Abstract
The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that belongs to the basic-helix-loop-helix (bHLH)–Per-ARNT-Sim (PAS) superfamily of transcription factors, mediates toxic response induced by environmental chemicals such as polycyclic aromatic hydrocarbons (PAH). AhR is expressed at high levels in several human breast carcinoma cell lines in direct correlation with the degree of their malignancy. Recent studies suggest a possible role for AhR in cancer independent of PAH. Therefore, we established stable AhR knockdown cells of the human breast cancer cell line MDA-MB-231 and analyzed their tumorigenic properties in in vitro and in vivo model systems. In addition we analyzed their response to radiation and chemotherapeutic treatment. AhR knockdown attenuated these cells tumorigenic properties in vitro including proliferation, anchorage independent growth, migration and apoptosis and reduced orthotopic xenograft tumor growth and lung metastasis in vivo. Notably, we observed that AhR knockdown enhanced radiation-induced apoptosis as well as significantly decreased cell clonogenic survival. Furthermore, AhR knockdown in MDA-MB-231 cells sensitized them to paclitaxel treatment, evident by a decrease in the required cytotoxic dose. Subsequent analysis revealed AhR knockdown significantly reduced phosphorylation of AKT, which impacts cell proliferation and survival. Apoptosis-focused gene expression analyses revealed an altered expression of genes regulating apoptosis in MDA-MB-231 cells. Collectively, our data identify AhR as a potential novel therapeutic target in the treatment of metastatic breast cancer.
Keywords: breast cancer, AhR knockdown, cell proliferation, apoptosis, bioluminescence imaging
Introduction
Breast cancer, the second leading cause of cancer deaths in women1, is the most common cancer among North American women, accounting for nearly 1 in 3 cancer cases diagnosed in U.S. women2. An estimated 40 percent of breast cancer patients relapse and develop metastatic disease and approximately 40,000 women die of breast cancer each year2, a mortality rate that is largely attributed to systemic metastatic disease3. Despite the recently reported decline in death rates the complexity of breast cancer makes treatment of many breast cancer subtypes difficult (reviewed in4–6). Therefore, identifying factors associated with breast cancer aggressiveness have the potential to serve as novel molecular targets for breast cancer therapy.
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that belongs to the basic helix-loop-helix (bHLH), Per-ARNT-Sim (PAS) superfamily of transcription factors7. It is historically known for its role in mediating the toxic and carcinogenic effect of environmental poly aromatic hydrocarbons (PAH)8,9. The AhR protein is predominantly cytoplasmic in the majority of normal tissues and it is found associated with chaperone proteins hsp90 and XAP210–12. Binding to exogenous ligands such as PAH, leads to conformational changes that result in nuclear translocation of AhR, where it heterodimerizes with the AhR nuclear translocator protein (ARNT)11,13. The heterodimer binds to a consensus DNA sequence xenobiotic responsive element (XRE), on the enhancer regions of target genes and increases their transcription; these target genes include the CYP1 family of genes, which encode enzymes responsible for activation of chemical carcinogens14,15. Following transcriptional activation, the AhR is exported back to the cytoplasm where it is degraded by calpains and proteasomes16,17.
Substantial evidence has shown that PAH-dependent activation of AhR plays a role in a variety of cancers including those in breast, liver and lung18,19. Activation of AhR leads to induction of CYP1A1, CYP1A2 and CYP1B1 genes, which encode for enzymes that metabolize PAH to mutagenic intermediates; resulting in cancer initiation15,18,20. Ligand-dependent activation of AhR not only plays a role in tumor initiation but also in tumor progression21–23. However, recent studies suggest a possible role for AhR in cancer independent of PAH24,25. Thus elevated and constitutively active levels of AhR have been found in advanced human breast tumors and breast cancer cell lines, with a strong correlation between expression of AhR and the degree of the tumor malignancy24,26. In a previously published study, we demonstrated that the overexpression of AhR in immortalized human mammary epithelial cells (HMEC) was sufficient to transform HMEC to exhibit malignant phenotypes26. We also demonstrated a significant correlation between AhR expression and carcinoma case type using tissue microarrays containing specimens of clinically defined stages of invasive breast cancer (unpublished data).
In the present study, we further investigated the role of AhR expression in breast cancer using RNA interference to stably knockdown AhR expression in the metastatic human breast cancer cell line MDA-MB-231. Utilizing in vitro and in vivo model systems, we demonstrate that reducing AhR expression attenuates cell proliferation, anchorage independent growth, migration and apoptosis (survival) in MDA-MB-231 in vitro, and reduced orthotopic xenograft tumor growth and pulmonary metastasis in vivo.
Materials and Methods
Cell Culture
The triple negative cell lines MDA-MB 231 and MDA-MB-468 were purchased from ATCC (Manassas, VA). Cells were cultured in the recommended medium [L-15 medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin] in a humidified atmosphere at 37°C with 5% CO2. Stable knockdowns were cultured in media supplemented with 2.5μg/ml puromycin (Sigma-Aldrich, St. Louis, MO). For animal experiments, cells were engineered by methods previously described27 to constitutively express firefly luciferase using pLenti-III-luc2 lentiviruses (In Vivo Imaging Solutions, Cheyenne, WY) and cultured in media supplemented with 500 μg/mL zeocin (Invitrogen, Carlsbad, CA).
