Abstract
As liquid liposomal formulations are prone to chemical degradation and aggregation, these formulations often require freeze drying (e.g. lyophilization) to achieve sufficient shelf-life. However, liposomal formulations may undergo oxidation during lyophilization and/or during prolonged storage. The goal of the current study was to characterize the degradation of 1, 2-dilinolenoyl-sn-glycero-3-phosphocholine (DLPC) during lyophilization, and to also probe the influence of metal contaminants in promoting the observed degradation. Aqueous sugar formulations containing DLPC (0.01 mg/ml) were lyophilized, and DLPC degradation was monitored using HPLC/UV and GC/MS methods. The effect of ferrous ion and sucrose concentration, as well as lyophilization stage promoting lipid degradation, was investigated. DLPC degradation increased with higher levels of ferrous ion. After lyophilization, 103.1% ± 1.1%, 66.9% ± 0.8%, and 28.7% ± 0.7% DLPC remained in the sucrose samples spiked with 0.0 ppm, 0.2 ppm and 1.0 ppm ferrous ion, respectively. Lipid degradation predominantly occurs during the freezing stage of lyophilization. Sugar concentration and buffer ionic strength also influence the extent of lipid degradation, and DLPC loss correlated with degradation product formation. We conclude that DLPC oxidation during the freezing stage of lyophilization dramatically compromises the stability of lipid-based formulations. In addition, we demonstrate that metal contaminants in sugars can become highly active when lyophilized in the presence of a reducing agent.
Keywords: DLPC, Lyophilization, liposomes, chemical stability, metal contamination, unsaturated lipids, oxidation, freezing, freeze-drying
1. Introduction
Liposomal delivery systems have been used to improve the delivery of small molecule pharmaceuticals; there are currently twelve liposomal products on the market [1]. In the case of liposomal formulations designed to deliver siRNA, unsaturated lipids have proven superior to saturated lipids for promoting intracellular nucleic acid delivery [2–5]. Unfortunately, unsaturated lipids are highly susceptible to being oxidized which limits their use in lipid-based commercial products. As liquid liposomal formulations are prone to chemical degradation and aggregation, these formulations are often lyophilized to achieve stability compatible with a marketable product. Furthermore, the increased immunogenicity associated with the use of viral vectors and the resulting safety concerns underscore the need to further develop non-viral delivery systems [6–8]. Liposomal vectors also have the potential to provide a possible avenue for the treatment of diseases that are not responsive to existing therapeutics. Tuberculosis, for example, has become increasingly difficult to treat due to a significant increase in drug resistant strains [9]. While the use of liposomes to deliver small molecule drugs to treat tuberculosis (e.g., Rifampicin) appears promising, the technology has not been fully developed [10]. Hence, additional research efforts to improve stability and efficiency of liposomal delivery systems are needed.
The chemical and physical stability of aqueous liposomal formulations, which have been studied extensively, are limited due to the occurrence of peroxidation, hydrolysis and aggregation [11, 12]. It has been shown that as the number of unsaturated bonds increases, the extent of peroxidation also increases [12]. The decreased bond dissociation energy at the carbon-hydrogen bonds in the bis-allylic position underlies the increased oxidizability of polyunsaturated lipids [13, 14]. Previous studies have assessed oxidative lipid degradation by measuring reactive aldehyde products, conjugated dienes, hydroperoxides and/or oxygen consumption [15, 16].
One common approach to improve the overall stability of liposomal formulations is freeze-drying (i.e., lyophilization). Lyophilization of liposomes often incorporates the use of sugars, such as sucrose or trehalose, which form glassy matrices that limit mobility (vitrification hypothesis), forms hydrogen bonds (water replacement theory) and/or separates the individual liposomes (particle isolation hypothesis) [17–20]. Even in the case of pharmaceutical-grade sugars, the presence of transition metal contaminants is a concern. Transition metals, such as iron, can catalyze the oxidative degradation of unsaturated lipids in the dried state and may also lead to co-degradation of liposomal cargos (e.g., DNA degradation) [21, 22]. Saturated and hydrogenated lipids are less prone to oxidative degradation, and therefore these lipids are often utilized in lipid-based therapeutics (e.g., Doxil®, DaunoXome®, and Ambisome®) [1]. Singly unsaturated lipids such as 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) are also less susceptible to oxidative degradation than polyunsaturated lipids and have been used in commercial liposomal products such as DepoCyt® [1]. Although polyunsaturated lipids are significantly more susceptible to oxidative degradation, the use of these lipids in liposomal pharmaceutical preparations is being investigated. Recently, it was demonstrated that lipids with two or more unsaturations were more fusogenic relative to lipids with fewer unsaturated bonds, and this promotes endosomal escape [2]. The requirement for endosomal escape is a major hindrance when delivering nucleic acids to the interior of the cell. Furthermore, the increased endosomal escape has been shown to correlate with increased siRNA delivery and luciferase silencing in vitro [2].
In the current study, we have utilized a triply unsaturated lipid, DLPC, and evaluated the acute degradation occurring during lyophilization. While lyophilization is generally a strategy to minimize the degradation of liposome formulations, the lyophilization process exposes liposomal formulations to freezing and drying stresses [23–25]. We investigated the effects of sugar (i.e. sucrose, trehalose or hydroxyethyl starch) and ferrous ion concentration on DLPC degradation during lyophilization. In an attempt to mimic transition metal contaminants known to be present in pharmaceutical-grade sugars, we spiked ferrous ion into the DLPC samples at iron levels that are commonly found in pharmaceutical-grade sugars [26, 27]. Although the effect of transition metals, such as ferric and ferrous ions, on the oxidative degradation of lipids has been studied, a significant proportion of studies which examined iron catalyzed lipid peroxidation were focused on the stability of consumable foods and the progression of various diseases [28–30]. We believe this to be the first study to address the stability of pharmaceutically-relevant unsaturated lipids during lyophilization.
2. Materials and Methods
2.1 Materials
1, 2-Dilinolenoyl-sn-glycero-3-phosphocholine (DLPC; 18:3 (Cis) PC) (>99%, Lot # 183-12li) was purchased from Avanti Polar Lipids (Alabaster, AL). Sucrose (Ultrex© ultrapure, Lot # J25H00) was purchased from JT Baker (Center Valley, PA). Trehalose (100%, Lot # 28549A) was purchased from Ferro Pfanstiehl (Waukegan, IL). Hydroxyethyl starch (HES, Batch # 17081421) was purchased from Fresenius Kabi (Linz, Austria). 5-(2-Carboxyphenyl)-5-hydroxy-1-((2, 2, 5, 5-tetramethyl-1-oxypyrrolidin-3-yl) methyl)-3-phenyl-2-pyrrolin-4-one potassium salt (proxyl fluorescamine) was obtained from Molecular Probes, Inc. (Eugene, OR). Tris [hydroxymethyl] amino-methane, iron (II; ferrous ion) chloride tetrahydrate, 2-thiobarbituric acid (TBA), 2,6-di-tert-butyl-4-methylphenol (BHT), 1,1,3,3-tetraethoxy propane (MDA), sodium sulfite and hydrochloric acid were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). 3-(2-Pyridyl)-5, 6-diphenyl-1, 2, 4-triazine-p, p′-disulfonic acid, disodium salt hydrate (ferroZine iron reagent) and propionic acid were purchased from Acros Organics (Geel, Belgium). Trichloroacetic acid 10% w/v aqueous solution was purchased from Ricca Chemical Company (Arlington, TX). Chloroform, methanol, water, (all of which were HPLC grade), LC/MS grade acetone, ascorbic acid and Eppendorf tubes (1.5 mL) were procured from Fisher Scientific (Pittsburgh, PA). High grade helium and nitrogen were purchased from Airgas (Radnor, PA). Amber glass vials (5 mL) were procured from West Pharmaceutical Services (Lionville, PA). Phenomenex Strata X solid phase extraction (SPE) tubes (1.0 mL) containing sorbent (30 mg; Lot # S300-139) were used (Torrance, CA).
