57 | .003 | .028 |
| ENSMUST00000099414 | Zinc finger protein (zfp) 955b | 1.51 | .038 | .047 |
| ENSMUST00000101951 | Small nucleolar RNA (SNORD) 115 | 1.50 | .003 | .028 |
ADP, adenosine diphosphate; AAV, adeno-associated virus; FC, fold change; FDR, false discovery rate; GTP, guanosine triphosphate; HD, hydrolase; PFC, prefrontal cortex; SAM, sterile alpha motif.
Some of the highest fold changes for genes implicated in cognitive impairment involved Ttr (Transthyretin), which is expressed at low levels in cortical neurons and thought to play a role in thyroid hormone transport and metabolism and regulation of beta-amyloid (52). Interestingly, the small subset of 26 genes with a >1.5-fold change in Hdac1 overexpressing mice (Table 1) showed significant Gene Ontology enrichments for transcripts associated with immune functions and major histocompatibility complex class II (MHC II)-related antigen presentation (Table 2), including H2-ab1 (histocompatibility 2, class II antigen A, beta 1), H2-aa (histocompatibility 2, class II antigen A, alpha), and H2-eb1 (histocompatibility 2, class II antigen E, beta 1) and the MHC II gamma chain and transporter, Cd74 (cluster of differentiation 74). Downregulation of these genes was confirmed by comparing additional samples of PFC from AAV-Hdac1 and AAV-LacZ mice as control animals, using quantitative reverse transcriptase-polymerase chain reaction (Figure 4C-G). This decrease in expression of immune-regulatory transcripts in the Hdac1-overexpressing PFC was highly specific, because neither the set of Hdac1-sensitive transcripts with 1.2-fold or 1.5-fold change was enriched for Gene Ontology categories involved in neuronal function and neurotransmission (yet, it should be mentioned the extended set of 317 Hdac1-sensitive transcripts included several neuronal genes such as Elf1, a beta-spectrin considered important for axonal growth [53] and Snap23 [synaptosomal-associated protein 23], Table S1 in Supplement 1).
Table 2. Gene Ontology Enrichment Showing Prominent Footprint of MHC II and Immune Regulation Among the Set of Transcripts with > 1.5-fold Change.
| Path (GO) | Path Name | Number of Genes Significant | Annotated | p Value | Gene Name |
|---|---|---|---|---|---|
| Biological Process | |||||
| GO:0019886 | Antigen processing and presentation of exogenous peptide antigen via MHC class II | 4 | 16 | 2.74E-05 | H2-Aa, H2-Ab1, H2-Eb1, Cd74 |
| GO:0002504 | Antigen processing and presentation of peptide or polysaccharide antigen via MHC class II | 3 | 10 | 3.73E-04 | H2-Aa, H2-Ab1, H2-Eb1 |
| GO:0048002 | Antigen processing and presentation of peptide antigen | 2 | 3 | 5.00E-04 | H2-Aa, H2-Ab1 |
| GO:0019882 | Antigen processing and presentation | 4 | 32 | 1.20E-03 | H2-Aa, H2-Ab1 H2-Eb1, Cd74 |
| GO:0006955 | Immune response | 6 | 150 | 1.54E-02 | H2-Aa, H2-Ab1 H2-Eb1, Cd74 Cxcl9, Tnfs10 |
| GO:0045582 | Positive regulation of T cell differentiation | 2 | 12 | 1.07E-01 | H2-Aa, Cd74 |
| GO:0051607 | Defense response to virus | 2 | 43 | .00055 | Cxcl9, Samhd1 |
| GO:0045087 | Innate immune response | 2 | 118 | .0098 | Serping1 Samhd1 |
| Cellular Component | |||||
| GO:0042613 | MHC class II protein complex | 3 | 10 | 3.73E-04 | H2-Aa, H2-Ab1 H2-Eb1 |
| GO:0005771 | Multivesicular body | 2 | 20 | 5.44E-01 | H2-Ab1, Cd74 |
| GO:0005615 | Extracellular space | 6 | 810 | .032 | Serping1, Cp Cxcl9, Tnfsf10 Ttr, Igfbp7 |
| GO:0009897 | External side of plasma membrane | 4 | 227 | .0016 | H2-Aa, H2-Ab1 Cd74, Cxcl9 |
| Molecular Function | |||||
| GO:0042605 | Peptide antigen binding | 2 | 20 | 5.44E-01 | H2-Aa, H2-Ab1 |
| GO:0005125 | Cytokine activity | 2 | 200 | .039 | Cxcl9, Tnfsf10 |
GO, Gene Ontology; MHC II, major histocompatibility complex class II.