Stable Knockdown of AhR using RNA interference
For stable knockdown of AhR gene expression in MBA-MD-231 and MDA-MB-468 human breast carcinoma (HBC) cell lines, cell lines were infected with shRNA retroviral particles produced in Phoenix cells targeted to human AhR mRNA as previously described26. AhRshRNA specific plasmids were generated using the pSuper.Retro.puromycin vector (OligoEngine Inc., Seattle, WA) and inserted oligonucleotides designed against full length human AhR mRNA targeting the 3′ untranslated region (3′ UTR) using the shRNA design program as previously described28. For AhR sh-1; Sense 5′-GAT CTC CGC ACC CAT TTC AAT GTA TAT TCA AGA GAT ATA CAT TGA AAT GGG TGC TTT TTG GAA A-3′ and Anti-Sense 5′-AGC TTT TCC AAA AAG CAC CCA TTT CAA TGT ATA TCT CTT GAA TAT ACA TTG AAA TGG GTG CGG A-3′. AhR sh-2; 5′-GAT CTC CCC AGA GGT CTA GAT TAG TAT TCA AGA GAT ACT AAT CTA GAC CTC TGG TTT TTG GAA A-3′ and Anti-Sense 5′-AGC TTT TCC AAA AAC CAG AGG TCT AGA TTA GTA TCT CTT GAA TAC TAA TCT AGA CCC TGG GGA-3′. Limited cell dilution was performed with Scr control and AhRshRNA cells to obtain single cell clones. Multiple clones of Scr control with similar AhR protein expression as the parental heterogeneous Scr control cells, were pooled, expanded and used throughout the study to represent the Scr control (supplemental Figure 1 b).
RNA Isolation, Real-time RT–PCR, Immunoblotting, and Immunofluorescence Microscopy
Total RNA isolation, cDNA synthesis and PCR amplification for AhR and GAPDH (internal control), electrophoresis, immunoblotting and quantification of immuno-detectable bands were performed as previously described26. Primary antibodies used include: anti-AhR, anti-cyclin D2, anti-Cdk4, anti-cyclin E, and anti-Cdk2 (Santa Cruz, Dallas, TX), anti-β-Actin (Sigma-Aldrich, St. Louis, MO) anti-AKT, anti-phosphoAKT(Ser473), anti-phosphoRB(Ser795) anti-phosphoRB(Ser807/811), mouse anti-RB, anti-Bak, anti-Bcl2 (Cell Signaling, Danvers, MA). PCR primers used for AhR (5′ to 3′) were: CCCTGACCTCTGTCTTACTTGTGGA and ACGTCAGATGGTGCCAGCAATA, CYP1A1 (5′ to 3′) were: TAGACACTGATCTGGCTGCAG and GGGAAGGCTCCATCAGCATC, and CYP1B1 (5′ to 3′) were: AACGTCATGAGTGCCGTGTGT and GGCCGGTACGTTCTCCAAATC.
Cell Proliferation and Cell Cycle Analysis
Cell proliferation assay was performed as previously described. For cell cycle analysis, cells were plated and the following day serum-starved (in medium supplemented with 0.05% FBS) for 24 hours followed by replacement of complete medium for an additional 24 hours. The remainder of the procedures was done as described previously26.
Soft Agar Colonization
A 0.6% base agar was prepared by combining a 1:1 dilution of 1.2% agar and 2X complete L-15 medium. The 0.6% base agar was added to the bottoms of a 6-well plate, 1 ml/well, and allowed to solidify at room temperature. A 0.3% top agarose was prepared by combining a 1:1 dilution of 0.6% agarose and 2X complete L-15 medium. Cells were trypsinized and cell suspensions of 4000 cells/ml prepared; 100 μl of each cell suspension was added to 4ml of 0.3% agarose. Suspensions were thoroughly mixed and 1ml was added on top of the base agar and allowed to solidify at room temperature. The plates were incubated at 37°C in a humidified incubator with 5% CO2; media was replenished every three days. After 30 days of incubation, colonies were counted.
Migration Assay
Migration potential was assessed by a previously described scratch assay29.
Apoptosis Assays
For the apoptosis assay, cells were carefully detached with 0.05% trypsin and stained using TACS Annexin V Kit (Trevigen, Gaithersburg, MD) according to the manufacturer’s instructions, and flow cytometric analysis was conducted using a Guava EasyCyte Plus flow cytometer (Millipore Corporation, Billerica, MA).
Radio-sensitization experiments and clonogenic survival assay
Cells exponentially growing in tissue culture flasks were irradiated at room temperature with a single dose of 0, 2 or 4 Gy using a Mark 1 137Cs irradiator. Immediately following irradiation, cells were trypsinized and plated for clonogenic survival assay; 24 h after irradiation, cells were processed for apoptosis analyses.
For the clonogenic assay, irradiated cells were prepared as described above for soft agar colonization assays and plated 100 cells/well. Colonies with >50 cells were scored 30 days later as surviving colonies. The plating efficiency (PE) was calculated as the number of colonies observed/the number of cells plated for un-irradiated control cells.
Chemo-sensitization and cytotoxicity assay
Cells were plated in triplicate (10,000/well) in 96 well plates and treated with 0.1% DMSO or 1, 5, 10, 50 nM paclitaxel in DMSO for 18 h. As a positive control, cells were treated with 30 ng TNF/ml for 30 min. Cell death was measured using the CytoTox-Glo cytotoxicity following manufactures instructions. Dead cells luminescence signal was measured using a Luminoskan Ascent luminometer (Thermo Electronic Corp, Vantaa, Finland).
Animals
All animal protocols were conducted at Vanderbilt animal care facility with full ethical approval by Vanderbilt University Institutional Animal Care and Use Committee and according to NIH guidelines. Six to seven week old female athymic nude mice obtained from Harlan Laboratories (Indianapolis, IN) were used for tumor xenograft and experimental metastasis experiments. For tumor xenograft experiments, 2.5×106 cells suspended in a volume of 100 μL PBS were injected into the inguinal mammary gland fat pad (MFP). For experimental metastasis experiments, 1.0×106 cells suspended in a volume of 100 μL PBS were injected into the lateral tail vein of mice. Tumor growth in MFP and formation of lung metastasis was monitored by noninvasive bioluminescence imaging (BLI).
Procedures for BLI and data analysis, and histopathological and immunohistochemical analyses are included as supplemental material.
Statistical Analysis
All of the experiments were performed at least three times, using cells at different passage numbers; AhR levels were always verified by Western blotting prior to every analysis. Statistical comparisons were made using a paired two-tailed Student’s t-test using GraphPad Prism software (Graphpad, La Jolla, CA). A p-value of ≤ 0.05 was considered statistically significant.
