Abstract
BACKGROUND AND OBJECTIVES
Studies in mice suggest that rapid transfusions of red blood cells (RBCs), refrigerator stored for longer durations, induce a pro-inflammatory cytokine response. Studies in human neonates confirm these findings; however, to date, adult human studies have failed to replicate these findings. We used healthy research dogs to begin to examine the factors affecting the cytokine response to transfusion.
MATERIALS AND METHODS
In a prospective study, healthy dogs were randomized for two autologous packed RBC transfusions after 7 (i.e. “fresh”) and 28 (“old”) days of storage, or after 28 and 7 days of storage, with or without pre-storage leukoreduction (LR).
RESULTS
No significant differences were observed between LR and non-LR transfusions for all circulating analytes measured following transfusion. A pro-inflammatory cytokine response, exemplified by monocyte chemoattractant protein-1, was observed 6 hours after only old RBC transfusions, irrespective of infusion rate (P<0.001). This response was accompanied by increased neutrophil counts (P<0.001) and decreased platelet counts (P<0.001).
CONCLUSION
In healthy dogs, old RBC transfusions induce inflammation, which is unaffected by infusion rate.
Keywords: Dog, RBC transfusion, non-transferrin bound iron, monocyte chemoattractant protein-1
INTRODUCTION
The potential adverse affects of transfusing RBCs after longer durations of refrigerated storage is a major issue in human transfusion medicine [1]. Large observational studies evaluating critically ill individuals, including cardiac surgery [2], pediatric intensive care [3], and trauma patients [4], found associations between transfusions of “older” RBCs (generally defined as >14 days of storage) with increased mortality, multiple organ dysfunction, and/or sepsis. A recent meta-analysis concluded that transfusions of older, stored RBCs correlate with a significantly increased risk of death [5]. These studies raise important concerns regarding the risk-benefit ratio of RBC transfusion in certain clinical settings.
The mechanisms by which transfusions of older, stored RBCs may increase morbidity and mortality are not yet known, although multiple hypotheses are being intensely investigated. Potential mediators accumulating in the RBC supernatant during storage include free hemoglobin, microvesicles, and other compounds [6, 7]. In addition, the “iron hypothesis” proposes that rapid clearance of storage-damaged RBCs acutely delivers a bolus of hemoglobin iron to the reticuloendothelial system, thus impairing host defenses [8]. Multiple mediators may also act in combination to manifest the clinical effects attributed to older, stored RBC transfusions.
In mouse models and human volunteers, transfusions of older, stored leukoreduced (LR) RBCs produce extravascular hemolysis and circulating non-transferrin-bound iron. In addition, non-transferrin-bound iron levels correlate well with enhanced pathogen proliferation in serum samples in vitro [9, 10]. Rapid infusion of older, stored RBCs also induces a dose-responsive pro-inflammatory cytokine response in mice [10]. Although a pro-inflammatory cytokine response was seen after transfusion in preterm neonates [11], this was not observed in healthy human volunteers slowly transfused with one unit of RBCs [9]. These findings suggest that recipient factors, along with transfusion dose and infusion rate, may affect the resulting inflammatory response.
To this end, healthy research dogs were used to examine: (i) whether stored RBC transfusions induce an inflammatory response, (ii) whether the infusion rate is relevant, and (iii) whether the extent of extravascular hemolysis correlates with the inflammatory response. Furthermore, because the clinical benefits of LR are still debatable, with some centers not practicing universal leukoreduction [12], and because this issue has not been fully explored in veterinary practice, we also evaluated whether pre-storage LR affects the degree of hemolysis (both ex vivo in the unit and in vivo) and the inflammatory response.
MATERIALS AND METHODS
Study design
In a prospective, crossover study, 20 healthy research dogs were randomized to two autologous RBC transfusion events, one after 7 days (i.e., “fresh”) and one after 28 days (i.e., “old”) of storage or vice versa. Fresh and old RBC transfusions were separated by at least a one-month washout period. Dogs were also randomized to LR (n = 10) or non-LR (n = 10) for both transfusions. As a control, 4 untransfused and 4 transfused (at least one month following the second autologous transfusion) healthy research dogs received saline infusions instead of RBCs.
Dogs
Healthy research dogs of various mixed breeds (8 males, 16 females), maintained in a research colony at the University of Pennsylvania, were used (4 were only infused with saline). Dogs ranged in age from 6 months to 8 years and weighed 18–34 kg. To minimize the potential for lipemia-induced hemolysis in vitro [13], dogs were fasted overnight before collecting whole blood units or administering autologous RBC transfusions or saline infusions, and were not fed until 2 hours after transfusion; thereafter, dogs were fed ad libitum. Dogs were not sedated for any procedure. The Institutional Animal Care and Use Committee of the University of Pennsylvania approved the study protocol.
Blood collection and processing
Blood (15 mL/kg; 20% blood volume) was collected from the jugular vein using a standard triple blood-pack unit containing anticoagulant citrate-phosphate-dextrose solution and RBC preservative solution (Adsol/AS-1; Fenwal). The volumes of CPD and Adsol were adjusted for the smaller dogs to maintain CPD:whole blood and Adsol:whole blood ratios of 1:7 and 1:4.5, respectively. For pre-storage LR, a RS-2000 whole blood filter (Fenwal) was used [14]; an additional 50 mL of blood was collected from dogs in the LR group to compensate for losses during filtering. Residual leukocytes were enumerated by flow cytometry (BD Leucocount kit, BD Biosciences). Aliquots (2 mL) of packed RBCs in Adsol were obtained prior to and following storage to examine storage changes in analytes; supernatants were stored at −80°C until analysis. Plasma fHb was measured using a cyanmethemoglobin assay [15].