Hdac1-Downregulated Target Genes Show Significant Overlap with Transcripts Altered in PFC of Subjects Diagnosed with Schizophrenia or Bipolar Disorder
To determine whether the Hdac1 sensitive transcripts in mouse PFC match any genes expressed at altered levels in postmortem PFC of subjects diagnosed with schizophrenia or bipolar disorder, we compared our dataset of significantly altered transcripts in AAV-Hdac1 (mouse) PFC with published microarray datasets of US and Australia-based postmortem cohorts that showed increased HDAC1 expression in PFC of diseased subjects (28,29), with p < .1 as filter for the clinical datasets (Table S1 in Supplement 1). We note that from a total of 203 genes subject to significant down-regulation in the AAV-Hdac1 mice, 60 matched a transcript that was expressed at decreased (n = 30) or increased (n = 30) levels in at least one, or both, microarray datasets from the schizophrenia and/or bipolar disorder subjects (28,29). This list included TTR, CD74, and other MHC II-related transcripts and genes related to neuronal signaling including ELF1 and NPY. In contrast, only 12 of 121 transcripts subject to a significant increase in AAV-Hdac1 mice were expressed at decreased (n = 4) or increased (n = 8) levels in the clinical specimens. This much larger overlap of the Hdac1-downregulated genes with transcriptome of subjects on the psychosis spectrum, compared with the Hdac1-upregulated genes, was highly significant (χ215.06 [df 1], p < .001).
Medication Effects and Developmental Regulation
Hdac1 levels became downregulated during the course of normal PFC development, because in C57BL/6J wild-type mice, levels in adult PFC had decreased by 90%, compared with newborn animals (Figure S7 in Supplement 1), suggesting that supranormal HDAC1 expression in adult PFC of schizophrenia and bipolar disorder subjects could reflect altered developmental regulation.
Of note, none of the three studies reporting abnormally high levels of HDAC1 transcript in schizophrenia brain could find conclusive evidence for antipsychotic drug- or other medication-related effect (28–30). However, the large majority of postmortem cases were exposed to APD treatment at some point in their lifetime; hence, drug-mediated effects are difficult to study postmortem owing to a paucity of drug-free cases. To explore the role of APD for the regulation of cortical HDAC1 expression in the patients' brains, we systemically treated adult mice over the course of 21 days with once daily injections of the D2-receptor antagonist and conventional antipsychotic, haloperidol (.5 mg/kg); the atypical antipsychotic, clozapine (5 mg/kg); or saline, then harvested the brain and measured via quantitative reverse transcriptase-polymerase chain reaction the RNA levels for Hdac1 and the Hprt (hypoxanthine-guanine phosphoribosyltransferase) housekeeping gene as a control. These APD doses are sufficiently high to elicit changes in the animals' locomotor activity (54) and histone methylation changes in the cerebral cortex (4). However, in each treatment group, changes in Hdac1 RNA levels were less than .2-fold compared with saline-treated control animals and without significance (Figure S8A in Supplement 1). Interestingly, some of the transcripts that showed a robust, 2.8fold downregulation after AAV-Hdac1 injection, including the aforementioned Ttr and multiple MHC II transcripts, such as Cd74, H2-Aa, H2-Ab1, and H2-Eb1 (Table S1 in Supplement 1), were upregulated after APD, particularly in clozapine-treated mice (Figure S8B–F). These drug-induced increases in gene expression were specific, because RNA levels for the neuropeptide Npy were downregulated after clozapine treatment (Figure S8G in Supplement 1), consistent with previous reports (55). Expression of guanylate binding protein 4 (Gbp4), which is decreased in AAV-Hdac1 PFC (Table S1 in Supplement 1), remained virtually unchanged in clozapine-treated mice but was significantly downregulated after haloperidol (Figure S8H in Supplement 1).