Results
Stable knockdown of AhR in two triple negative breast cancer cells by RNAi
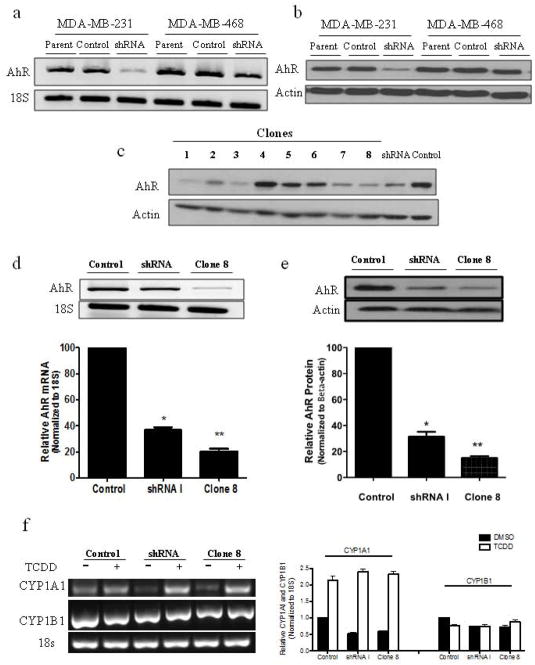
Our previous studies showed that AhR is overexpressed and constitutively active in advanced HBC cell lines including two triple negative breast cancer (TNBC) cell lines, MDA-MB-231 and MDA-MB-46830. As both of these cells have elevated levels of constitutively activated AhR, we targeted them for this study. Thus we stably knocked down AhR expression using vector-based generation of shRNAs (sh-1 and sh-2) retroviruses (Figure 1). Sh-1 knocked down AhR expression more efficiently than sh-2 (supplemental data S1). RT-PCR (Fig. 1a) and immunoblot analyses (Fig. 1b) confirmed that the resultant stable AhR-shRNA cells have suppressed AhR mRNA and protein expression, compared to their scramble (Scr) and parental control cells.
Figure 1.
Stable knockdown of AhR mRNA and protein expression in two triple negative breast cancer (TNBC) cell lines using shRNA. Stable knockdown was validated at the (a) mRNA level by RT-PCR and (b) at the protein level by immunoblot analysis. (c) Single cell clones (1–8) generated by limited cell dilution of MDA-MB-231 shRNA I cells. Clone 8 (C8) cells were selected for evaluation, and AhR expression was further evaluated at the (d) mRNA and (e) protein level compared to control and shRNA I cells. (f) RT-PCR analysis of CYP1A1 and CYP1B1 expression following treatment with 1 nM TCDD or 0.1% DMSO for 3 h. Bar graphs are mean ± s.d. from three independent experiments (* p<0.05; ** p<0.01).
As MDA-MB-231 cells exhibited the greatest degree of receptor knockdown (KD), they were chosen for the study. Cells infected with either Scr control viruses or AhRshRNA were subjected to cloning by limited cell dilution. As expected, AhR expression remained unchanged in clones generated from Scr control (supplemental data S1); subsequently, the control clonal cells were pooled, expanded, and maintained as a Scr control polyclonal populations, which were used as control for all studies. The AhRshRNA generated clonal cell lines with varying degrees of AhR expression, reduced AhR expression levels in these clones ranged from 50–80% compared to control cells (Fig. 1c). Clone 8 cells, in particular, exhibited one of the highest degrees of AhR KD (~80%) and were chosen for subsequent studies. Quantitative comparison of AhR mRNA (Fig. 1d) and protein (Fig. 1e) showed levels in the heterogeneous shRNA of ~40% and clone 8 ~20% compared to control MDA-MB-231 cells (100%).
In the absence of ligand, AhR is predominantly cytoplasmic, however AhR overexpression has been reported to produce constitutive activation, as evidenced by the presence of nuclear receptor in the absence of exogenous ligand 31,32. This was confirmed by immunocytochemical staining and confocal-fluorescence imaging in which a substantial amount of AhR was seen in nuclei of Scr control cells in the absence of exogenous ligand and which was reduced as a result of AhR KD (supplemental data S2). To determine whether this nuclear AhR was transcriptionally active we measured the expression of the CYP1A1 gene, which represents a direct readout of AhR transcriptional activity. RT-PCR analysis showed a substantial expression of CYP1A1 mRNA in control cells that was reduced in clone 8 (Fig. 1f). Expression of CYP1A1 mRNA was further enhanced in control and clone 8 cells following exposure to AhR agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). These results support the notion that in MDA-MB-231, AhR is constitutively active and AhR KD results in subsequent attenuation of this activation. CYP1B1 mRNA is constitutively expressed in all cells and TCDD treatment did not affect its expression levels.
Stable AhR knockdown attenuates tumorigenic properties of MDA-MB-231 cells
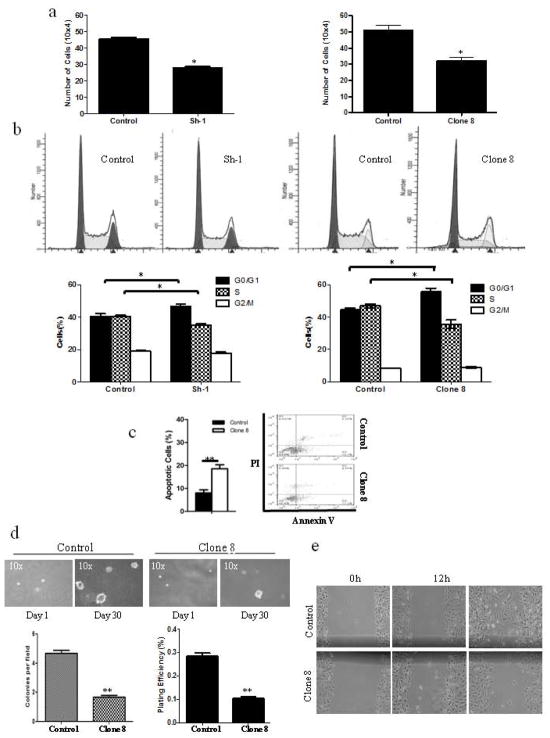
We then investigated the effect of stable AhR KD on the tumorigenic properties of MDA-MB-231 cells (Figure 2). Proliferation assays revealed that AhR KD reduced cell numbers compared to control cells (Fig. 2a). Analysis of cell growth kinetics estimated a population doubling time (PDT) for clone 8 to be 30.5 hours compared to 27.8 hours for the control MDA-MB-231 cells. Assessment of cell cycle distribution was used to better understand the increase in the PDT in clone 8 cells. There was an accumulation of cells in G0/G1 phase indicating delayed entry into S-phase in clone 8 cells. There is also a substantial sub-G0 fraction in the clone 8 cell population, representing apoptotic cells (Fig. 2b). This was confirmed by Annexin V staining and flow cytometry; showing higher percentage of apoptotic cells in clone 8 compared to control cells (Fig. 2c). Thus, stable AhR KD dramatically inhibited growth and promoted apoptosis in MDA-MB-231 cells.