Transfusions and post-transfusion recovery (PTR)
Immediately following RBC labeling (see below), packed RBCs were administered via an 18g cephalic vein catheter over 20–30 minutes. Normal saline (15 ml/kg) was infused over 25 minutes by infusion pump. Dogs were monitored for vital and clinical signs during and for 2 hours following transfusion/infusion, and at each sampling time point.
The proportion of transfused RBCs circulating after transfusion (i.e., the post-transfusion recovery [PTR]) was determined using a dual-labeling method. Thus, fresh blood (10 mL) was collected from the recipient before transfusion and labeled with chloromethylbenzamido 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI; Vybrant CM-DiI cell-labeling solution, Invitrogen), and an 8-mL aliquot of the stored packed RBC unit was labeled with 3,3'-dihexadecyloxacarbocyanine perchlorate (DiO; Invitrogen) [10]. At defined time points after transfusion (0, 1, 2, and 24 hours), blood (0.5 mL) was obtained to quantify labeled RBCs by flow cytometry (FACSCalibur flow cytometer, BD Biosciences). Percent RBC recovery is the ratio of DiO to DiI-labeled RBCs in the blood samples divided by the ratio in the transfusate.
Laboratory measurements
Blood samples were collected primarily from the jugular vein at baseline and at 2, 6, 12, 24, 48, and 72 hours after transfusion/infusion. Complete blood counts were performed using a Cell-Dyn 3700 analyzer. Serum total bilirubin was measured using a Vitros 350 analyzer (Ortho-Clinical Diagnostics).
Plasma interleukin (IL)-6, IL-8, and monocyte chemoattractant protein (MCP)-1 levels were quantified using high-sensitivity ELISA kits (Canine DuoSet ELISA, R&D Systems). Serum non-transferrin-bound iron was measured using an ultrafiltration assay [10]. Transferrin saturation was measured by the Veterinary Diagnostic Laboratory, Kansas State University (Raichem reagents, Hemagen Diagnostics).
Effect of transfusion rate of old RBCs on MCP-1 levels
To determine if “slow” transfusions dampened the MCP-1 response seen with old RBCs transfused at a “fast” rate (over 20–30 minutes), units were collected from 3 dogs exhibiting high post-transfusion MCP-1 levels. At least 2 months after their last transfusion, they were phlebotomized, their RBCs were stored for 28 days and then transfused over two hours. A 4 hour post-transfusion sample was added.
Statistical analysis
Results are presented as median ± interquartile range. Data were analyzed by 1-way ANOVA and Bonferroni post-tests, Kruskal-Wallis and Dunns post-tests, or 2-way ANOVA with repeated measures and Bonferroni post-tests as appropriate. Pearson correlations and segmental linear regression models were performed to determine predictors of the MCP-1 response. A P value of <0.01 was considered significant. Prism 5 (GraphPad Software) was used for statistical analyses.
RESULTS
Characterization of packed RBC supernatants in vitro
LR was effective, yielding <5×106 WBCs/unit and <4 ×109/L platelets/unit (not shown). Supernatant hemoglobin levels and hemolysis rate in RBC units during refrigerated storage were significantly greater at 28 days than at 7 days of storage (Table 1). In addition, the mean hemolysis rate was <0.8% at 28 days of storage for all units. No significant differences in supernatant hemoglobin level or hemolysis rate were observed between the LR and non-LR groups. No significant accumulations in vitro of MCP-1, IL-6, or IL-8 were observed during storage in LR units (Table 1). In contrast, mean MCP-1 levels in vitro significantly increased in non-LR units from Day 0 (23.1 ± 12.9 pg/mL; mean ± SD) to Day 28 (89.9 ± 57.9 pg/mL; P <0.001). Finally, mean IL-8 levels were significantly higher in non-LR than LR units on Day 0 and after 28 days of storage (P <0.001; Table 1).
Table 1.
Changes in hemolysis rate and cytokine levels ex vivo in RBC unit supernatants with leukoreduction and time.
| Non-LR units | LR units | |||||||
|---|---|---|---|---|---|---|---|---|
| Fresh | Old | Fresh | Old | |||||
| Day 0 | Day 7 | Day 0 | Day 28 | Day 0 | Day 7 | Day 0 | Day 28 | |
| Free Hb (mg/dL) | 39.2 ± 11.6 | 127.5 ± 32.9** | 47.9 ± 25.4 | 242.4 ± 80.7***, ††† | 61.2 ± 37.9 | 187.3 ± 51.0*** | 52.1 ± 22 | 302.3 ± 55.6***, ††† |
| Hemolysis rate (%) | 0.12 ± 0.03 | 0.29 ± 0.05** | 0.13 ± 0.04 | 0.49 ± 0.14***, †† | 0.15 ± 0.06 | 0.41 ± 0.11*** | 0.16 ± 0.06 | 0.62 ± 0.11***, †† |
| MCP-1 (pg/mL) | 20.4 ± 6.5 | 30.6 ± 11.9 | 23.1 ± 12.9 | 89.9 ± 57.9***, †††, ‡‡‡ | 20.8 ± 8.4 | 23.1 ± 8.8 | 19.8 ± 11.8 | 32.1 ± 11.8 |
| IL-6 (pg/mL) | 102.3 ± 245.6 | 65.2 ± 129.8 | 80.0 ± 171.1 | 72.4 ± 110.7 | 21.7 ± 31.5 | 14.9 ± 22.1 | 84.0 ± 173.1 | 71.5 ± 131.1 |
| IL-8 (pg/mL) | 1034.5 ± 845.6‡‡ | 1103.2 ± 1356.1 | 1268.4 ± 819.8‡‡ | 1286.7 ± 1314 | 25.2 ± 39.2 | 27.3 ± 40.0 | 54.8 ± 70.1 | 54.9 ± 63.6 |
Values are mean ± S.D.