Discussion
Summary of Results
In the present study, 3 weeks after virus vector injection into the PFC, increased expression of the protein (lysine) deacetylase, HDAC1/KDAC1, resulted in significant behavioral alterations, including impaired working memory, enhanced repetitive behavior, and a blunted and nonadaptive response to novel environments. These behavioral alterations were specific because long-term memory remained unaffected in the same set of animals. Increased Hdac1 expression in young adult PFC was associated with subtle alterations (mostly downregulation) of hundreds of gene transcripts, with more robust changes limited to a small subset of 26 genes, including multiple members of the MHC II complex. Further, Hdac1 levels declined during the course of postnatal PFC development and were not increased after APD exposure. However, APD had opposite effects on MHC II complex genes than Hdac1 overexpression. Thus, it might be conceivable that developmental defects could have contributed to increased HDAC1 levels in microarray datasets from PFC and hippocampus of subjects with schizophrenia (28–30), while APD could ameliorate downstream molecular pathology. Because the Hdac1 transgene was overexpressed in the PFC for several weeks, our study is difficult to complement by pharmacologic approaches using histone deacetylase (HDAC) inhibitors and other chromatin modifying drugs.
Neuroanatomical Considerations
The findings from the present study strongly suggest that fine-tuning of Hdac1 expression and activity in the adult brain is pivotal for orderly function of PFC. However, the AAV9-based transduction system was not limited to the brain territory occupied by the PFC but involved a small portion of the underlying white matter, portions of the dorsal and lateral septum, and scattered cells in rostral thalamus and hippocampus. This labeling pattern could, in part, be due to local spread. However, given that each of the above areas are directly interconnected with the PFC and function as important nodes in prefrontal-cortical-limbic circuitry (56), retrograde transport as previously reported for the AAV9 capsid (32,57) is a more likely possibility. Therefore, the neuroanatomical substrate for the impairments in cognition and behavior as reported here could involve the wider prefrontal circuitry, including neurons located in the septum, anterior thalamus, and hippocampus.
HDAC Multiplicity of Functions
The molecular link between upregulated Hdac1 expression and impaired cognition remains to be explored further. Importantly, one defining feature of HDACs is their pleiotropism, due to the fact that these enzymes cleave off lysine acetyl-groups in a wide variety of proteins. Thus, HDAC1, which in brain is located predominantly but not exclusively in the nucleus (58), also regulates, in addition to its classical substrate, the nucleosomal core histones, adenosine monophosphate-activated protein kinase as a key regulator for cell signaling and metabolism (59), multiple proteins of the pre-messenger RNA 3′end processing machinery (60), and several transcriptional activators or repressors including p53, NFkB, MyoD, and E2F (61). Therefore, AAV-Hdac1 mediated changes in the PFC transcriptome could result from repressive chromatin remodeling at promoters (33,51), which is a very plausible hypothesis, given the majority of gene expression changes in our microarray studies were defined by a decrease from baseline. Alternatively, increased HDAC1 activity could have resulted in alterations in transcription factor activity (61), differential regulation of polyadenylation and other pre-messenger RNA processing (60), or adenosine monophosphate-activated protein kinase-mediated shifts in transcripts regulating energy homeostasis (59,62) or a combination thereof.