Figure 2.
AhR knockdown attenuates tumorigenic properties of MDA-MB-231 cells. (a) Cell proliferation of AhR KD vs. control MDA-MB-231 cells. (b) Histogram plot shown is a FACS analysis (left panel) and bar graph of the percentage of distributed cells in each phase of the cell cycle (right panel) of AhR KD vs. control MDA-MB-231 cells. (c) Representative scatter plot of Annexin V apoptosis assay, with the % apoptotic cells quantitatively expressed. (d) Representative micrograph of colony formation. The mean number of colonies/field and plating efficiency are presented. (e) Representative micrographs of wound closure at 0, 12 and 24 h following the induced wound. Bar graphs are mean ± s.d. from three independent experiments, each done in duplicate (* p<0.05; ** p<0.01).
We also examined the effect of AhR KD on anchorage independent growth and motility of MDA-MB-231 cells. AhR KD reduced both colony numbers and plating efficiency compared to control cells (Fig. 2d). We investigated cell migration using a wound healing assay (Fig. 2e). Both clone 8 and control cells migrated to the wound area within 12 hours. Although complete wound closure was not observed within 24 hours, control cells exhibited more wound closure than clone 8 cells at that time. These results collectively indicate that stable AhR KD remarkably attenuated tumorigenic properties of MDA-MB-231 cells, including anchorage independent growth and migration.
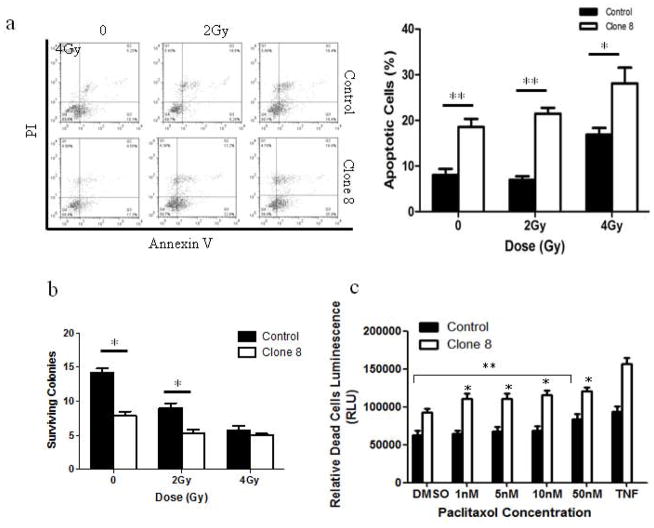
Stable AhR knockdown enhances radio- and chemo-sensitization
Because of the frequently encountered resistance of metastatic breast cancers to radiation and chemotherapy, we examined the effect of stable AhR KD on the sensitivity of MDA-MB-231 cells to either increasing doses of ionizing radiation (IR) or chemotherapeutic agent, paclitaxel (Figure 3). Annexin V assay revealed AhR KD increased the percentage of cells undergoing apoptosis in response to IR (Fig. 3a). To further examine the effect of AhR on radiosensitivity, a clonogenic assay was performed to assess the survival of MDA-MB-231 cells after IR. AhR KD reduced clonogenic growth (expressed as % surviving colonies) compared to control cells (Fig. 3b). However, independent of irradiation, clone 8 had only ~40% of clonogenic growth compared to controls.
Figure 3.
AhR knockdown enhances radio- and chemo- sensitization of MDA-MB-231 cells. (a) Representative scatter plot of Annexin V apoptosis assay, bar graphs represent percentage of early apoptotic cells. (b) Bar graph is average surviving colonies per field 30 days after IR. (c) Paclitaxel (1, 5, 10, 50 nM) induced cytotoxicity as assessed by Cyto-Glo assays after 18 h. Treatment with TNFα for 30 min served as a positive control. Bar graphs are mean ± s.d. from three independent experiments done in triplicate (* p<0.05; ** p<0.01).
Paclitaxel is a commonly used chemotherapeutic agent for treatment and management of breast cancer. We therefore examined the effect of AhR KD on MDA-MB-231 cellular sensitivity to paclitaxel. Results showed that AhR KD sensitized MDA-MB-231 cells to paclitaxel. Interestingly, AhR resulted in a different dose-response than controls; while at 1nM drug concentration clone 8 cells underwent significant cytotoxicity compared to vehicle treatment, control cells did not show cytotoxicity until 50 nM paclitaxel concentration (Fig. 3c).