2.2 DLPC Sample Preparation
The lipid (DLPC) stock, originally dissolved in chloroform (10.0 mg/ml), was concentrated using a slow stream of nitrogen. The lipid was then further dried under vacuum (559 torr) for approximately one hour. The lipid (DLPC) was then suspended in Tris buffer (0.5 mM, pH 7.4) to afford a final DLPC concentration of 0.1 mg/ml. The rehydrated lipid (DLPC) was then sonicated (30 sec) and vortex mixed (5 sec). The resulting DLPC liposome suspension was used immediately after additional dilution with Tris buffer to 0.04 mg/ml. Stock aqueous ferrous ion solutions (400 ppm) were prepared immediately before use in HPLC grade water (Fisher Scientific W5-4); this stock solution was then diluted and added into the study samples. Sucrose, trehalose and HES were initially prepared as 10% w/v solutions in Tris buffer (0.5 mM, pH 7.4). The sugars were added to the samples such that the final sugar concentration was 2.0%. The final samples (1.0 mL) were prepared in amber glass vials (5 mL) and contained 0.01 mg/ml DLPC, 2% sugar (sucrose, trehalose or HES), and 0.0 ppm, 0.2 ppm or 1.0 ppm ferrous ion. In the experiments evaluating the effect of the oxidation state of the transition metal contaminants, stock sodium sulfite solution was freshly prepared in 0.5 mM Tris buffer and samples prepared as described previously to contain sodium sulfite (2.0 μg/ml). When the effects of buffer ionic strength were tested, samples were also prepared as described above except that the concentrations of buffer, ferrous ion, and DLPC lipid were increased to mimic the concentrations in the frozen state. The volume of the freeze concentrate was calculated to determine to what extent the sample solutes were concentrated in the frozen state [31]. These samples were stored at 4 ± 1 °C overnight.
2.3 Lyophilization Protocol
Sample vials were placed directly on the shelf of an FTS Durastop lyophilizer (Stone Ridge, NY). The lyophilization cycle involved freezing samples to −30°C (8 h, cooled at 0.4°C/min) and the pressure of the chamber was reduced to 30–60 mTorr. Next, the primary drying occurred at −20 °C (15 h, 2°C/min), the secondary drying occurred at 30°C (30 h, 1°C/min) and then at 35°C (1.5 h, 1°C/min). Sample temperatures were monitored by inserting a thermocouple into a representative sample prior to starting the lyophilization cycle. When samples were removed during freezing, time zero was considered to be 81 min after the shelf temperature had reached −30°C; at this point the sample temperature was stable. After sample lyophilization was completed, the vials were stoppered while the chamber remained under vacuum.
2.4 HPLC-UV Analysis
To measure DLPC, a Shimadzu analytical HPLC system (LC-20AB, DGU-20A, CTO-20A, Sil-20A HT) equipped with SPD-20A UV-VIS detector was used (Shimadzu Scientific Instruments, Inc.; Columbia, MD). An Aligent zorbax extended-C18 50 x 4.6 mm (5 micron) column equipped with a guard column was used (Santa Clara, CA). The column temperature was maintained at 40 °C with a flow-rate was 0.4 mL/min. An isocratic method (20 min run time; 19:1 methanol:water mobile phase) was used, and UV detection was monitored at 205 nm. A 10 μL volume was injected for both samples and standards.
2.5 Solid Phase Extraction Procedure
Analysis of DLPC degradation products via GC/MS required the removal of the sugar component; a solid phase extraction (SPE) procedure was implemented. SPE was accomplished immediately after rehydrating lyophilized DLPC-sugar samples in 0.05 M HClaq. The SPE tubes were initially conditioned with acetone (1.0 mL) and equilibrated with HClaq (0.05M; 1.0 mL). Next, the sample was loaded onto the SPE device and washed using HClaq (0.05M; 1.0 mL). Using a 1.0 mL plastic syringe, air was pushed through the SPE device to expel any remaining solution retained in the SPE resin. Acetone (1.0 mL) was used to elute degradation products which were subsequently analyzed via GC/MS. An internal standard solution, propionic acid diluted in acetone, was freshly prepared and spiked into samples to afford a final concentration of 5.0 μg/ml.
2.6 GC/MS Analysis
A Shimadzu GC/MS-QP2010 Plus gas chromatograph mass spectrometer (Shimadzu Scientific Instruments, Inc.; Columbia, MD) was used to monitor DLPC degradation products. The GC2010 was equipped with a Phenomenex ZB-FFAP column (30m x 0.25mm x 0.25 mm). To prevent accumulation of non-volatile molecules within the analytical column, a 5 meter guard column (Phenomenex, Torrance, CA) was used in tandem with the analytical column. The settings were as follows: column temperature was initially set at 50 °C with an injector temperature of 250 °C; the temperature was held at 50 °C for 6.0 min and then ramped at 8 °C min to 200 °C and held for 35 min. The ion source temperature was set at 210 °C and the interface at 230 °C. Helium was used and the column flow rate was 1.0 ml/min. The purge flow was 5.0 ml/min and the total flow was 26.1 ml/min. The pressure was 543 torr. A split ratio of 2 was utilized and the detector delay was set to 4.0 min. The event time was set to 0.5 sec and the scan speed was set to 769. The solvent cut off time was 1.0 min and the injection volume was 1.0 μL. Three ions were specifically monitored for the propanoic internal standard (74.1, 57.1, and 55.1) and the main product (Product A) detected in lyophilized DLPC samples (105.1, 122.1, and 77.0).
2.7 Thiobarbituric Reactive Substances (TBARS) Assay
The thiobarbituric acid reactive substances (TBARS) assay was utilized to evaluate the extent of lipid peroxidation in the samples as previously described [32–34]. Freeze-dried samples were rehydrated with distilled water (1.0 mL). An aliquot (150 μl) of each sample was mixed with 10% trichloroacetic acid (250 μl), 2% BHT (25 μl), and 0.67% TBA (500 μl). These mixtures were then heated at 85°C (20 min), and cooled at 4 °C (15 min). An aliquot (200 μl) from each sample was transferred into a black polystyrene 96-well plate and fluorescence (EX: 532 nm, EM: 550 nm) read in a SpectraMAX Gemini EM fluorescence microplate reader (Sunnyvale, CA). The TBARS concentrations were calculated from a calibration curve of fluorescence intensities from 12 MDA standards ranging from 33.0 μM to 66.7 mM (R2 = 0.93).