Implications for the Neurobiology and Treatment of Schizophrenia and Bipolar Disorder
In the present study, robust impairments in working memory and additional behavioral alterations were observed at 3 weeks after viral vector-mediated overexpression of Hdac1 in young adult PFC. This finding strongly suggests that increased HDAC1 expression in schizophrenia and bipolar brains (28–30) is not an epiphenomenon but instead a key event in the pathophysiological cascade that ultimately could contribute to psychiatric symptoms. According to the present study, a failure to down-regulate HDAC1 levels during development could be one of the possible mechanisms (Figure S7 in Supplement 1). Developmental downregulation of Hdac1 is particularly pronounced in neurons, while astrocytes continue to express Hdac1 at moderate levels throughout adulthood (63). Furthermore, during the course of neuronal maturation, synaptic transmission becomes much less sensitive to the effects of an experimental knockdown of Hdac1 (64). The preclinical findings in PFC (present study) and hippocampus (33,50), in conjunction with clinical observations (28-30), suggest that orderly brain function depends on a delicate balance of Hdac1 expression. Consequently, supranormal HDAC1 expression in cortical-limbic circuitry could then lead to molecular, cellular, and behavioral manifestations of disease. While the precise molecular mechanisms linking HDAC1 excess to psychiatric phenotypes remain to be elucidated, we were surprised to find multiple immune regulatory transcripts among the small set of 26 genes with a more robust (>1.5-fold) change in expression after Hdac1 transduction. Among these Hdac1-sensitive immune transcripts were three genes, H2-Aa, H2-Ab1, and H2-Eb1 (which are known in humans as HLA-DQA1, HLA-DQB1, and HLA-DRB1) that are positioned within a prominent schizophrenia and bipolar disorder susceptibility locus at the MHC II gene cluster on chromosome 6p21.3-22.1. Furthermore, these three genes are the closest ones to several schizophrenia-associated single nucleotide polymorphisms at this risk locus (65-67) (Figure S9 in Supplement 1). For example, rs9271850 is positioned 50 kilobase (kb) downstream from HLA-DRB1 and 10 kb upstream of HLA-DQA1, rs9272219 is 3 kb upstream of HLA-DQA1, and rs927535 is positioned in the first intron of HLA-DQA1 and 20 kb upstream of HLA-DQB2(65). Each of these single nucleotide polymorphisms was found in a meta-analysis of three European ancestry schizophrenia datasets (Molecular Genetics of Schizophrenia, International Schizophrenia Consortium, and SGENE) with high statistical significance (65-67) and in two independent bipolar disorder datasets, the Systematic Treatment Enhancement Program for Bipolar Disorder and the Wellcome Trust Case Control Consortium (68,69).
The HLA-DRB1, HLA-DQA1, and HLA-DQB1 genes encode MHC class II proteins that play a central role in the immune system by presenting peptides derived from extracellular proteins. Major histocompatibility complex II proteins are produced in microglia under diverse pathologic conditions (inflammation, neuronal injury, neurodegeneration) (70). Notably, neuronal activity and neurotrophin factors may suppress MHC class I and II expression in microglia and astrocytes (70-74), and similar mechanisms may explain decreased expression of H2-Ab1/H2-Aa/H2-Eb1 transcripts as reported here. Interestingly, MHC II transcripts were subject to a robust upregulation after (sub)chronic exposure to clozapine (Figure S8C-F in Supplement 1), a drug considered the gold standard among antipsychotics due to its exceptional efficacy in treating positive symptoms in patients diagnosed with treatment-resistant schizophrenia (75,76).
Some studies suggested that AAV-mediated expression of nonself protein in brain parenchyma could trigger an immune response (77). In the present study, there was no activation of microglia as the brain's immune surveillance system (Figure S10 in Supplement 1). Instead, the Hdac1 transgene induced down-regulated expression of the MHC II complex (which is normally expressed in astrocytes [77]) in conjunction with behavioral changes.
While APD did not significantly alter Hdac1 expression in the present study, atypical APD decreased HDAC2 occupancy at the metabotropic glutamate receptor 2 promoter (78) and increased open chromatin-associated histone methylation at gamma-aminobutyric acidergic genes (4). Given this multiplicity of APD-mediated epigenetic effects and the variabilities in therapeutic response and side effects (79,80), it would be interesting to explore blood-based histone acetylation levels as biomarkers for treatment response or side effects in individual patients diagnosed with schizophrenia or bipolar disorder (81,83).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (SA) and the German Research Foundation (MJ).