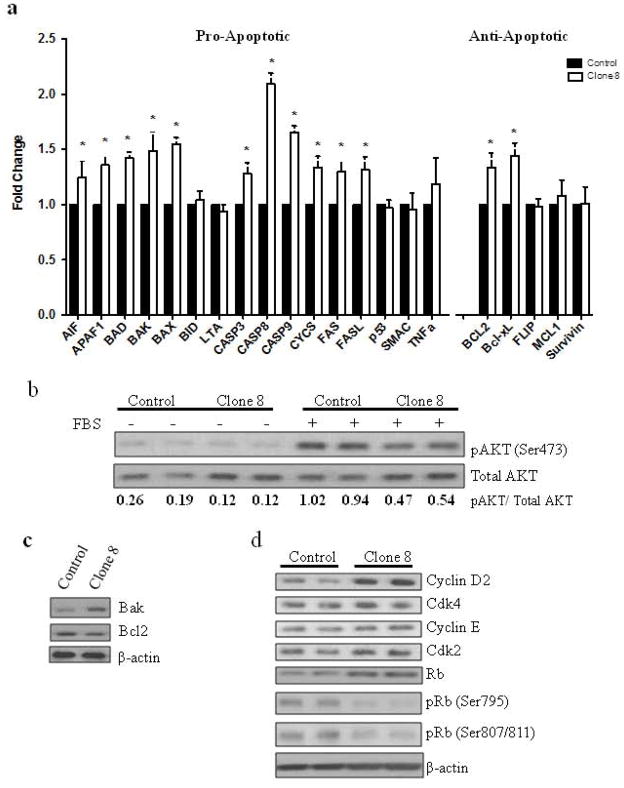
Effect of stable AhR knockdown on the expression of regulators of apoptosis and cell cycle
To further explain the apparent higher tendency of clone 8 for apoptosis, a panel of genes regulating apoptosis was examined. Out of the 16 pro-apoptotic genes investigated, 11 genes had significantly higher expression in clone 8 cells compared to control cells; 3 of 5 anti-apoptotic genes had no significant change and two showed increased expression in clone 8 cells compared to control cells (Fig. 4a). Since AKT signaling controls cell survival and proliferation, we examined the effect of AhR KD on AKT activation in MDA-MB-231 cells. In the presence of mitogenic stimuli (10% serum) AhR KD reduced AKT phosphorylation at Ser473, the predominant site for AKT activation, compared to control cells. Serum-free conditions served to establish a baseline AKT activation. Densitometric analysis of relative levels of AKT phosphorylation (pAKT/AKT) revealed a 50% inhibition in AKT phosphorylation in response to AhR KD (Fig. 4b). The expression of pro-apoptotic protein Bak was increased and anti-apoptotic Bcl-2 expression was decreased compared to control cells (Fig. 4c).We next examined the expression of several proteins involved in cell cycle progression. Cyclin D and Rb expressions were increased while phosphorylation of Rb at Ser 795 and Ser807/811 was decreased in clone 8 cells. Cyclin E, Cdk4 and Cdk2 expression remained unchanged (Fig. 4d).
Figure 4.
Stable AhR knockdown affects the expression of regulators of apoptosis and cell cycle. (a) AhR knockdown alters expression of pro- and anti-apoptotic genes. The expression of these genes was measured using a human apoptosis cDNA plate array as described in Materials and Methods. Relative mRNA expression was normalized to internal housekeeping genes and displayed as the fold-change relative to control cells from three independent experiments done in triplicate *p<0.05. Immunoblot analysis of (b) phospho- and total AKT numbers below are the relative levels of phospho-AKT normalized to total, (c) regulators of apoptosis and (c) cell cycle.
Stable AhR knockdown in MDA-MB-231 cells reduces tumor growth in orthotopic and experimental lung metastases in athymic nude mouse xenograft models
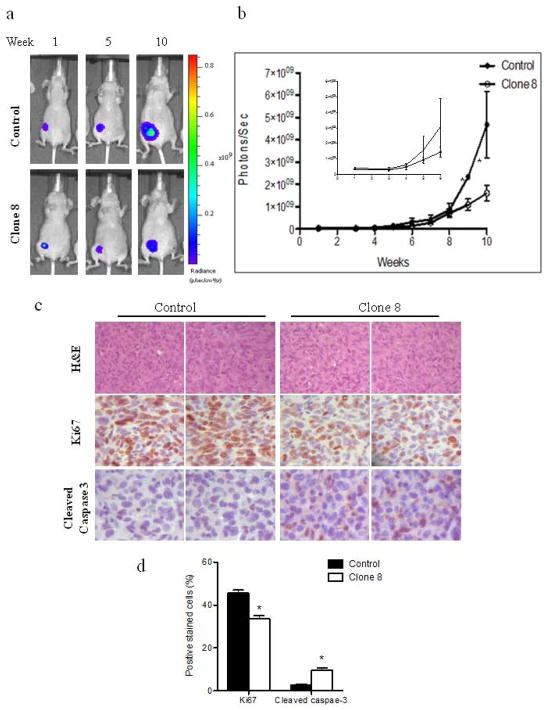
Because AhR KD attenuated tumorigenic properties of the metastatic MDA-MB-231 cells in vitro, we examined the effect of AhR KD on tumor growth and metastasis in vivo (Figures 5–6). To allow for non-invasive imaging, we established control and clone 8 cells that stably express constitutive luciferase enabling tumor growth and metastasis to be monitored by BLI. Tumor growth was assessed by injecting target cells into mammary fat pads of athymic nude mice with growth monitored weekly by BLI. Prior to injection, cell lines were analyzed by western blot analysis to confirm AhR status in addition, luciferase activity was measured in vitro to insure comparable activity (data not shown). All animals in both groups exhibited comparable bioluminescence initially and no significant difference in photon flux was observed at week 1 (Fig. 5a). Control cells gave rise to larger tumors whereas mice bearing AhR KD cells developed much smaller tumors, as measured by region of interest (ROI) analysis of BLI images (Fig. 5b). Except for the apparent higher density of cells in tumors from control xenografts, analyses of H&E-stained slides from harvested tumors revealed no obvious morphologic differences between tumors formed from control cells and clone 8 cells (Fig. 5c). IHC staining for Ki-67 revealed lower numbers of nuclear staining-positive cells in clone 8 xenograft tumors, whereas cells staining for cleaved caspase 3 were higher in number, indicating more apoptosis in consistence with our in vitro observations (Fig. 5d). Levels of AhR protein in cell lines established from excised xenograft tumors showed no difference in AhR expression from pre-xenograft cells, demonstrating that the xenograft tumors retained their cell line expression of AhR (supplemental data 2a).
Figure 5.