2.8 Reactive Oxygen Species Measurement
The fluorogenic spin-trap proxyl fluorescamine was used to detect superoxide and hydroxyl radicals as previously described [35]. Proxyl flourescamine (5.0 mg) was dissolved in tri-distilled water (1.0 ml) and aliquots (132 μl) transferred into Eppendorf tubes (1.5 mL). The solutions were then dried using an Eppendorf vacufuge (Hauppauge, NY). Immediately prior to use, the material was rehydrated to afford a final proxyl fluorescamine concentration of 10.3 mM. Samples were prepared as described above except the proxyl fluorescamine solution (4.4 μL) was added to samples prior to lyophilization at a final proxyl fluorescamine concentration was 45 uM. Aliquots (200 μLs) of each sample were then transferred into a black polystyrene 96 well plate and analyzed using a SpectraMAX Gemini EM fluorescence microplate reader (Sunnyvale, CA; EX: 385nm and EM: 485 nm).
2.9 Ferrozine assay
Ascorbic acid and ferrozine were dissolved in 1.0 M HCl so that the final concentrations were 200 mM and 20 mM, respectively. In experiments evaluating the effect of increasing the concentration of HCl on measured iron values, a concentrated stock of HCl (12.1 M, approximately 36%) was appropriately diluted. An aliquot (20 μL) of the ascorbic acid/ferrozine solution was added to each sample (340 μL), mixed and heated (60°C, 20 min). The reagent/sample mixture was then cooled to room temperature (12 hr), and the absorbance measured at 562 nm. A standard curve was generated using ferric citrate standards which encompassed a concentration range of 0–800 ppb (R2 = 0.91).
2.10 Data analysis and statistical methods
Prism 5.02™ (GraphPad Software, Inc.; San Diego, CA) was used to graph and perform statistical analysis. The statistical differences were compared using a one-tailed unpaired t test at the 95% confidence level.
3. Results
3.1 Degradation of Lyophilized DLPC Samples
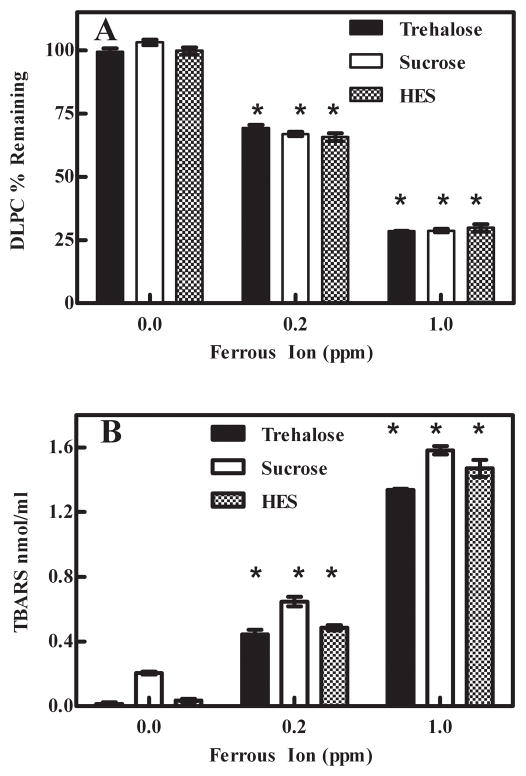
To measure the acute degradation of DLPC from lyophilized samples, a C18 reverse phase HPLC-UV method was developed. The HPLC/UV method was very reliable and DLPC could be readily quantified. For every experiment, freshly prepared standards containing DLPC (0.001 mg/ml to 0.1 mg/ml) were used to generate a standard curve (see Supplementary Material S-Fig. 1). Reconstituted lyophilized samples, which varied in terms of sugar and ferrous ion concentration, were analyzed. The sugar (sucrose, trehalose or HES) present in the samples did not have a significant effect on DLPC degradation. However, the ferrous ion concentration significantly influenced DLPC stability. We observed that DLPC degradation was a function of ferrous ion concentration with approximately 101%, 67% and 29% of the DLPC remaining after lyophilization in samples spiked with 0 ppm, 0.2 ppm and 1.0 ppm ferrous ion, respectively (Fig. 1A). While the presence of sucrose and trehalose did not cause any complications, we found that the HPLC column was significantly spoiled after analyzing approximately 20 HES samples.
Fig. 1.
Degradation of lyophilized DLPC samples containing ferrous ion. A- Measurement of DLPC remaining in lyophilized samples. Increasing DLPC degradation was observed as the ferrous ion concentration increased. The values represent the mean ±1 SEM of triplicate or quadruplicate determinations. * indicates statistical significance. p < 0.0001 B- TBARS measurement of degradation products in lyophilized samples. Increased product concentrations were detected as the concentration of ferrous ion increased. The values represent the mean ±1 SEM of triplicate or quadruplicate determinations. * indicates statistical significance. p < 0.0001
3.2 Analysis of DLPC Degradation Products
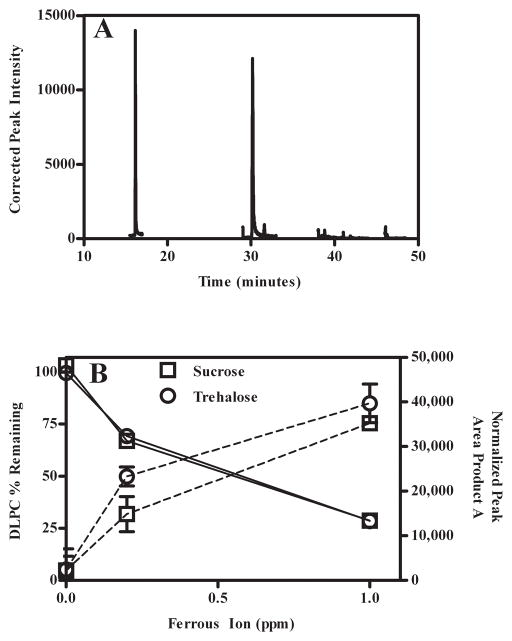
We initially used the TBARS assay as an indirect method to measure DLPC degradation products. As summarized in Fig. 1B, DLPC degradation products increased with an increase in ferrous ion concentration. Control samples containing sugar and 1.0 ppm Fe2+ only (i.e., no DLPC) exhibited low TBARS values comparable to 0 ppm samples, and thus degradation products arising from the sugar excipient do not contribute significantly to the degradation observed in the presence of ferrous ion (data not shown). In addition to measuring the DLPC degradation products formed during lyophilization, we were also interested in measuring DLPC degradation products formed during storage. When TBARS was utilized to measure DLPC degradation products from samples stored at 60°C, it was apparent that the products formed during storage were not detectable using the TBARS assay. Because the TBARS is a non-specific assay that measures several different lipid degradation products, and also due to the inadequacy mentioned above, we utilized GC/MS. Samples required that the sugar be first removed using SPE preparation (section 2.5). However, analogous to issues observed when conducting studies with the HPLC C18 column (section 3.1) samples containing HES were not compatible with the SPE sample preparation protocol and therefore were not analyzed. One predominant GC/MS degradation product, referred herein as Product A, was observed (S-Fig. 2). A representative chromatogram displaying the internal standard (propionic acid; tR = 16.2 min) and Product A (tR = 30.2 min) are depicted in Fig. 2A. As summarized in Fig. 2B, we observed by HPLC/UV and GC/MS that as ferrous ion concentrations were progressively enhanced, the amount of DLPC remaining decreased, and the relative amount of Product A increased. While an absolute structure has not been fully elucidated, we have proposed (see Supplementary Material section) a tentative structure using GC/MS and LC/MS-MS data (S-Fig. 3 and S-Fig. 4). Samples containing sucrose without spiked ferrous ion displayed essentially no DLPC degradation; whereas samples containing 0.2 ppm and 1.0 ppm of spiked ferrous ion exhibited 68% and 29% of the initial DLPC, respectively.
