Abstract
The aim of this paper is to explore the efficacy of whole brain radiotherapy (WBRT) versus WBRT concurrent with erlotinib in patients with multiple brain metastases of lung adenocarcinoma. WBRT was administered at 30Gy/10f in both arms. In the combination arm, 150 mg erlotinib was given each day, starting the first day of radiotherapy and continuing for 1 month following the end of radiotherapy. Thereafter, pemetrexed or docetaxel monotherapy or the best supportive therapy was given to both arms. The intracranial objective response rate and the local progression-free survival (LPFS) were primary endpoints. Toxicity, progression-free survival (PFS) and overall survival (OS) were secondary endpoints. Thirty-one patients in the WBRT group and 23 patients in the combination group were enrolled from November 2009 to December 2011. In the WBRT and the combination arms, respectively, the objective response rate was 54.84% and 95.65% (P = 0.001), the median local progression-free survival was 6.8 months and 10.6 months (P = 0.003), the median PFS was 5.2 months and 6.8 months (P = 0.009), and median OS was 8.9 months and 10.7 months (P = 0.020). In the combination group, there were no differences of LPFS, PFS, and OS between the epidermal growth factor receptor (EGFR) mutation patients and EGFR wild-type patients. No Grade 4 or higher side effects were observed in either group. A multivariate analysis indicated that erlotinib was the most important prognostic factor for a prolonged survival. Data showed that erlotinib in combination with WBRT had a tolerable toxicity profile and prolonged the LPFS, PFS, and OS of lung adenocarcinoma patients with multiple brain metastases compared with WBRT monotherapy.
Keywords: WBRT, tyrosine kinase inhibitor, radiosensitizer, erlotinib
Introduction
Whole brain radiotherapy (WBRT) is the standard treatment for patients with multiple brain metastases.1,2 It is theoretically reasonable to treat lung adenocarcinoma patients with multiple brain metastases with erlotinib, and preliminary clinical results demonstrated the efficacy of erlotinib in lung adenocarcinoma patients with multiple brain metastases.3–7 However, in most of these studies, WBRT and erlotinib were not combined, and there have been few clinical trials focusing on lung adenocarcinoma patients, of which only some were classified as prospective controlled studies. The difference in efficacy of erlotinib in combination with WBRT versus WBRT monotherapy has not been clearly defined. Patients with lung adenocarcinoma often suffer from brain metastasis. Erlotinib has been widely used in clinical practice. Therefore, clinical studies are necessary to provide data to support the treatment of lung adenocarcinoma patients with multiple brain metastases with the combination of WBRT and erlotinib. In this prospective cohort study, we compared the efficacy of WBRT versus erlotinib in combination with WBRT in lung adenocarcinoma patients with multiple brain metastases. The results will be of great clinical significance for the treatment of lung adenocarcinoma patients with multiple brain metastases.
Patient evaluation
All enrolled patients were required to sign a study-specific informed consent, and Tianjin Medical University Cancer Institute and Hospital ethical requirements were met. Pretreatment evaluations included a complete patient history, physical examination, electrocardiogram, upper abdomen and neck ultrasound B, bone scan, and brain magnetic resonance imaging (MRI) or intensified computed tomography (CT; for patients who could not undergo an MRI due to internal metal objects). Laboratory evaluations included hematology, renal and hepatic function analysis, and measurements of blood glucose, electrolytes, and lung cancer markers. The 6th edition American Joint Committee on Cancer tumor-nodes-metastasis staging system was used for disease staging. If a patient had received epidermal growth factor receptor (EGFR) mutation status testing (but did not necessarily have an EGFR mutation), he/she received WBRT concurrent with erlotinib; if the EGFR mutation status was unknown, he/she received WBRT monotherapy. For this reason, the number of patients in the two groups differed. The mutation detection included 18,19,20,21 exons of EGFR, and k-ras mutation. The direct sequencing method was used for mutation detection. The tissue for detection was from the primary tumors. The criteria for inclusion, exclusion, removal, and discontinuation are summarized in Table 1.
Table 1.
Eligibility criteria, exclusion criteria, rejection criteria, and suspension criteria
| WBRT group | WBRT + erlotinib group | |
|---|---|---|
| Eligibility criteria | Lung adenocarcinoma | Equal to WBRT group |
| First-line chemotherapy failure Multiple brain metastases diagnosed by CT/MRI RPA class 1 or 2 | Having EGFR mutation detection | |
| Expected survival time ≧6 months Over 4 weeks after blood-brain barrier-crossing cytotoxic drugs medication | ||
| Hb ≧ 90; granulocyte count ≧ 1.5 × 109/L; Platelet count ≧ 100 × 109/L; Serum bilirubin ≦1.5 × ULN; AST and/or ALT ≦2× ULN; Serum creatinine ≦1.5 × ULN | ||
| Exclusion criteria | Double or multiple primary cancer | Equal to WBRT group |
| Presence of unstable systemic disease RPA class 3 | Unable to take erlotinib | |
| Leptomeningeal metastases | ||
| Patient is unable to complete WBRT | ||
| Treated with SRS after WBRT | ||
| Previous use of TKI | ||
| Rejection criteria | Taking blood–brain barrier-crossing cytotoxic drugs in the period of WBRT or follow-up | |
| Application of stereotactic radiotherapy in follow-up or WBRT | ||
| Not following the implementation of this program | ||
| Suspension criteria | Patient requirement | |
| Serious adverse reactions | ||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; EGFR, epidermal growth factor receptor; Hb, hemoglobin; MRI, magnetic resonance imaging; RPA, recursive partitioning analysis; SRS, stereotaxic radiosurgery; TKI, tyrosine kinase inhibitors; ULN, upper limit of normal; WBRT, whole brain radiotherapy.