P<0.01,
P<0.001 compared to Day 0 of the same LR group.
P<0.01,
P<0.001 compared to Day 7 of the same LR group.
P<0.01,
P<0.001 compared to same time point of the opposite LR group.
Clinical assessment of transfusion recipients
Fresh and old RBC transfusions were well tolerated, with no adverse events noted during the 72-hour monitoring period for any of the transfusions/infusions. There was no significant difference in mean rectal temperature between transfused dogs (fresh vs. old, or LR vs. non-LR) and saline controls at any time point (not shown).
Effect of storage duration on complete blood cell counts in vivo
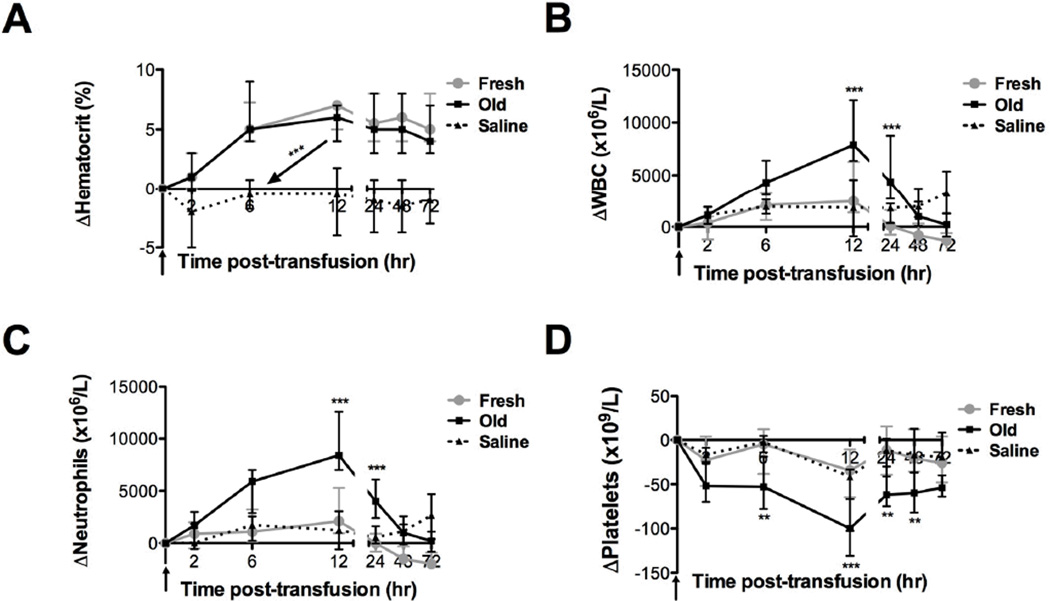
No differences between LR and non-LR groups were observed in any analyte measured after transfusion; therefore, LR and non-LR data were combined for all subsequent analyses. At 24 hours after transfusion, mean hematocrit increased by 6.2 ± 2.6% and 5.2 ± 3.0% (mean ± SD; difference not statistically significant) following fresh and old RBC transfusions, respectively (Figure 1A).Old RBC transfusions induced increased mean total WBC counts and, specifically, absolute neutrophils counts peaking 12 hours after transfusion (P <0.001; Figure 1B–C). Mean platelet counts decreased following old RBC transfusions, reaching a nadir 12 hours post-transfusion (P <0.001; Figure 1D).
Figure 1. Transfusions of old RBCs result in neutrophilia and decreased platelet counts.
Data are median ± interquartile range for increases in (A) hematocrit, (B) WBC count, (C) neutrophil count, and (D) platelet count from baseline levels up to 72 hours after fresh (gray circles; n=20) or old (black squares; n=20) RBC transfusions or saline infusions (dotted line; n=8). **P<0.01, ***P<0.001 comparing only the fresh and old RBC transfusions at each time point.