We thank Yin Guo and Hsien-Sung Huang for providing brain tissue from antipsychotic drug-treated mice and Phyllis Spatrick for valuable advice on the microarray experiments.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.03.020.
References
- 1.Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdolmaleky HM, Cheng KH, Faraone SV, Wilcox M, Glatt SJ, Gao F, et al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15:3132–3145. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwamoto K, Bundo M, Yamada K, Takao H, Iwayama-Shigeno Y, Yoshikawa T, Kato T. DNA methylation status of SOX10 correlates with its downregulation and oligodendrocyte dysfunction in schizophrenia. J Neurosci. 2005;25:5376–5381. doi: 10.1523/JNEUROSCI.0766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulha HP, Cheung I, Whittle C, Wang J, Virgil D, Lin CL, et al. Epigenetic signatures of autism: Trimethylated H3K4 landscapes in prefrontal neurons. Arch Gen Psychiatry. 2012;69:314–324. doi: 10.1001/archgenpsychiatry.2011.151. [DOI] [PubMed] [Google Scholar]
- 7.Akbarian S, Ruehl MG, Bliven E, Luiz LA, Peranelli AC, Baker SP, et al. Chromatin alterations associated with down-regulated metabolic gene expression in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2005;62:829–840. doi: 10.1001/archpsyc.62.8.829. [DOI] [PubMed] [Google Scholar]
- 8.Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PloS One. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F, et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet. 2011;20:4786–4796. doi: 10.1093/hmg/ddr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melas PA, Rogdaki M, Osby U, Schalling M, Lavebratt C, Ekstrom TJ. Epigenetic aberrations in leukocytes of patients with schizophrenia: Association of global DNA methylation with antipsychotic drug treatment and disease onset. FASEB J. 2012;26:2712–2718. doi: 10.1096/fj.11-202069. [DOI] [PubMed] [Google Scholar]
- 11.Petronis A, Gottesman II, Kan P, Kennedy JL, Basile VS, Paterson AD, Popendikyte V. Monozygotic twins exhibit numerous epigenetic differences: Clues to twin discordance? Schizophr Bull. 2003;29:169–178. doi: 10.1093/oxfordjournals.schbul.a006988. [DOI] [PubMed] [Google Scholar]
- 12.Numata S, Ye T, Hyde TM, Guitart-Navarro X, Tao R, Wininger M, et al. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90:260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labrie V, Pai S, Petronis A. Epigenetics of major psychosis: Progress, problems and perspectives. Trends Genet. 2012;28:427–435. doi: 10.1016/j.tig.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology. 2013;38:220–236. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30:13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res. 2012;140:175–184. doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci U S A. 2008;105:16356–16361. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: A novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris CV, DiNieri JA, Szutorisz H, Hurd YL. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur J Neurosci. 2011;34:1574–1583. doi: 10.1111/j.1460-9568.2011.07884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen D, Lazar G, Couvert P, Desportes V, Lippe D, Mazet P, Héron D. MECP2 mutation in a boy with language disorder and schizophrenia. Am J Psychiatry. 2002;159:148–149. doi: 10.1176/appi.ajp.159.1.148-a. [DOI] [PubMed] [Google Scholar]
- 23.Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawk JD, Florian C, Abel T. Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory. Learn Mem. 2011;18:367–370. doi: 10.1101/lm.2097411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Sharma RP, Grayson DR, Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: Analysis of the National Brain Databank microarray collection. Schizophr Res. 2008;98:111–117. doi: 10.1016/j.schres.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008;1239:235–248. doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Jakovcevski M, Guo Y, Su Q, Gao G, Akbarian S. rAAV9–a human-derived adeno-associated virus vector for efficient transgene expression in mouse cingulate cortex. Cold Spring Harb Protoc. 2010;2010;5:417. doi: 10.1101/pdb.prot5417. pdb.prot. [DOI] [PubMed] [Google Scholar]