Fig. 2.
Measurement of DLPC degradation products using GC/MS. A- Representative chromatogram depicting the peaks corresponding to the internal standard (propionic acid; tR = 16.2 min) and ‘Product A’ (tR = 30.2 min). B- Loss of DLPC (solid line, error bars are smaller than the symbols) and accumulation of Product A (dotted line) as the ferrous ion concentration is increased.
3.3 Effect of Freezing and Drying on DLPC Degradation
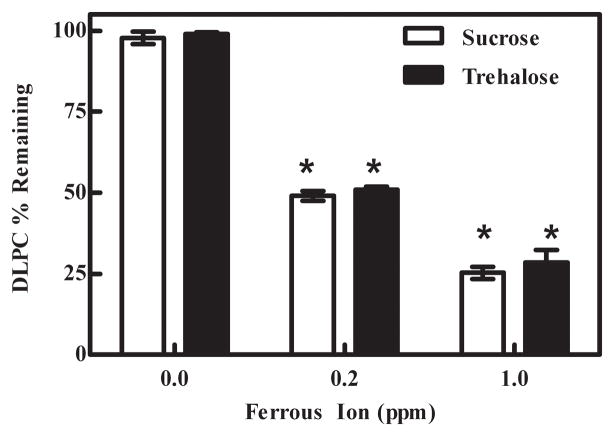
As we wanted to establish the influence that each lyophilization stage (i.e. freezing, primary drying, or secondary drying) had on DLPC stability, DLPC samples were removed from the lyophilization chamber after freezing, primary drying and secondary drying. We observed that approximately 100%, 50% and 27% of the DLPC remained after freezing samples (8 hours) containing 0 ppm, 0.2 ppm and 1.0 ppm ferrous ion, respectively (Fig. 3). After the freezing phase, we did not observe any statistically significant DLPC loss during the primary or secondary drying phases (data not shown). We next sought to address if relative amounts of DLPC degradation that occurred during the cooling (i.e., freezing stage) was a function of the freezing process and/or a function of the amount of time spent in the frozen state prior to proceeding to the primary drying phase. We did not observe any statistically significant DLPC loss with time (monitored at 4, 8, 24, 48 and 72 hr post freezing) once the samples had reached the frozen state (data not shown). Hence, DLPC degradation predominantly occurs during the freezing stage of lyophilization.
Fig. 3.
Effect of freezing on DLPC degradation. Degradation of DLPC occurred during freezing when samples were spiked with ferrous ion. The values represent the mean ±1 SEM of quadruplicate determinations. * indicates statistical significance. p < 0.0001
3.4 Effect of Sucrose Concentration
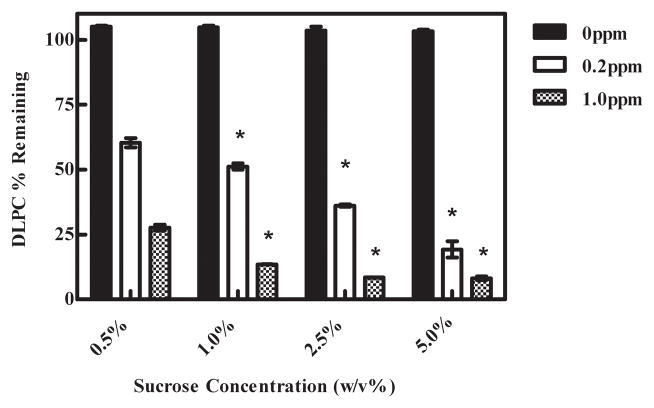
To evaluate if freeze concentration was affecting DLPC degradation, we varied the sucrose concentration (0.5, 1.0, 2.5 and 5.0%) and the ferrous ion concentrations, but kept DLPC content constant. Consequently, these experiments effectively altered the non-ice fraction volume; increasing sucrose concentration results in a larger non-ice fraction volume and thereby dilutes DLPC and ferrous ion. Regardless of sucrose concentration, Fig. 4, DLPC samples not spiked with ferrous ion were essentially stable. However, samples spiked with ferrous ion displayed sucrose concentration-dependent DLPC degradation.
Fig. 4.
Effect of freeze concentration on DLPC degradation. DLPC degradation increased as the sucrose concentration increased from 0.5% to 5.0% (w/v) in frozen samples. The values represent the mean ±1 SEM of triplicate determinations. The DLPC degradation of samples containing 1.0%, 2.5%, and 5.0% were compared to the samples containing 0.5% sucrose. * indicates statistical significance. 0ppm p > 0.05, 0.2ppm p < 0.05, 1.0ppm p < 0.0001
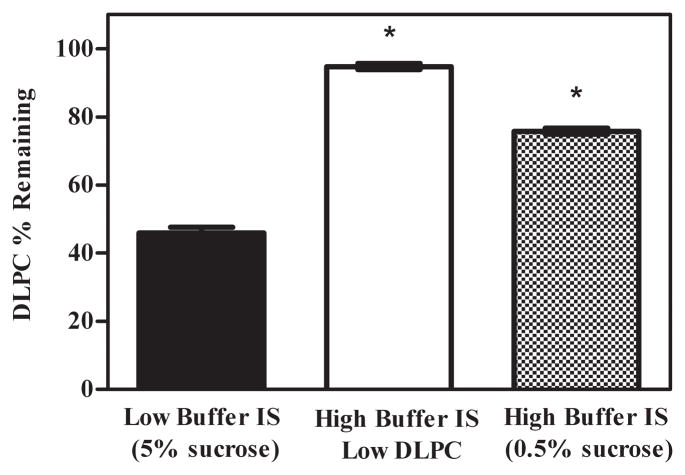
3.5 Effect of Buffer Ionic Strength on DLPC Degradation
We next sought to evaluate the effect of ionic strength on DLPC degradation. Samples containing DLPC, ferrous ion, and Tris buffer were prepared such that the concentrations of these components mimicked the concentrations predicted in the non-ice fraction of maximally freeze-concentrated samples containing 0.5% and 5.0% sucrose (section 3.4). It follows that a higher Tris buffer concentration should be associated with the samples containing the lower concentration of sucrose, i.e. smaller non-ice fraction volume. Calculations revealed that samples mimicking the extent of freeze concentration expected in the presence of 5.0% sucrose would have solute concentrations 17.2 times higher than the starting solution, with solute concentrations estimated at 172 μg/ml DLPC, 3.44 ppm ferrous ion, and 8.6 mM Tris. Samples mimicking the freeze concentration expected in 0.5% sucrose solutions would have solute concentrations 170.2 times higher; sample solute concentrations are estimated to be 1720 μg/ml DLPC, 34.4 ppm ferrous ion, and 86 mM Tris. These values reflect that the volume of the non-ice fraction is 10 times smaller in the samples containing 0.5% sucrose relative to the samples containing 5.0% sucrose.