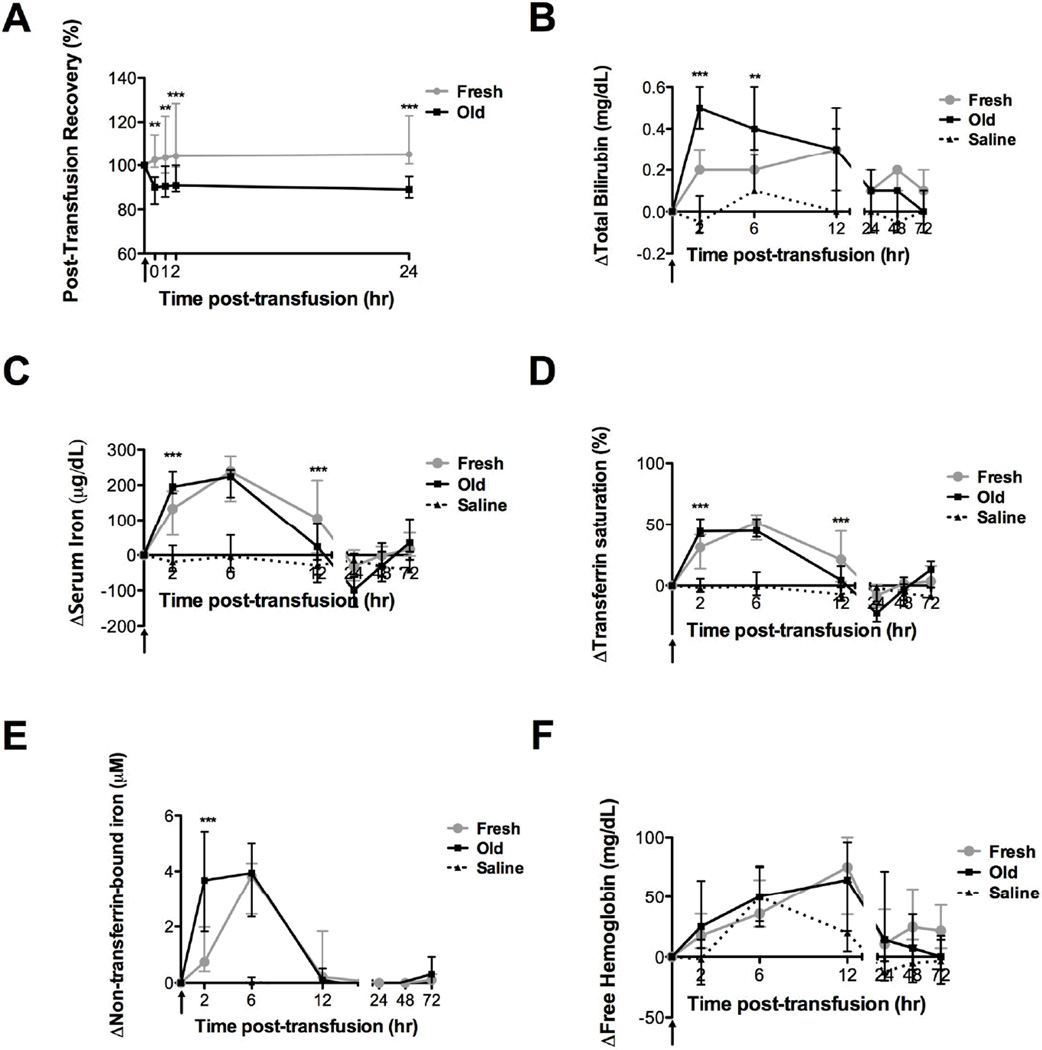
Prolonged storage duration reduces PTR
Compared with fresh RBC transfusions, the PTR decreased significantly (Figure 2A) following old RBC transfusions at all time points. Storage for 28 days resulted in a 19.4% decrease in 24-hour PTR (P <0.001), as compared to 7 days of storage. Furthermore, no difference was detected between the immediate and 24-hour PTR for old RBCs (91.1 ± 11.9% vs. 92.7 ± 17.0%, respectively; mean ± SD). However, the mean PTR for fresh RBC transfusions was greater than 100% and increased from immediately after transfusion to 24-hours after transfusion (105.9 ± 9.8% vs. 112.1 ± 16.4%, respectively; mean ± SD; P <0.05). Of note, the percentage of circulating DiI-labeled control RBCs, which were assumed to have a 100% PTR for the dual label study, decreased over time (to 93.4 ± 5.6% of the immediate post-transfusion level by 24 hours post-transfusion; mean ± SD), suggesting that the labeling method probably damaged the RBCs; therefore, the dual-label PTR data overestimate the true recovery. However, we believe that the differences between the fresh and old RBC transfusions (i.e., a mean of 19.4%) are unaffected, because both transfusions are subject to the same bias.
Figure 2. Transfusions of fresh and old RBCs both increase markers of hemolysis.
Data are median ± interquartile range for the (A) post-transfusion RBC recovery from immediately to 24 hours post-transfusion of fresh of old RBCs, and the change in (B) total bilirubin, (C) serum iron, (D) transferrin saturation, (E) non-transferrin-bound iron, and (F) plasma free hemoglobin from baseline levels up to 72 hours after fresh (gray circles; n=20) or old (black squares; n=20) RBC transfusions or saline infusions (dotted line; n=8). **P<0.01, ***P<0.001 comparing only the fresh and old RBC transfusions at each time point.
Extravascular hemolysis following fresh and old transfusions
Compared with fresh RBCs, old RBC transfusions demonstrated significantly increased serum total bilirubin with a mean peak increase at 2 hours after transfusion (Figure 2B; P <0.001). Furthermore, although serum iron, transferrin saturation, and non-transferrin-bound iron levels were unchanged from baseline after saline, all three analytes peaked at 6 hours after transfusion of either fresh or old RBCs (Figure 2C–E). However, as compared with fresh RBCs, all three analytes were significantly elevated at 2 hours, but not at 6 hours, after transfusion of old RBCs (P <0.001). Furthermore, as compared with fresh RBCs, serum iron and transferrin saturation were significantly decreased at 12 hours after transfusion of old RBCs (P <0.001). There were no significant differences in plasma fHb levels among the fresh RBCs, old RBCs, or saline groups at any time (Figure 2F).