AhR knockdown reduces orthotopic growth of MDA-MB-231cells in xenograft nude mice model. (a) Representative BLI images and (b) photon flux of xenograft tumors of mice injected orthotopically with control or clone 8 cells. (c) Representative H&E and immunohistochemical analysis of Ki67 and cleaved caspase-3 stained xenograft tumor sections. (d) Bar graph represents means ± s.d. of percentages of Ki67 (proliferating cells) and cleaved caspase-3 (apoptotic cells) positive stained cells. * p<0.05.
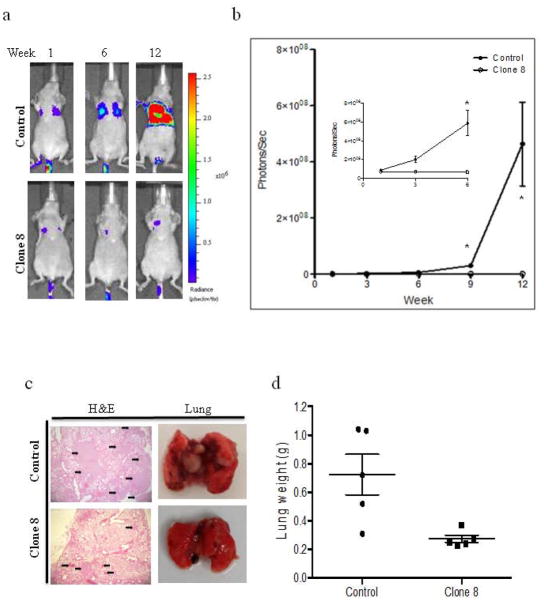
Figure 6.
AhR knockdown in MDA-MB-231cells reduces lung metastasis in nude mice experimental metastasis model. (a) Representative BLI images and (b) photon flux of lung metastasis of mice at indicated time points following intravenous injection with control or clone 8 cells. Results are expressed as means ± s.d. * p<0.05. (c) Representative H&E stained lung sections (left) and gross images of excised lungs (right) from the two groups. Arrows indicate micro-metastatic nodules. (d) At the termination of the experiment mice were sacrificed and lungs from each mouse were weighed. The mean lung weight was plotted for each group. Error bars represent 95% confidence intervals.
To examine whether AhR KD affects metastatic potential of MDA-MB-231 cells; cells were injected intravenously through the tail veins of athymic nude mice. As shown in Figure 6a both cell lines formed nodules; however AhR KD resulted in reduced nodule formation evident from BLI. As measured by ROI analysis of BLI images of lungs, normalized photon flux from clone 8 mice was significantly lower compared to control mice (Fig. 6b), demonstrating that AhR KD may affect extravasation and survival of circulating tumor cells. Visible metastatic nodules in the lungs of control mice were higher in number and size than those in clone 8 mice which resulted in increase of lung weights in control mice compared to clone 8 xenograft mice (Fig. 6c-d). In addition, visual inspection showed control cells produced nodules that occupied a higher percentage of the total lung area, while nodules of clone 8 cells produce discrete smaller dark foci. Metastases identified with BLI in vivo were confirmed in selected mice by ex vivo imaging of excised lungs (supplemental data 2b). No detectable macrometastases were observed on visual inspection at autopsy, in any of mice that didn’t show BLI. As control and clone 8 cells stably expressing luciferase have comparable levels of luciferase activity, these data indicate that AhR KD reduced tumor growth in mammary fat pads and experimental lung metastasis of MDA-MB-231 cells in athymic nude mice.
Discussion
In this study, both in vitro and in vivo analyses confirmed that AhR KD significantly decreased tumorigenic properties of MDA-MB-231 breast cancer cell line. AhR KD by approximately 80% in MDA-MB-231 attenuated cell proliferation, anchorage independent growth, migration and apoptosis in vitro and reduced xenograft tumor growth in orthotopic and experimental metastasis in vivo. AhR KD also led to enhanced sensitivity to both radiation and paclitaxel cytotoxicity. The enhanced sensitivity to both radiation and chemotherapeutic agents was mainly due to enhanced ability of cells to undergo apoptosis subsequent to AhR KD. Reports from our laboratory and others have supported the notion that AhR has oncogenic properties33–35. Using human breast cancer tissue microarrays, we found a strong association between AhR overexpression and advanced stages of invasive breast carcinomas and metastatic case types (unpublished data). In addition, our laboratory has shown previously that ectopic overexpression of AhR in HMEC results in malignant transformation; leading to cell cycle abrogation, increased proliferation, induction of the EMT, anchorage-independent growth and increased invasive potential26. These findings suggested that AhR drives the progression of breast cancer from an early, pre-malignant to malignant stages.
Therefore, it was intriguing to consider what affect the loss-of-function of AhR in an already advanced metastatic breast cancer cell line, would have on their malignant phenotypes. For this purpose we chose the triple negative metastatic HBC cell line, the MDA-MB-231, in which the AhR protein is overexpressed and constitutively active 30. The AhR functions as a regulator of cell cycle and apoptosis and the coordination and balance between cell proliferation and cell death plays an important role in cancer development 36–38. Stable AhR KD resulted in slowing down the cells’ proliferation rate, concomitant with a pronounced delay of their entry into S-phase and accumulation in G1 phase. These observations are in agreement with Safe’s group who reported growth inhibition in MCF7 HBC cells following treatment with AhR siRNA 39. This delay can be attributed to an increase in tumor suppressor RB expression and decrease in its phosphorylation (Figure 4). RB serves as a key restriction point switch by regulating cells transition from G1 to S phase and loss of function is often associated with poor prognosis in cancer patients. In addition to the inhibitory effect on proliferation, AhR KD resulted in an increase in apoptosis (Figure 2). Therefore, the slower growth of these cells can be attributed to inhibition of proliferation and an increase in apoptosis secondary to the lack of AhR, consistent with previous reports 40,38.