This study was approved by Tianjin Medical University Cancer Institute and Hospital ethics committee. All clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. All participants provided written consent from themselves and their family members to participate in this study. A treating physician, a hospital ethics committee staff member, and a medical record department staff member were present at follow-up to document the process. Confidentiality of records was always respected.
Study design and treatment plan
Lung adenocarcinoma patients who progressed after first-line chemotherapy and had multiple brain metastases diagnosed by imaging were enrolled in the study. WBRT was prescribed at a dose of 30 Gy/10f in both arms for 5 days per week, up to 2 weeks. In the combination arm, 150 mg erlotinib was administered once daily from the first day of WBRT to a month after the end of radiotherapy. Patients in both groups were visited 1 month after the end of WBRT; subsequent treatment decisions were based on changes in physical status and symptoms. If the performance status (PS) score was ≤1, the patient received pemetrexed or docetaxel monotherapy. The dose was adjusted based on the patient’s physical status. If the PS score was ≥2, the patient received the best supportive care.
Follow-up
All patients were closely monitored for toxicity. To evaluate the response, all patients received a whole-body examination 1 month after WBRT and once every 2–3 months thereafter. At each follow-up, a detailed medical history, physical examination, electrocardiogram, chest CT, upper abdomen and neck ultrasound B, bone scan, and brain MRI or intensified CT (for patients who could not receive an MRI due to internal metal objects) were obtained. Laboratory evaluations included hematology, renal and hepatic function analysis, and measurements of blood glucose, electrolytes, and carcinoembryonic antigen. Common Terminology Criteria for Adverse Events version 3.08,9 was used to assess toxicities, and Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.0 was used to evaluate the tumor response.10,11
Endpoints and statistical analysis
In this study, the intracranial objective response rate (ORR) and local progression-free survival (LPFS) were primary endpoints. Toxicity, progression-free survival (PFS) and overall survival (OS) were secondary endpoints. ORR was based on the best response measured according to the RECIST 1.0. LPFS was defined as the time from treatment initiation to the first occurrence of intracranial progression or death. PFS was defined as the time from treatment initiation to the first occurrence of disease progression or death. OS was defined as the time from treatment initiation to the date of death from any cause. In estimating the PFS and the OS, if progression was observed, the patient was censored at the date of the last progression-free tumor assessment. Statistical Product and Service Solutions version 17 (IBM Corporation, Armonk, NY, USA) was utilized to perform independent t-tests to compare patient characteristics and toxicities. The direct method was used to calculate the survival rate, and Kaplan–Meier survival curves were generated. A Cox regression analysis of prognostic factors was utilized for single and multiple factor tests. A linear correlation analysis was used to identify correlations between variables.
Results
Patient characteristics
From November 2009 to December 2011, 31 patients were included in the WBRT group, and 23 patients entered the combination group. Among the patients in the combination group, 12 patients had no exons mutations; eleven patients had 19/21 exons mutations, and no patients had k-ras mutations. All enrolled patients were treated with the allocated regimens and received follow-up evaluations. The follow-up rate was 100%. In the WBRT arm, 19 patients received the consecutive chemotherapy, while 12 patients had no further chemotherapy due to poor physical condition. In the combination arm, 14 patients received consecutive chemotherapy and nine patients had no further chemotherapy (P > 0.05, compared with WBRT arm). The baseline characteristics of enrolled patients are summarized in Table 2.
Table 2.