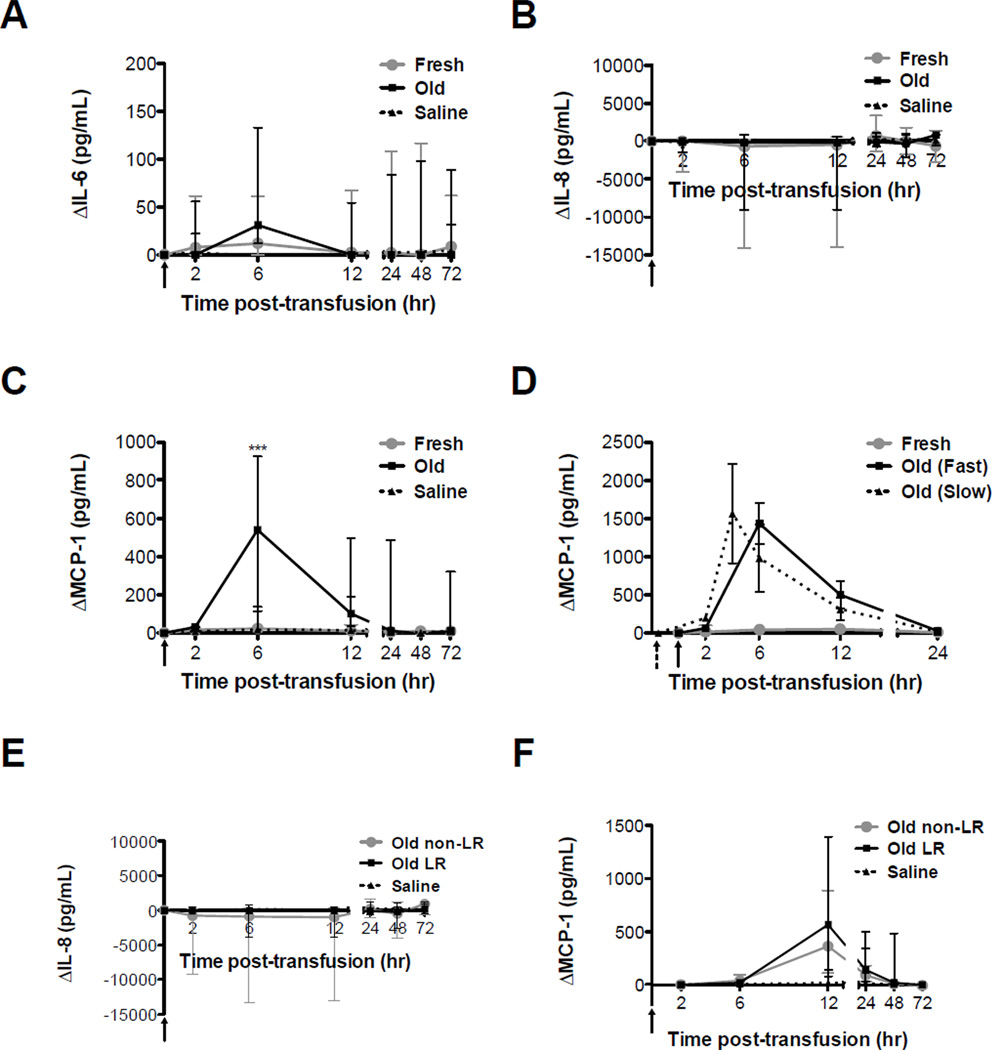
Rapid and slow transfusions of old RBCs induce MCP-1
There were no significant differences between fresh and old RBC transfusion groups, or the saline infusion group, at any time point in changes in IL-6 (Figure 3A) or IL-8 (Figure 3B) levels from baseline. However, as compared to fresh RBC transfusions or saline infusion, mean MCP-1 levels peaked at 6 hours after old RBC transfusions (P <0.001; Figure 3C). To test whether infusion rate affects cytokine response, 3 dogs with robust MCP-1 increases at 6 hours after transfusion, were transfused again with old RBCs; however, on this occasion, the transfusion rate was 2 units over 2 hours rather than 20–30 minutes. To avoid missing the peak MCP-1 concentration, a 4-hour time point was added to this group. When comparing slow and rapid transfusions, there were no significant differences in peak MCP-1 levels (Figure 3D). Finally, although IL-8 and MCP-1 levels in vitro were significantly higher in non-LR RBCs, IL-8 and MCP-1 levels did not differ significantly in vivo after LR and non-LR RBC transfusions (Figure 3E, F).
Figure 3. Both slow and rapid transfusions of old RBCs increase MCP-1 levels.
Data are median ± interquartile range for the changes in: (A) IL-6, (B) IL-8, and (C) MCP-1 from baseline levels up to 72 hours after fresh (gray circles; n=20) or old (black squares; n=20) RBC transfusions or saline infusions (dotted line; n=8); (D) MCP-1 levels for the old RBC transfusions in 3 selected dogs transfused rapidly (i.e. over 20–30 minutes; solid line) or slowly (i.e. over 2 hours; dotted line); and (E) IL-8 and (F) MCP-1 from baseline levels up to 72 hours after old non-LR (gray circles; n=10) or old LR (black squares; n=10) RBC transfusions, or after saline infusions (dotted line; n=8). Solid vertical arrows in all panels indicate the pre-transfusion time point for rapid transfusions; dotted arrows indicate the pre-transfusion time point for slow transfusions. ***P<0.001 comparing only the fresh and old RBC transfusions at each time point.