The apoptotic process is highly regulated by a balance between pro-apoptotic and anti-apoptotic genes; an imbalance of these genes can either promote or inhibit apoptosis. Bcl-2 family members (e.g., Bcl-xL and Bcl-2) are overexpressed in a number of cancers; functioning to inhibit apoptosis, whereas other members (e.g., Bak and Bad) which promote apoptosis are expressed at low levels. Bcl-2 family members are involved in regulating mitochondria integrity and deficiency in AhR led to mitochondrial dysfunction and increase in basal caspase 3 activity which resulted in apoptosis 41. We show here that AhR KD significantly increased expression of several pro-apoptotic genes, including BAD, BAK and BAX (Fig. 4). Initiator Caspases 8 and 9 and effector Caspase 3 gene expression also increased significantly, which suggests that both the mitochondria mediated (intrinsic) and death receptor mediated (extrinsic) pathways are activated, (reviewed in 42).
Both radiation and chemotherapy are important treatment modalities in breast cancer and clinical trials have demonstrated major benefits from their use43,44. Our present results demonstrated that AhR KD sensitizes MDA-MB-231 cells to radiation. It is noteworthy that independent of radiation, the cells with AhR KD showed a significantly higher margin of apoptosis and loss of clonogenic survival, underscoring the vast role of AhR as a direct and independent molecular target. A major obstacle for chemotherapeutic treatment and the primary cause of cancer chemotherapy failure is multi-drug resistance (MDR). MDR allows cancer cells to actively expel chemotherapeutic agents through transporters thereby maintaining intracellular drug concentrations below the cell-killing threshold 45. Paclitaxel, a chemotherapeutic agent used in the treatment of metastatic breast cancer is highly efficacious against breast cancer however; its dose-limiting toxicity and the development of drug resistance often limit its clinical potential. AhR KD sensitized MDA-MB-231 cells to paclitaxel, thereby improving the drug effectiveness and reduced concentration needed to induce cytotoxicity.
To gain insight into the possible mechanism by which AhR KD induced apoptosis we examined AKT activation, which modulates a number of signaling pathways. Deregulation of AKT signaling, resulting in enhanced AKT activation, is one of the most frequent changes in cancer, and is associated with poor prognosis and resistance to chemotherapeutic agents. Our results showed that AhR KD reduced AKT phosphorylation and increased its protein level in MDA-MB-231, consistent with previous reports indicating that lack of AhR leads to impaired activation of AKT 46.
Classic hallmarks of cancer include the ability of cancer cells to sustain chronic proliferation and metastasize. Metastasis is a multistep process that includes cancer cell entry and exit from circulation, and survival and proliferation at the distant site. AhR KD significantly decreased the metastatic potential of MDA-MB-231 cells, which can be attributed to the increased ability of cells to undergo apoptosis and decreased proliferation following AhR KD. This was clearly manifested as lower cellularity in the H&E staining, lower Ki67 staining and substantially higher activated caspase-3 staining of AhR KD cell xenografts.
In conclusion, the present study reveals a vital role for AhR in MDA-MB-231 cells and a potential mechanism by which AhR overexpression contributes to breast cancer. Our findings highlight the potential of targeting AhR for metastatic breast cancer therapy alone or in combination with radiation or chemotherapy. Our data identify AhR as being essential for breast tumor survival, progression, and metastasis, which is mediated, at least in part, by activation of the PI3K-AKT pathway.
Supplementary Material
Novelty and Impact.
In this work we tested the effect of suppressing the expression of AhR protein in a metastatic human breast cancer cell line that inherently expressed elevated levels of AhR. Results showed that suppressing AhR expression effectively reduced the growth and metastatic potential both in vitro and in vivo. The current study provides evidence for the feasibility of targeting AhR in the treatment of metastatic breast cancer alone, or in combination with ionizing radiation or chemotherapeutic agents.
Acknowledgments
We are grateful for the critical review of the manuscript by Drs. Ifeanyi Arinze and Diana Marver and the technical assistance of Ms. Rawia Abukalam. This research was supported by NIH grants G12RR003032 and SC1CA153326 to SEE. GG was supported by training grants: R25GM059994, T32HL007735, U24CA126588 and P30CA068485.
Abbreviations
- AhR
Aryl hydrocarbon receptor
- BLI
Bioluminescence imaging
- HMEC
human mammary epithelial cells
- HBC
human breast cancer
- IR
ionizing radiation
- KD
knockdown
- PAH
polyaromatic hydrocarbons
- TNBC
triple negative breast cancer
Footnotes
Conflict of Interest
The authors declare no conflict of interest
References
- 1.DeSantis C, Siegel R, Bandi P, et al. Breast cancer statistics, 2011. CA: a cancer journal for clinicians. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Alteri R, Bandi P, Brinton L, et al. Breast Cancer Facts & Figures 2011–2012. American Cancer Society; 2011. [Google Scholar]
- 3.Robinson BD, Sica GL, Liu YF, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:2433–2441. doi: 10.1158/1078-0432.CCR-08-2179. 1078-0432.CCR-08-2179 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blagosklonny MV. Why therapeutic response may not prolong the life of a cancer patient: selection for oncogenic resistance. Cell cycle. 2005;4:1693–1698. doi: 10.4161/cc.4.12.2259. 2259 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. 0092-8674(94)90518-5 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Parton M, Dowsett M, Smith I. Studies of apoptosis in breast cancer. Bmj. 2001;322:1528–1532. doi: 10.1136/bmj.322.7301.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Critical reviews in toxicology. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 9.Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annual review of pharmacology and toxicology. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 10.Pollenz RS, Sattler CA, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Molecular pharmacology. 1994;45:428–438. [PubMed] [Google Scholar]
- 11.Chen HS, Perdew GH. Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex. The Journal of biological chemistry. 1994;269:27554–27558. [PubMed] [Google Scholar]
- 12.Petrulis JR, Kusnadi A, Ramadoss P, et al. The hsp90 Co-chaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. The Journal of biological chemistry. 2003;278:2677–2685. doi: 10.1074/jbc.M209331200M209331200. [pii] [DOI] [PubMed] [Google Scholar]