After incubating these samples at 4°C overnight, we observed that samples having a greater buffer ionic strength afforded lower DLPC lipid degradation (Fig. 5). In contrast, DLPC degradation was higher in samples mimicking the 5.0% sucrose conditions with lower buffer ionic strength. An additional sample contained constant DLPC and ferrous ion concentrations (172 μg/ml and 3.44 ppm, respectively), while Tris buffer concentration was increased to 86 mM. Under these conditions, significantly lower DLPC degradation was observed, consistent with the notion that buffer ionic strength influences DLPC degradation during freezing.
Fig. 5.
Effect of buffer ionic strength on DLPC degradation. DLPC samples containing ferrous ion, DLPC and Tris concentrations mimicking the freeze concentrated state. Increased buffer ionic strength decreased DLPC degradation. The values represent the mean ± 1 SEM of triplicate determinations. * indicates statistical significance. p < 0.0001
3.6 Effect of Sodium Sulfite on DLPC Degradation
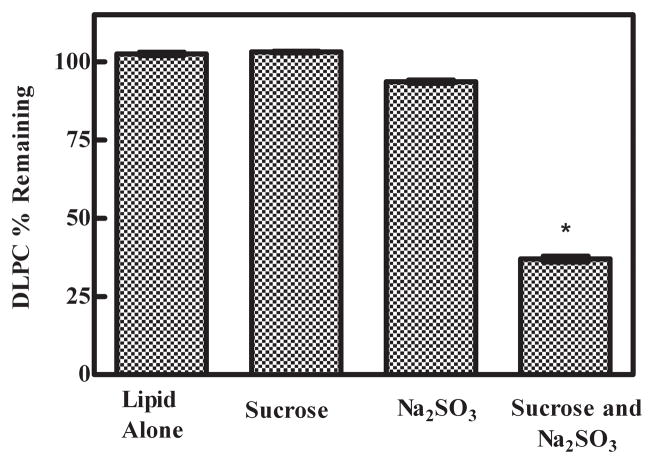
It is well known that transition metals (e.g. Fe+3 and Fe+2) catalyze lipid peroxidation (i.e. Fenton Chemistry) [21, 36–38]. As ferric ion (Fe+3) is more stable in air than Fe+2, the iron contaminants present in sucrose are far more likely to be in the Fe+3 state due to exposure to ambient atmosphere during processing, packaging and storage. Consequently, depending upon the amount of moisture present, the Fe+3 is likely in the form of iron (III) oxide-hydroxides and/or sugar iron oxide complexes. Prior to lyophilization and upon rehydration, these ferric ion contaminants may be undergoing in situ Fenton Chemistry with DLPC [39]. It is also well known that upon the addition of sodium sulfite to a solution containing Fe+3 ions, redox reactions will readily produce Fe+2 and H+, and transiently change the Fe+3/Fe+2 ratio [40, 41]. Consequently, we investigated DLPC degradation after freezing Tris buffer solutions containing DLPC, DLPC + 2% sucrose, DLPC + Na2SO3, and DLPC + 2% sucrose + Na2SO3; these experiments were conducted without spiking any exogenous ferrous ion. Only when Na2SO3 was added to the sample containing sucrose did we observe significant DLPC degradation (Fig. 6). These results are consistent with a model where the sucrose used in the formulation possesses trace quantities of Fe+3 contaminants, and the added Na2SO3 participates in redox reactions with the sucrose-Fe+3 complex to produce Fe+2 + H+ (i.e., a more acidic environment) and/or displaces the Fe+3 species, which then undergoes in situ redox reactions to produce the more reactive Fe+2 ion which ultimately catalyzes DLPC degradation.
Fig. 6.
Reduction of the transition metal contaminants in sucrose affected DLPC stability. Increased DLPC degradation occurred in the presence of the reduced transition metal contaminants. The DLPC degradation in samples containing sucrose and Na2SO3 was significantly different relative to the DLPC samples containing sucrose only and Na2SO3 only. The values represent the mean ±1 SEM of triplicate or quadruplicate determinations. Error bars are not visible on the scale of the figure. * indicates statistical significance. p < 0.0001
3.7 Measurement of Iron Contaminants in Pharmaceutical-Grade Sugars
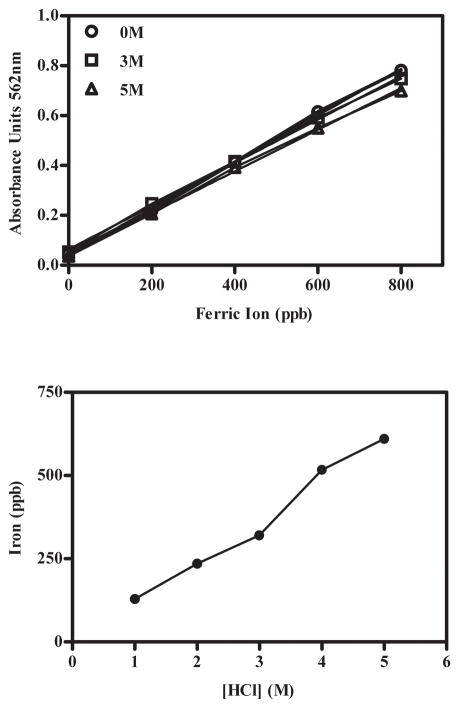
The ferrozine assay is commonly used to evaluate the iron contaminants in pharmaceutical-grade sugars. Using this assay to analyze the sucrose, trehalose and hydroxyethyl starch used in the current studies, we found that the amount of iron detected via this assay was a dependent on the amount of HClaq present during the ferrozine reaction; higher quantities of HClaq resulted in higher values for iron content (Fig. 7). In contrast, when aqueous iron standards were mixed with the ferrozine reaction in the presence of higher HClaq, the absorbance did not increase. Therefore, these results are consistent with the phenomena previously observed (section 3.6) wherein the production of protons (H+; i.e., more acidic environment) liberates Fe+3 from the sucrose-Fe+3 complex (e.g., sugar-iron oxide complexes) which subsequently reacts with the ferrozine assay reagents.