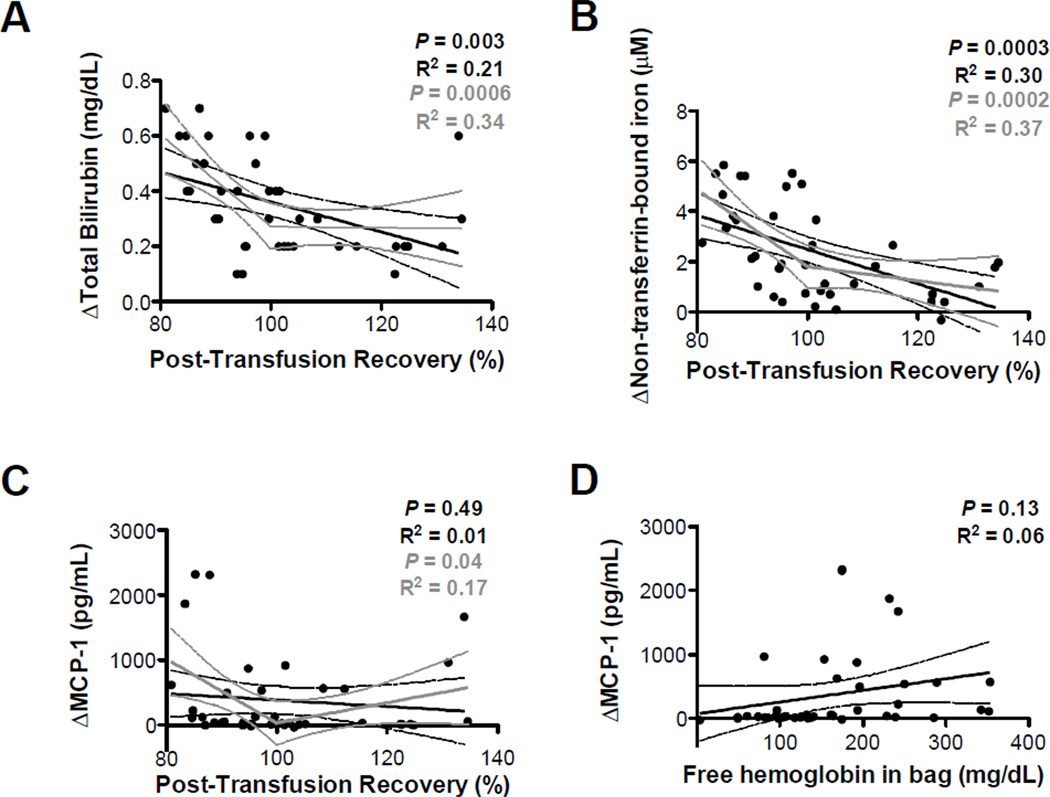
Predictors of the MCP-1 response to RBC transfusion
To determine whether the degree of post-transfusion extravascular hemolysis predicts the MCP-1 response, we assessed potential predictors by univariate linear regression. There were significant, but relatively weak, inverse correlations between the 2-hour PTR and total bilirubin (R2=0.21; P <0.01; Figure 4A) and non-transferrin-bound iron levels (R2=0.30; P <0.001; Figure 4B), supporting the presence of extravascular hemolysis. However, there was no correlation between the PTR and the peak MCP-1 response at 6 hours after transfusion (Figure 4C). To explore the possibility of a threshold effect, in which, below a certain PTR (e.g., 100%), there is a more dramatic correlation between PTR and hemolysis markers and/or MCP-1, segmental linear regression models were generated constraining the PTR at 100%. In this case, the R2 value increased for correlations between PTR and total bilirubin (R2=0.34; Figure 4A, gray lines), non-transferrin-bound iron (R2=0.37; Figure 4B, gray lines), and MCP-1 (R2=0.17; Figure 4C, gray lines). Finally, the fHb in the unit supernatant did not correlate with the peak MCP-1 level (Figure 4D; R2=0.06, P=0.13).
Figure 4. Hemolysis markers correlate poorly with peak MCP-1 levels.
Pearson correlations (black lines) and segmental linear regression constraining the PTR at 100% (gray lines) were used to determine the relationships between the 2-hour post-transfusion RBC recovery and the (A) increases in total bilirubin at 2 hours post-transfusion, (B) increases in non-transferrin-bound iron at 2 hours post-transfusion, and (C) peak increases in MCP-1 at 6 hours post-transfusion. (D) Pearson correlations were used to determine the relationships between the fHb in the RBC unit supernatant pre-transfusion and the peak increase in MCP-1 at 6 hours post-transfusion. The P value for the segmental linear regression models is the result of an extra sum-of-squares F test to compare the goodness of fit of the segmental model to a horizontal line. Dotted lines represent 95% confidence intervals.
DISCUSSION
We provide evidence that old RBC transfusions induce an MCP-1 response, accompanied by increased neutrophils and decreased platelets. Interestingly, LR did not affect these responses; in addition, slowing the transfusion rate did not affect the MCP-1 response. Because LR removes platelets and WBCs, this suggests that storage-induced changes of the RBCs and/or the supernatant are responsible for these effects. Furthermore, stored autologous canine RBC transfusions led to extravascular hemolysis, producing substantial amounts of circulating non-transferrin-bound iron. Although circulating iron and bilirubin increase more rapidly following transfusion of 28 day (old), as compared to 7 day (fresh), stored RBCs, both induce substantial hemolysis.
As in humans [16], LR reduced IL-8 and MCP-1 in canine RBC unit supernatants (Table 1); however, no differences in inflammation or other outcomes were seen in 20 LR and 20 non-LR transfusions. Nonetheless, measuring additional cytokines or inflammation markers may have yielded different results. Importantly, no adverse clinical events were observed.