- 13.Denison MS, Pandini A, Nagy SR, et al. Ligand binding and activation of the Ah receptor. Chemico-biological interactions. 2002;141:3–24. doi: 10.1016/s0009-2797(02)00063-7. S0009279702000637 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Smolowitz RM, Schultz ME, Stegeman JJ. Cytochrome P4501A induction in tissues, including olfactory epithelium, of topminnows (Poeciliopsis spp.) by waterborne benzo[a]pyrene. Carcinogenesis. 1992;13:2395–2402. doi: 10.1093/carcin/13.12.2395. [DOI] [PubMed] [Google Scholar]
- 15.Whitlock JP., Jr Induction of cytochrome P4501A1. Annual review of pharmacology and toxicology. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. The Journal of biological chemistry. 1999;274:28708–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 17.Dale YR, Eltom SE. Calpain mediates the dioxin-induced activation and down-regulation of the aryl hydrocarbon receptor. Molecular pharmacology. 2006;70:1481–1487. doi: 10.1124/mol.106.027474. [DOI] [PubMed] [Google Scholar]
- 18.Nebert DW, Dalton TP, Okey AB, et al. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. The Journal of biological chemistry. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200R400004200. [pii] [DOI] [PubMed] [Google Scholar]
- 19.Schlezinger JJ, Liu D, Farago M, et al. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biological chemistry. 2006;387:1175–1187. doi: 10.1515/BC.2006.145. [DOI] [PubMed] [Google Scholar]
- 20.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer research. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 21.Diry M, Tomkiewicz C, Koehle C, et al. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25:5570–5574. doi: 10.1038/sj.onc.1209553. 1209553 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Peng TL, Chen J, Mao W, et al. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009;10:27. doi: 10.1186/1471-2121-10-27. 1471-2121-10-27 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010;31:1319–1328. doi: 10.1093/carcin/bgq028. bgq028 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong PS, Li W, Vogel CF, et al. Characterization of MCF mammary epithelial cells overexpressing the Arylhydrocarbon receptor (AhR) BMC cancer. 2009;9:234. doi: 10.1186/1471-2407-9-234. 1471-2407-9-234 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Liu D, Murray TJ, et al. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24:7869–7881. doi: 10.1038/sj.onc.1208938. 1208938 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Brooks J, Eltom SE. Malignant transformation of mammary epithelial cells by ectopic overexpression of the aryl hydrocarbon receptor. Current cancer drug targets. 2011;11:654–669. doi: 10.2174/156800911795655967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virostko J, Radhika A, Poffenberger G, et al. Bioluminescence imaging in mouse models quantifies beta cell mass in the pancreas and after islet transplantation. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging. 2010;12:42–53. doi: 10.1007/s11307-009-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matveeva OV, Kang Y, Spiridonov AN, et al. Optimization of duplex stability and terminal asymmetry for shRNA design. PloS one. 2010;5:e10180. doi: 10.1371/journal.pone.0010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. nprot.2007.30 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Eltom SE, Gasmelseed AA, Guentri DS. The aryl hydrocarbon receptor is over-expressed and constitutively activated in advanced breast carcinoma. Proc Amer Assoc Cancer Res. 2006;1730 [Google Scholar]
- 31.Hayashibara T, Yamada Y, Mori N, et al. Possible involvement of aryl hydrocarbon receptor (AhR) in adult T-cell leukemia (ATL) leukemogenesis: constitutive activation of AhR in ATL. Biochemical and biophysical research communications. 2003;300:128–134. doi: 10.1016/s0006-291x(02)02793-6. S0006291X02027936 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Chang CY, Puga A. Constitutive activation of the aromatic hydrocarbon receptor. Molecular and cellular biology. 1998;18:525–535. doi: 10.1128/mcb.18.1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson P, McGuire J, Rubio C, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9990–9995. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moennikes O, Loeppen S, Buchmann A, et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer research. 2004;64:4707–4710. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 35.McGuire J, Okamoto K, Whitelaw ML, et al. Definition of a dioxin receptor mutant that is a constitutive activator of transcription: delineation of overlapping repression and ligand binding functions within the PAS domain. The Journal of biological chemistry. 2001;276:41841–41849. doi: 10.1074/jbc.M105607200. [DOI] [PubMed] [Google Scholar]
- 36.Marlowe JL, Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. Journal of cellular biochemistry. 2005;96:1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- 37.Marlowe JL, Fan Y, Chang X, et al. The aryl hydrocarbon receptor binds to E2F1 and inhibits E2F1-induced apoptosis. Molecular biology of the cell. 2008;19:3263–3271. doi: 10.1091/mbc.E08-04-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elizondo G, Fernandez-Salguero P, Sheikh MS, et al. Altered cell cycle control at the G(2)/M phases in aryl hydrocarbon receptor-null embryo fibroblast. Molecular pharmacology. 2000;57:1056–1063. [PubMed] [Google Scholar]
- 39.Abdelrahim M, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Molecular pharmacology. 2003;63:1373–1381. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez FJ, Fernandez-Salguero P. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug metabolism and disposition: the biological fate of chemicals. 1998;26:1194–1198. [PubMed] [Google Scholar]
- 41.Baglole CJ, Maggirwar SB, Gasiewicz TA, et al. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. The Journal of biological chemistry. 2008;283:28944–28957. doi: 10.1074/jbc.M800685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elmore S. Apoptosis: a review of programmed cell death. Toxicologic pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McArdle CS, Crawford D, Dykes EH, et al. Adjuvant radiotherapy and chemotherapy in breast cancer. Br J Surg. 1986;73:264–266. doi: 10.1002/bjs.1800730407. [DOI] [PubMed] [Google Scholar]
- 44.Hiley C, Tutt A, Torres M, et al. Adjuvant radiotherapy for breast cancer. Bmj. 2008;337:a2843. doi: 10.1136/bmj.a2843. [DOI] [PubMed] [Google Scholar]
- 45.Ullah MF. Cancer multidrug resistance (MDR): a major impediment to effective chemotherapy. Asian Pacific journal of cancer prevention: APJCP. 2008;9:1–6. [PubMed] [Google Scholar]
- 46.Wu R, Zhang L, Hoagland MS, et al. Lack of the aryl hydrocarbon receptor leads to impaired activation of AKT/protein kinase B and enhanced sensitivity to apoptosis induced via the intrinsic pathway. The Journal of pharmacology and experimental therapeutics. 2007;320:448–457. doi: 10.1124/jpet.106.111773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.