- 33.Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bahr M, et al. HDAC1 regulates fear extinction in mice. J Neurosci. 2012;32:5062–5073. doi: 10.1523/JNEUROSCI.0079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 35.Pothuizen HH, Davies M, Aggleton JP, Vann SD. Effects of selective granular retrosplenial cortex lesions on spatial working memory in rats. Behav Brain Res. 2010;208:566–575. doi: 10.1016/j.bbr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Morellini F, Sivukhina E, Stoenica L, Oulianova E, Bukalo O, Jakovcevski I, et al. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex. 2010;20:2712–2727. doi: 10.1093/cercor/bhq017. [DOI] [PubMed] [Google Scholar]
- 37.Davis HP, Spanis CW, Squire LR. Inhibition of cerebral protein synthesis: Performance at different times after passive avoidance training. Pharmacol Biochem Behav. 1976;4:13–16. doi: 10.1016/0091-3057(76)90168-4. [DOI] [PubMed] [Google Scholar]
- 38.Weible AP, Rowland DC, Monaghan CK, Wolfgang NT, Kentros CG. Neural correlates of long-term object memory in the mouse anterior cingulate cortex. J Neurosci. 2012;32:5598–5608. doi: 10.1523/JNEUROSCI.5265-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentim AM, Olsson IA, Antunes LM. The anaesthetic combination of ketamine/midazolam does not alter the acquisition of spatial and motor tasks in adult mice. Lab Anim. 2013;47:19–25. doi: 10.1258/la.2012.011179. [DOI] [PubMed] [Google Scholar]
- 41.Takeuchi H, Yatsugi S, Yamaguchi T. Effect of YM992, a novel antidepressant with selective serotonin re-uptake inhibitory and 5-HT 2A receptor antagonistic activity, on a marble-burying behavior test as an obsessive-compulsive disorder model. Jpn J Pharmacol. 2002;90:197–200. doi: 10.1254/jjp.90.197. [DOI] [PubMed] [Google Scholar]
- 42.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albelda N, Joel D. Animal models of obsessive-compulsive disorder: Exploring pharmacology and neural substrates. Neurosci Biobehav Rev. 2012;36:47–63. doi: 10.1016/j.neubiorev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Whitney GD. Vocalization of mice: A single genetic unit effect. J Hered. 1969;60:337–340. doi: 10.1093/oxfordjournals.jhered.a108009. [DOI] [PubMed] [Google Scholar]
- 45.Whitney G. Ontogeny of sonic vocalizations of laboratory mice. Behav Genet. 1970;1:269–273. doi: 10.1007/BF01074658. [DOI] [PubMed] [Google Scholar]
- 46.Ramos A, Mormede P. Stress and emotionality: A multidimensional and genetic approach. Neurosci Biobehav Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 47.Dracheva S, Lyddon R, Barley K, Marcus SM, Hurd YL, Byne WM. Editing of serotonin 2C receptor mRNA in the prefrontal cortex characterizes high-novelty locomotor response behavioral trait. Neuropsychopharmacology. 2009;34:2237–2251. doi: 10.1038/npp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granon S, Changeux JP. Deciding between conflicting motivations: What mice make of their prefrontal cortex. Behav Brain Res. 2012;229:419–426. doi: 10.1016/j.bbr.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Bertos NR, Wang AH, Yang XJ. Class II histone deacetylases: Structure, function, and regulation. Biochem Cell Biol. 2001;79:243–252. [PubMed] [Google Scholar]
- 50.Ficner R. Novel structural insights into class I and II histone deacetylases. Curr Top Med Chem. 2009;9:235–240. doi: 10.2174/156802609788085304. [DOI] [PubMed] [Google Scholar]