In mice, the intact transfused RBCs initiate an inflammatory cytokine response following rapid transfusion of 2 units of old, stored mouse RBCs [10]. Recent neonatal studies provide evidence for an inflammatory response following transfusion [11]. However, studies of healthy adult humans have, thus far, failed to replicate these findings, following either slow transfusion of 1 unit of 40–42 day old RBCs [9] or rapid transfusion of 2 units of freshly cryopreserved RBCs [17]. Our current results do not support the hypothesis that RBC transfusion rate affects cytokine response; no differences in peak MCP-1 levels were observed when 2 units of old RBCs were transfused rapidly or slowly (Figure 3D); however, one-unit transfusions, to address dose-response issues, were not performed. In addition, although univariate linear regression did not reveal significant correlations between the MCP-1 response and the PTR (Figure 4D), PTR did correlate with bilirubin, non-transferrin-bound iron, and MCP-1 in a segmental linear regression model (Figure 4A–C; gray lines). This suggests a threshold effect is likely, in which, beyond a certain level of hemolysis, compensatory systems are overwhelmed, resulting in increased markers of hemolysis and inflammation. However, the weak correlation of PTR and MCP-1 (R2=0.17) suggests that other undiscovered factors affect this response.
Serum MCP-1 level is a marker of disease severity in critically ill dogs (e.g., sepsis) ranging from 145 to 1723 pg/mL (median 578 pg/mL) [18]; in our study, using the same ELISA, healthy dogs receiving old RBCs had MCP-1 levels of 29–2361 pg/mL (median 506 pg/mL) at 6 hours post-transfusion. Thus, in dogs, old RBC transfusions and sepsis similarly increase circulating MCP-1 levels.
In this study, given the evidence of extravascular hemolysis after old RBC transfusions (e.g., increased total bilirubin and iron parameters without increased fHb), it is not surprising that the 24-hour PTR was 19.4% less in dogs receiving old RBCs, as compared to fresh RBCs. However, despite some extravascular hemolysis following fresh RBC transfusions, it is surprising that the 24-hour PTR was >100% in this setting. RBC labeling with lipophilic dyes was used in a murine RBC storage and transfusion model [10], with results comparable to those obtained by radiolabeling [19]. A limitation of the current study is that lipophilic dye labeling of canine RBCs was not compared to traditional methods. However, the mean 24-hour PTR obtained with lipophilic dyes (e.g., 92.7%) for 28 day RBCs stored in Adsol are higher than those reported using biotinylation (80%) and radiolabeling (81%) for canine RBCs stored in Adsol for 35 days [20]. We suspect that our control, fresh RBC aliquots labeled with DiI were somewhat damaged during labeling, washing, and centrifugation steps, leading to an over-estimation of PTR for both 7 and 28 day old units. In addition, a recent study supports our finding of non-optimal canine PTR following 7 days of storage: two of four dogs transfused with 7 day old RBCs had a radiolabeled 24-hour PTR of <75% [7].
We examined osmotic fragility of canine RBCs labeled with DiI or DiO, but did not detect any differences as compared to a saline control group (not shown); however, RBC damage induced by the labeling process, but not detected by osmotic fragility testing, could cause rapid clearance of these RBCs by macrophages. Despite the potential bias caused by RBC damage during labeling, the bias probably affects fresh and old transfusions similarly; thus, the 19.4% difference in 24-hour PTR between the fresh and old transfusions is meaningful. Importantly, the immediate and 24-hour PTRs are similar. Thus, the clearance of these storage-damaged RBCs is very rapid; most storage-damaged RBCs are cleared from the circulation within 30 minutes of transfusion [21, 22] (of note, our RBCs were infused over 20–30 minutes, in contrast to the IV push used in traditional studies).
There were no differences in mean fHb concentrations between RBC transfusions and saline infusions at any time point; however, samples were not collected in a manner that would eliminate iatrogenic and/or artifactual hemolysis in vitro. Furthermore, fHb was not measured immediately after transfusion, when fHb levels are expected to be highest. We also observed increased fHb in all dogs, including the saline controls, at 6–12 hours post-transfusion/infusion, when serum samples were obtained after food ingestion, which occurred after the 2 hour post-transfusion sample. We attribute these fHb levels to post-prandial lipemia, which was visibly apparent; canine RBCs are fragile in lipemic plasma and can hemolyze in vitro [13].
RBC transfusions can cause leukocytosis in critically-ill humans, with kinetics similar to those observed in the current study [23]; however, to our knowledge, the effects on leukocytosis of LR and RBC storage duration were not addressed. In dogs, 28 day-old non-LR and LR RBC transfusions resulted in median increases of 7,710 ×106/L and 7,900 ×106/L over baseline, respectively, as compared to median increases of 2,800 ×106/L over baseline for fresh RBC transfusions and 1,835 ×106/L for saline infusions. Thus, RBC storage duration, rather than WBCs in the units, is a key factor associated with post-transfusion leukocytosis. However, species differences may exist, as no consistent differences in WBC or neutrophil counts were seen in healthy humans receiving fresh or old RBCs [9]. Similarly, the median platelet count of dogs transfused old RBCs decreased from baseline by 98 ×109/L at 12 hours after transfusion, as compared to decreases of 37 and 42 ×109/L for the fresh RBC and saline groups, respectively. Although the cause of this effect is unknown, old RBC transfusions may activate the endothelium [6], resulting in platelet consumption.
The mean lifespan of canine RBCs is approximately 105 days [24], similar to human and ovine RBCs [25]. Although the Food and Drug Administration mandates the maximal allowable shelf life of stored human RBCs, there is no similar oversight of canine blood products. The allowable outdate of canine RBCs varies among veterinary blood banks, but is typically 28–42 days. The decision to use a 28-day storage time for “old” RBC units in our study was based on standard practice for canine patients at our institution. Although increases in serum bilirubin, non-transferrin-bound iron, and MCP-1 levels would likely have been more dramatic in dogs receiving RBCs stored for 42 days, the inflammation and non-transferrin-bound iron production evident after 28 days of storage is impressive and raises the question of whether even 28 days of storage is appropriate for critically-ill dogs. The current study used healthy dogs receiving autologous transfusions to eliminate confounding factors associated with allogeneic RBCs and underlying illness. The results provide evidence that in a large animal model, transfusion of older RBCs, but not fresh RBCs, produce a pro-inflammatory cytokine response. Further studies are required to determine whether inflammation and non-transferrin-bound iron contribute to increased morbidity and mortality.