Fig. 7.
Measurement of iron contaminants in pharmaceutical-grade sucrose. A-Absorbance of iron standards as the hydrochloric acid concentration was increased. n = 1 B- Increased concentrations of iron were detected in 10% sucrose solution as hydrochloric acid concentration was increased. n = 2
4. Discussion
Liposomal delivery systems have been successfully utilized to deliver small molecule pharmaceuticals; however, one disadvantage has been that unsaturated lipids are highly susceptible to oxidation which limits their use in lipid-based commercial products. DLPC (1, 2-dilinolenoyl-sn-glycero-3-phosphocholine) is a triply unsaturated lipid and significantly more prone to oxidative degradation than the analogous saturated lipid (DSPC 1, 2-distearoyl-sn-glycero-3-phosphocholine). Despite the ability of unsaturated lipids to improve intracellular delivery by promoting endosomal escape, their intrinsic reactivity has thus far precluded their use in marketed pharmaceutical products. The goal of the current study was to characterize DLPC degradation during lyophilization, and investigate the influence of metal contaminants in promoting degradation.
After developing appropriate methods for analyzing the degradation of DLPC and the formation of degradation products (Figs. 1, 2), we performed experiments to determine the stage of lyophilization during which degradation was occurring. Our results demonstrate that degradation occurs almost entirely in the freezing stage (Fig. 3), with undetectable losses during primary and secondary drying. Because our freezing protocol cools samples to temperatures very close to the Tg′ of sucrose (≈ −30°C) [31], we conducted additional experiments wherein samples were frozen at −20°C, or plunged directly into liquid nitrogen. Since each of these experiments resulted in DLPC losses comparable to that depicted in Fig. 3, we concluded that cooling rate and glass formation played a minimal role in lipid degradation. Further experiments were performed to determine whether the concentrating effect of ice formation might create high concentrations of reactants that promote degradation in the non-ice fraction. Surprisingly, dilution of Tris-HCl, iron, and DLPC in a larger non-ice fraction resulted in increased degradation (Fig. 4). This counterintuitive result suggested that the increased buffer ionic strength in the freeze concentrate might play a protective role. We then conducted experiments at Tris-HCl concentrations approximating that in the freeze concentrate to evaluate their effect on degradation in the absence of freezing (Fig. 5). The results clearly demonstrate that the increased Tris-HCl buffer ionic strength has a stabilizing effect on DLPC. Considering that iron ions are known to interact with lipid bilayers (i.e., liposomes and microsomes) through electrostatic interactions with the negatively charged phosphate in the lipid head group [42], the increased concentration of ions (ionized Tris molecules and chloride ions) may inhibit interactions that promote lipid degradation. Alternatively, the reduced DLPC degradation may be due to the formation of iron-chloride complexes which in turn decreased the iron-lipid interactions; previous studies have shown that chloride ions can reduce iron-catalyzed lipid oxidation [43–46]. Another possibility involves the Tris salts playing a more direct role in decreasing DLPC peroxidation. In this regard, it is important to note that Tris has been reported to react with hydroxyl radicals formed during lipid peroxidation to a greater extent than fatty acids, such as linoleate [47]. Experiments utilizing proxyl fluorescamine to assess the potential role of radicals in DLPC degradation did not detect reactive oxygen species (ROS) during freezing, suggesting that oxygen radicals may not play a major role in the observed degradation (data not shown). Consistent with this finding, previous studies have asserted that ROS do not always play a significant role in lipid peroxidation [48, 49]. Although the effect of salts on iron-catalyzed lipid oxidation has been previously studied, this phenomenon is not fully understood and contradictory reports have been cited in the literature [44, 50].
Transition metals, such as iron ions (Fe+2), are known to more readily catalyze the peroxidation of lipids (i.e., Fenton Chemistry) if present in the reduced form [21, 36–38]. When ferric ion catalyzes the formation of the lipid alkyl radical, an electron is transferred to ferric ion (Fe3+) [51]. The reduction of ferric ion can also occur during lipid hydro-peroxide decomposition [51] or as a result of reaction with superoxide [52]. We hypothesized that the reduction of the transition metal contaminants (e.g., iron) through the reactions discussed above during lipid peroxidation may allow these contaminants to catalyze DLPC degradation. To test this hypothesis, it was necessary to design an experiment where transition metal contaminants would be reduced without significantly altering solution pH. To the best of our ability, we wanted to avoid both acidic and basic conditions as they would promote lipid hydrolysis. As sodium sulfite is known to reduce transition metals (iron, copper, cobalt) without causing a dramatic pH change [40, 41], we investigated DLPC degradation after freezing in the presence of Na2SO3; these experiments were conducted without spiking any exogenous ferrous ion. Only when Na2SO3 was added to the sample containing sucrose did we observe significant DLPC degradation (Fig. 6). These results are consistent with the suggestion that the sucrose used in the formulation possesses trace quantities of Fe+3 contaminants such that addition of Na2SO3 undergoes redox with the sucrose-Fe+3 complex to produce Fe+2 and/or displaces the Fe+3 species which then undergoes in situ redox reactions to produce the more reactive Fe+2 ion which ultimately catalyzes DLPC degradation. Although the reduction of the iron contaminants in the sucrose by sodium sulfite is more extreme, it is somewhat analogous to the reduction of the iron contaminants during lipid peroxidation. It is proposed that although the ferric ion contaminants alone did not affect DLPC stability, the reduction of the ferric ion contaminants during lipid peroxidation may ultimately contribute to DLPC degradation. Such an effect may be especially relevant to formulations incorporating reducing agents that may promote oxidation by metal contaminants present in other excipients.
Lastly, as the ferrozine assay is often utilized to measure iron concentration in solutions and biological samples [53, 54], we used this assay to analyze the sucrose, trehalose and hydroxyethyl starch employed in our studies. Our observation that the amount of iron detected via this assay was dependent on the amount of HClaq present during the ferrozine reaction is consistent with previous studies documenting the formation of iron-sugar complexes [55, 56]. These complexes are affected by pH, and acidic conditions can cause the dissociation of the iron-sucrose complexes [57]. Therefore, these results are consistent with the suggestion that metal contaminants in sugars are present in a less reactive form that can be liberated/activated by other formulation components, and ultimately contribute to the observed DLPC degradation. Moreover, this mechanism is relevant to the shelf-life of any pharmaceutical preparation, especially those incorporating sugars as excipients.