- 51.Park SW, Huq MD, Loh HH, Wei LN. Retinoic acid-induced chromatin remodeling of mouse kappa opioid receptor gene. J Neurosci. 2005;25:3350–3357. doi: 10.1523/JNEUROSCI.0186-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Masliah E, Reixach N, Buxbaum JN. Neuronal production of transthyretin in human and murine Alzheimer's disease: Is it protective? J Neurosci. 2011;31:12483–12490. doi: 10.1523/JNEUROSCI.2417-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang Y, Katuri V, Iqbal S, Narayan T, Wang Z, Lu RS, et al. ELF a beta-spectrin is a neuronal precursor cell marker in developing mammalian brain; structure and organization of the elf/beta-G spectrin gene. Oncogene. 2002;21:5255–5267. doi: 10.1038/sj.onc.1205548. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang XF, Deng C, Zavitsanou K. Neuropeptide Y mRNA expression levels following chronic olanzapine, clozapine and halo-peridol administration in rats. Neuropeptides. 2006;40:213–219. doi: 10.1016/j.npep.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 57.Elmallah MK, Falk D, Lane MA, Conlon TJ, Lee KZ, Shafi NI, et al. Retrograde gene delivery to hypoglossal motoneurons using adeno-associated virus serotype 9. Hum Gene Ther Methods. 2012;23:148–156. doi: 10.1089/hgtb.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia H, Pallos J, Jacques V, Lau A, Tang B, Cooper A, et al. Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Hunting-ton's disease. Neurobiol Dis. 2012;46:351–361. doi: 10.1016/j.nbd.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin YY, Kiihl S, Suhail Y, Liu SY, Chou YH, Kuang Z, et al. Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK. Nature. 2012;482:251–255. doi: 10.1038/nature10804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Shimazu T, Horinouchi S, Yoshida M. Multiple histone deacetylases and the CREB-binding protein regulate pre-mRNA 3'-end processing. J Biol Chem. 2007;282:4470–4478. doi: 10.1074/jbc.M609745200. [DOI] [PubMed] [Google Scholar]
- 61.Reichert N, Choukrallah MA, Matthias P. Multiple roles of class I HDACs in proliferation, differentiation, and development. Cell Mol Life Sci. 2012;69:2173–2187. doi: 10.1007/s00018-012-0921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramamurthy S, Ronnett G. AMP-activated protein kinase (AMPK) and energy-sensing in the brain. Exp Neurobiol. 2012;21:52–60. doi: 10.5607/en.2012.21.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuroglial development. Dev Dyn. 2008;237:2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- 64.Akhtar MW, Raingo J, Nelson ED, Montgomery RL, Olson EN, Kavalali ET, Monteggia LM. Histone deacetylases 1 and 2 form a developmental switch that controls excitatory synapse maturation and function. J Neurosci. 2009;29:8288–8297. doi: 10.1523/JNEUROSCI.0097-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- 71.Neumann H, Misgeld T, Matsumuro K, Wekerle H. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: Involvement of the p75 neurotrophin receptor. Proc Natl Acad Sci U S A. 1998;95:5779–5784. doi: 10.1073/pnas.95.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 73.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 76.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 77.Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS, Forsayeth J. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol Ther. 2013;21:158–166. doi: 10.1038/mt.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL, et al. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci. 2012;15:1245–1254. doi: 10.1038/nn.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tandon R. Antipsychotics in the treatment of schizophrenia: An overview. J Clin Psychiatry. 2011;72(suppl 1):4–8. doi: 10.4088/JCP.10075su1.01. [DOI] [PubMed] [Google Scholar]
- 80.Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- 81.Gavin DP, Sharma RP. Chromatin from peripheral blood mononuclear cells as biomarkers for epigenetic abnormalities in schizophrenia. Cardiovasc Psychiatry Neurol. 2009;2009:409562. doi: 10.1155/2009/409562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavin DP, Kartan S, Chase K, Grayson DR, Sharma RP. Reduced baseline acetylated histone 3 levels, and a blunted response to HDAC inhibition in lymphocyte cultures from schizophrenia subjects. Schizophr Res. 2008;103:330–332. doi: 10.1016/j.schres.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasan A, Mitchell A, Schneider A, Halene T, Akbarian S. Epigenetic dysregulation in schizophrenia: molecular and clinical aspects of histone deacetylase inhibitors [published online ahead of print February 5] Eur Arch Psychiatry Clin Neurosci. 2013 doi: 10.1007/s00406-013-0395-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.