Acknowledgments
The authors thank Dr. Mark E. Haskins for generously providing dogs for this study, Patricia S. Chavey at the Veterinary Diagnostic Laboratory at Kansas State University for measuring serum iron and total iron binding capacity, and Dr. Steven L. Spitalnik for support and encouragement.
This work was supported in part by grants from the NIH (RR02512 to Mark E. Haskins and K08-HL103756 to E.A.H.) and a Louis V. Gerstner Scholars Award (to E.A.H.).
Footnotes
Disclosure: The authors declare no conflicts of interest relevant to the manuscript submitted to Vox Sanguinis.
References
- 1.Ness PM. Does transfusion of stored red blood cells cause clinically important adverse effects? A critical question in search of an answer and a plan. Transfusion. 2011;51:666–667. doi: 10.1111/j.1537-2995.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 2.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 3.Gauvin F, Spinella PC, Lacroix J, et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50:1902–1913. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 4.Hassan M, Pham TN, Cuschieri J, et al. The association between the transfusion of older blood and outcomes after trauma. Shock. 2011;35:3–8. doi: 10.1097/SHK.0b013e3181e76274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimshaw K, Sahler J, Spinelli SL, et al. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion. 2011;51:874–880. doi: 10.1111/j.1537-2995.2011.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Transfusion. 2011;51:881–885. doi: 10.1111/j.1537-2995.2011.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keir AK, McPhee AJ, Andersen CC, et al. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res. 2013;73:75–79. doi: 10.1038/pr.2012.144. [DOI] [PubMed] [Google Scholar]
- 12.Spinella PC, Dressler A, Tucci M, et al. Survey of transfusion policies at US and Canadian children's hospitals in 2008 and 2009. Transfusion. 2010;50:2328–2335. doi: 10.1111/j.1537-2995.2010.02708.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiss DJ, Tvedten H. The complete blood count, bone marrow examination, and blood banking. In: Willard MD, Tvedten H, editors. Small Animal Clinical Diagnosis by Laboratory Methods. Elsevier; 2012. p. 32. [Google Scholar]
- 14.Brownlee L, Wardrop KJ, Sellon RK, et al. Use of a prestorage leukoreduction filter effectively removes leukocytes from canine whole blood while preserving red blood cell viability. J Vet Intern Med. 2000;14:412–417. doi: 10.1892/0891-6640(2000)014<0412:uoaplf>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Moore GL, Ledford ME, Merydith A. A micromodification of the Drabkin hemoglobin assay for measuring plasma hemoglobin in the range of 5 to 2000 mg/dl. Biochem Med. 1981;26:167–173. doi: 10.1016/0006-2944(81)90043-0. [DOI] [PubMed] [Google Scholar]
- 16.McFaul SJ, Corley JB, Mester CW, et al. Packed blood cells stored in AS-5 become proinflammatory during storage. Transfusion. 2009;49:1451–1460. doi: 10.1111/j.1537-2995.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 17.Hult A, Malm C, Oldenborg PA. Transfusion of cryopreserved human red blood cells into healthy humans is associated with rapid extravascular hemolysis without a proinflammatory cytokine response. Transfusion. 2013;53:28–33. doi: 10.1111/j.1537-2995.2012.03710.x. [DOI] [PubMed] [Google Scholar]
- 18.Duffy AL, Olea-Popelka FJ, Eucher J, et al. Serum concentrations of monocyte chemoattractant protein-1 in healthy and critically ill dogs. Vet Clin Pathol. 2010;39:302–305. doi: 10.1111/j.1939-165X.2010.00228.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wardrop KJ, Tucker RL, Anderson EP. Use of an in vitro biotinylation technique for determination of posttransfusion viability of stored canine packed red blood cells. Am J Vet Res. 1998;59:397–400. [PubMed] [Google Scholar]
- 21.Jandl JH, Tomlinson AS. The destruction of red cells by antibodies in man. II. Pyrogenic, leukocytic and dermal responses to immune hemolysis. The Journal of clinical investigation. 1958;37:1202–1228. doi: 10.1172/JCI103710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollison PL. Haemolytic Transfusion Reactions. In: Klein HG, Anstee DJ, editors. Blood Transfusion in Clinical Medicine. Oxford: Blackwell Publishing; 2005. p. 474. [Google Scholar]
- 23.Fenwick JC, Cameron M, Naiman SC, et al. Blood transfusion as a cause of leucocytosis in critically ill patients. Lancet. 1994;344:855–856. doi: 10.1016/s0140-6736(94)92828-2. [DOI] [PubMed] [Google Scholar]
- 24.Novinger MS, Sullivan PS, McDonald TP. Determination of the lifespan of erythrocytes from greyhounds, using an in vitro biotinylation technique. Am J Vet Res. 1996;57:739–742. [PubMed] [Google Scholar]
- 25.Fung YL, Tung JP, Foley SR, et al. Stored blood transfusion induces transient pulmonary arterial hypertension without impairing coagulation in an ovine model of nontraumatic haemorrhage. Vox sanguinis. 2013 doi: 10.1111/vox.12032. [DOI] [PubMed] [Google Scholar]