5. Conclusions
DLPC peroxidation correlated with increased ferrous ion spiked into the formulation solution. In addition, our results demonstrate that DLPC degradation occurred during the freezing step of lyophilization and was affected by sucrose concentration and buffer ionic strength in the freeze concentrate. Recent studies have shown that particulate formulations are especially susceptible to damage immediately after ice formation due to the high concentration of solutes and relatively high temperatures prior to complete solidification [58]. In contrast, we report that the high solute concentration actually serves to stabilize lipids against metal-catalyzed degradation, presumably by disrupting the association with metal contaminants. Clearly, the roles which iron plays are complex, and the observations from this study further highlight this complexity. The fact that DLPC peroxidation did not occur unless ferrous ion or sodium sulfite was added to the formulation demonstrates that trace metal contaminants need to be activated/reduced to serve as effective catalysts for degradation, at least during acute lyophilization stress. While underscoring the importance of using high grade pharmaceutical sugars and avoiding contact with metal during manufacturing and processing, our results also demonstrate that the common practice of spiking samples with iron does not accurately simulate the conditions present in formulations incorporating excipients that are contaminated with trace metals.
Supplementary Material
Acknowledgments
This work was partially supported by NIH/NIBIB grant R01 EB006398-01A1 and NIH/NIGMS grant RO1GM093287-01A1. This research utilized services of the Medicinal Chemistry Core facility (MFW) housed within the Department of Pharmaceutical Sciences (DOPS). In part, the MCC has been funded via Colorado Clinical and Translational Sciences Institute grant 5UL1RR025780 from National Center for Research Resources at the National Institutes of Health (NCRR/NIH). We would also like to acknowledge Drs. Cortland Pierpont and James Ruth for extremely helpful discussions and critical insight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M.F. Wempe, Email: Michael.wempe@ucdenver.edu.
Jamie L. Betker, Email: Jamie.betker@ucdenver.edu.
T.W. Randolph, Email: Theodore.randolph@colorado.edu.
T.J. Anchordoquy, Email: Tom.anchordoquy@ucdenver.edu.
References
- 1.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International journal of nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced Gene Delivery and Mechanism Studies with a Novel Series of Cationic Lipid Formulations. J Biol Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 4.Wang M, Sun S, Alberti KA, Xu QB. A Combinatorial Library of Unsaturated Lipidoids for Efficient Intracellular Gene Delivery. Acs Synthetic Biology. 2012;1:403–407. doi: 10.1021/sb300023h. [DOI] [PubMed] [Google Scholar]
- 5.Zhi DF, Zhang SB, Wang B, Zhao YN, Yang BL, Yu SJ. Transfection Efficiency of Cationic Lipids with Different Hydrophobic Domains in Gene Delivery. Bioconjugate Chem. 2010;21:563–577. doi: 10.1021/bc900393r. [DOI] [PubMed] [Google Scholar]
- 6.Fox JL. Gene-therapy death prompts broad civil lawsuit. Nature biotechnology. 2000;18:1136–1136. doi: 10.1038/81104. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Anchordoquy T. Drug Delivery Trends in Clinical Trials and Translational Medicine: Challenges and Opportunities in the Delivery of Nucleic Acid-Based Therapeutics. J Pharm Sci-Us. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: A comprehensive review. Aaps J. 2005;7:E61–E77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muttil P, Wang CC, Hickey AJ. Inhaled Drug Delivery for Tuberculosis Therapy. Pharmaceutical research. 2009;26:2401–2416. doi: 10.1007/s11095-009-9957-4. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro M, Lucio M, Lima JLFC, Reis S. Liposomes as drug delivery systems for the treatment of TB. Nanomedicine-Uk. 2011;6:1413–1428. doi: 10.2217/nnm.11.122. [DOI] [PubMed] [Google Scholar]
- 11.Anchordoquy TJ, Koe GS. Physical stability of nonviral plasmid-based therapeutics. J Pharm Sci. 2000;89:289–296. doi: 10.1002/(SICI)1520-6017(200003)89:3<289::AID-JPS1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Samuni AM, Lipman A, Barenholz Y. Damage to liposomal lipids: protection by antioxidants and cholesterol-mediated dehydration. Chemistry and physics of lipids. 2000;105:121–134. doi: 10.1016/s0009-3084(99)00136-x. [DOI] [PubMed] [Google Scholar]
- 13.Wagner BA, Buettner GR, Burns CP. Free Radical-Mediated Lipid-Peroxidation in Cells-Oxidizability Is a Function of Cell Lipid Bis-Allylic Hydrogen Content. Biochemistry-Us. 1994;33:4449–4453. doi: 10.1021/bi00181a003. [DOI] [PubMed] [Google Scholar]
- 14.Yin HY, Xu LB, Porter NA. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chemical reviews. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 15.Gutteridge JM, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends in biochemical sciences. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 16.Mozuraityte R, Rustad T, Storrø I. The role of iron in peroxidation of polyunsaturated fatty acids in liposomes. Journal of agricultural and food chemistry. 2007;56:537–543. doi: 10.1021/jf0716073. [DOI] [PubMed] [Google Scholar]
- 17.Levine HSL. Another View of Trehalose for Drying and Stabilizing Biological Materials. BioPharm. 1992;5:35–40. [Google Scholar]
- 18.Allison SD, Molina MDC, Anchordoquy TJ. Stabilization of lipid/DNA complexes during the freezing step of the lyophilization process: the particle isolation hypothesis. Bba-Biomembranes. 2000;1468:127–138. doi: 10.1016/s0005-2736(00)00251-0. [DOI] [PubMed] [Google Scholar]
- 19.Crowe J, Crowe L, Carpenter J. Preserving dry biomaterials: The water replacement hypothesis, part 1. BIOPHARM-EUGENE- 1993;6:28–28. [Google Scholar]
- 20.Crowe J, Crowe L, Carpenter J. Preserving dry biomaterials: The water replacement hypothesis, part 2. BIOPHARM-EUGENE- 1993;6:40–40. [Google Scholar]
- 21.Molina MDC, Anchordoquy TJ. Metal contaminants promote degradation of lipid/DNA complexes during lyophilization. Bba-Biomembranes. 2007;1768:669–677. doi: 10.1016/j.bbamem.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Anchordoquy TJ. Effects of moisture content on the storage stability of dried lipoplex formulations. J Pharm Sci-Us. 2009;98:3278–3289. doi: 10.1002/jps.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Advanced drug delivery reviews. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Crowe JH, Carpenter JF, Crowe LM, Anchordoguy TJ. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology. 1990;27:219–231. [Google Scholar]
- 25.Molina MC, Allison SD, Anchordoquy TJ. Maintenance of nonviral vector particle size during the freezing step of the lyophilization process is insufficient for preservation of activity: insight from other structural indicators. J Pharm Sci. 2001;90:1445–1455. doi: 10.1002/jps.1096. [DOI] [PubMed] [Google Scholar]
- 26.Korenaga T. Spectrophotometric determination of iron at the ppm level in sugar samples with 2-nitroso-5-dimethylaminophenol. Microchimica Acta. 1977;68:419–424. [Google Scholar]
- 27.Škrbić B, Gyura J. Iron, copper and zinc in white sugar from Serbian sugar beet refineries. Food control. 2007;18:135–139. doi: 10.1080/02652030500306943. [DOI] [PubMed] [Google Scholar]
- 28.Catala A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chemistry and physics of lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Min B, Cordray JC, Ahn DU. Effect of NaCl, myoglobin, Fe(II), and Fe(III) on lipid oxidation of raw and cooked chicken breast and beef loin. Journal of agricultural and food chemistry. 2010;58:600–605. doi: 10.1021/jf9029404. [DOI] [PubMed] [Google Scholar]
- 30.Rolston RK, Perry G, Zhu X, Castellani RJ, Dwyer BE, Lee HG, Petersen RB, Smith MA. Iron: A Pathological Mediator of Alzheimer Disease? Agro food industry hi-tech. 2009;19:33–36. [PMC free article] [PubMed] [Google Scholar]
- 31.Franks F. Freeze drying: From empiricism to predictability, cryo-letters. 1990;11:91–110. [Google Scholar]
- 32.Ray S, Fariss M. Role of cellular energy status in to copheryl hemisuccinate cytoprotection against ethyl methane sulfonate-induced toxicity. Arch Biochem Biophys. 1994;311:180–190. doi: 10.1006/abbi.1994.1224. [DOI] [PubMed] [Google Scholar]
- 33.Stacey NH, Klaassen CD. Inhibition of lipid peroxidation without prevention of cellular injury inisolated rat hepatocytes. Toxicology and applied pharmacology. 1981;58:8–18. doi: 10.1016/0041-008x(81)90110-1. [DOI] [PubMed] [Google Scholar]
- 34.Tirmenstein MA, Pierce CA, Leraas TL, Fariss MW. A fluorescence plate reader assay for monitoring the susceptibility of biological samples to lipid peroxidation. Analytical biochemistry. 1998;265:246–252. doi: 10.1006/abio.1998.2907. [DOI] [PubMed] [Google Scholar]
- 35.Molina MD, Anchordoquy TJ. Formulation strategies to minimize oxidative damage in lyophilized lipid/DNA complexes during storage. J Pharm Sci. 2008;97:5089–5105. doi: 10.1002/jps.21365. [DOI] [PubMed] [Google Scholar]
- 36.Braughler JM, Chase RL, Pregenzer JF. Oxidation of ferrous iron during peroxidation of lipid substrates. Biochimica et biophysica acta. 1987;921:457–464. doi: 10.1016/0005-2760(87)90072-5. [DOI] [PubMed] [Google Scholar]
- 37.Fagali N, Catala A. Fe2+ and Fe3+ initiated peroxidation of sonicated and non-sonicated liposomes made of retinal lipids in different aqueous media. Chemistry and physics of lipids. 2009;159:88–94. doi: 10.1016/j.chemphyslip.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Hegenauer J, Saltman P, Ludwig D, Ripley L, Bajo P. Effects of supplemental iron and copper on lipid oxidation in milk. 1. Comparison of metal complexes in emulsified and homogenized milk. Journal of agricultural and food chemistry. 1979;27:860–867. doi: 10.1021/jf60224a048. [DOI] [PubMed] [Google Scholar]
- 39.Pignatello JJ, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Critical reviews in environmental science and technology. 2006;36:1–84. [Google Scholar]
- 40.Coichev N, van Eldik R. A Fascinating Demonstration of Sulfite Induced Redox Cycling of Metal Ions Initiated by Shaking. Journal of chemical education. 1994;71:767. [Google Scholar]
- 41.Moya HD, Neves EA, Coichev N, Niewahner J. A further demonstration of sulfite-induced redoxcycling of metal ions initiated by shaking. Journal of chemical education. 1999;76:930. [Google Scholar]
- 42.Braughler JM, Chase RL, Pregenzer JF. Stimulation and inhibition of iron-dependent lipid peroxidation by desferrioxamine. Biochemical and biophysical research communications. 1988;153:933–938. doi: 10.1016/s0006-291x(88)81317-2. [DOI] [PubMed] [Google Scholar]
- 43.Mei L, McClements DJ, Wu J, Decker EA. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl. Food chemistry. 1998;61:307–312. [Google Scholar]
- 44.Mozuraityte R, Rustad T. Oxidation of cod phospholipids in liposomes: Effects of salts, pH and zeta potential. European Journal of Lipid Science and Technology. 2006;108:944–950. [Google Scholar]
- 45.Mozuraityte R, Rustad T, Storrø I. Pro-oxidant activity of Fe2+ in oxidation of cod phospholipids in liposomes. European Journal of Lipid Science and Technology. 2006;108:218–226. [Google Scholar]
- 46.Ohyashiki T, Koshino M, Ohta A, Mohri T. The effect of ionic strength on the lipid peroxidation of porcine intestinal brush-border membrane vesicles. Biochimica et biophysica acta. 1985;812:84–90. doi: 10.1016/0005-2736(85)90524-3. [DOI] [PubMed] [Google Scholar]
- 47.Fiorentini D, Landi L, Barzanti V, Cabrini L. Buffers can modulate the effect of sonication on egg lecithin liposomes. Free Radical Research. 1989;6:243–250. doi: 10.3109/10715768909073477. [DOI] [PubMed] [Google Scholar]
- 48.Braughler JM, Duncan LA, Chase RL. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem. 1986;261:10282–10289. [PubMed] [Google Scholar]
- 49.Welch KD, Davis TZ, Aust SD. Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Arch Biochem Biophys. 2002;397:360–369. doi: 10.1006/abbi.2001.2694. [DOI] [PubMed] [Google Scholar]
- 50.Calligaris S, Nicoli MC. Effect of selected ions from lyotropic series on lipid oxidation rate. Food chemistry. 2006;94:130–134. [Google Scholar]
- 51.Schaich KM. Metals and lipid oxidation. Contemporary issues. Lipids. 1992;27:209–218. doi: 10.1007/BF02536181. [DOI] [PubMed] [Google Scholar]
- 52.Minotti G, Aust SD. Redox Cycling of Iron and Lipid-Peroxidation. Lipids. 1992;27:219–226. doi: 10.1007/BF02536182. [DOI] [PubMed] [Google Scholar]
- 53.Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Analytical biochemistry. 2004;331:370–375. doi: 10.1016/j.ab.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 54.Stookey LL. Ferrozine---a new spectrophotometric reagent for iron. Analytical Chemistry. 1970;42:779–781. [Google Scholar]
- 55.Gyurcsik B, Nagy L. Carbohydrates as ligands: coordination equilibria and structure of the metal complexes. Coordination chemistry reviews. 2000;203:81–149. [Google Scholar]
- 56.Nagy L, Burger K, Kurti J, Mostafa MA, Korecz L, Kiricsi I. Iron(III) Complexes of Sugar-Type Ligands. In org Chim a-Bioinor. 1986;124:55–59. [Google Scholar]
- 57.Somsook E, Hinsin D, Buakhrong P, Teanchai R, Mophan N, Pohmakotr M, Shiowatana J. Interactions between iron(III) and sucrose, dextran, or starch in complexes. Carbohydrate Polymers. 2005;61:281–287. [Google Scholar]
- 58.Kasper JC, Pikal MJ, Friess W. Investigations of polyplex stability during the freezing step of lyophilization controlled ice nucleation- The importance of residence time in the low viscosity fluid state. J Pharm Sci-Us. doi: 10.1002/jps.23419. (In Press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.