Patient and tumor characteristics
| Characteristic | WBRT group | WBRT + erlotinib group |
|---|---|---|
| Cases | 31 | 23 |
| Sex | ||
| Female | 18 (58) | 13 (57) |
| Male | 13 (42) | 10 (43) |
| Age (y) | ||
| Range | 43–81 | 37–76 |
| Median | 63 | 60 |
| PRA class | ||
| 1 | 3 (10) | 2 (9) |
| 2 | 29 (90) | 21 (91) |
| KPS score | ||
| Range | 70–100 | 70–100 |
| Median | 80 | 80 |
| Initial stage (cases) | ||
| I | 2 (7) | 1 (4) |
| II | 8 (26) | 6 (26) |
| III | 11 (35) | 8 (35) |
| IV | 10 (32) | 8 (35) |
| EGFR status | ||
| EGFR mutation | – | 11 (48) |
| EGFR wild-type | – | 12 (52) |
| EGFR unknown | 31 (100) | 0 (0) |
| Previous therapy | ||
| Chemoradiotherapy only | 16 (52) | 11 (48) |
| Chemoradiotherapy + surgery/surgery + chemoradiotherapy | 15 (48) | 12 (52) |
| Tumor control before WBRT | ||
| CR | 0 (0) | 0 (0) |
| PR | 5 (16) | 3 (13) |
| SD | 11 (36) | 9 (39) |
| PD | 15 (48) | 11 (48) |
| Brain metastases | ||
| Range | 2–12 | 2–35 |
| Median | 4 | 4 |
| Size of largest brain metastasis (cm) | ||
| Range | 0.70–4.10 | 0.80–4.70 |
| Median | 2.10 | 2.20 |
| Extracranial metastasis or uncontrolled primary tumor | ||
| Yes | 26 (84) | 21 (91) |
| No | 5 (26) | 2 (9) |
| Consecutive chemotherapy | ||
| Yes | 19 (61) | 14 (61) |
| No | 12 (39) | 9 (39) |
Notes: Data presented as number of patients, with percentages in parentheses. P > 0.05 comparing all the characteristics between the two groups.
Abbreviations: CR, complete response; EGFR, epidermal growth factor receptor; KPS, Karnofsky Performance scale; PD, progressive disease; PR, partial response; PRA, recursive partitioning analysis; SD, stable disease; WBRT, whole brain radiotherapy; y, year.
Acute toxicity and treatment compliance
Acute toxicities are summarized in Table 3. Regarding the skin, gastrointestinal tract, eyes, headache, nausea, respiratory system, hematology, and hepatic and renal function, no side effects of Grade 4 or higher were observed. The following side effects were more prevalent in the combination group than in the WBRT group: dry skin, nail change, skin pruritus, rash, anorexia and nausea; the incidence of these side effects was statistically significantly different (P < 0.05). However, all these side effects were well tolerated, and no patients required an erlotinib dose reduction. There was no difference in late neurotoxicity between the two arms.
Table 3.
Treatment-related toxicity
| NCI-CTC adverse event | Grade (cases: WBRT/WBRT +erlotinib)
|
|||||
|---|---|---|---|---|---|---|
| Any | 1 | 2 | 3 | 4 | 5 | |
| Constitutional symptoms | ||||||
| Acratia | 15/12 | 12/9 | 3/3 | |||
| Fatigue | 3/3 | 3/3 | ||||
| Weight loss | 13/12 | 11/10 | 2/2 | |||
| Dermatology | ||||||
| Cheilitis | 0/0 | 1/9 | ||||
| Dry skin | 1/9* | 0/3 | 0/1 | |||
| Nail changes | 0/4* | 0/9 | ||||
| Pruritus/itching | 0/9* | 0/9 | 0/4 | |||
| Rash | 0/13* | 4/5 | ||||
| Radiation dermatitis | 4/5 | |||||
| Gastrointestinal | ||||||
| Anorexia | 8/13* | 2/5 | 2/6 | 0/2 | ||
| Constipation | 8/10 | 8/8 | 0/2 | |||
| Diarrhea | 1/3 | 1/3 | ||||
| Dyspepsia | 1/3 | 1/3 | ||||
| Nausea | 7/11* | 6/7 | 1/4 | |||
| Vomiting | 5/6 | 1/4 | 0/2 | |||
| Mucositis | 1/1 | 1/1 | ||||
| Stomatitis | 1/2 | 1/2 | ||||
| Taste alteration | 3/3 | 3/3 | ||||
| Eyes | ||||||
| Xerophthalmia | 0/0 | |||||
| Conjunctivitis | 0/0 | |||||
| Headache | 6/5 | 5/1 | 1/4 | |||
| Dizziness | 6/11* | 5/10 | 0/0 | 1/1 | ||
| Pulmonary/upper respiratory | ||||||
| Cough | 5/8 | 5/7 | 0/1 | |||
| Dyspnea | 0/0 | |||||
| Pneumonitis | 7/7 | 7/7 | ||||
| Blood | ||||||
| Hemoglobin | 2/5 | 2/5 | ||||
| Leucocyte | 3/3 | 3/3 | ||||
| Platelet | 2/2 | 2/2 | ||||
| Liver/renal function | ||||||
| AST | 2/4 | 2/2 | 0/2 | |||
| ALT | 2/3 | 2/3 | ||||
| Bilirubin | 1/0 | 1/0 | ||||
| Serum creatinine | 2/2 | 2/2 | ||||
Note:
P < 0.05.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; NCI-CTC, National Cancer Institute-Common Toxicity Criteria; WBRT, whole brain radiotherapy.
Control of brain metastases
The intracranial control results were better in the combination group than in the WBRT monotherapy group. Based on the best intracranial efficacy, the WBRT monotherapy group had an ORR of 54.84% (CR [complete response]: 12.9%, PR [partial response]: 41.94%, SD [stable disease]: 38.71%, PD [progressive disease]: 6.45%), and the combination group had an ORR of 95.65% (CR: 34.78%, PR: 60.87%, SD: 4.35%, PD: 0.00%). During follow-up, only two patients (8.70%) in the combination group experienced local progression more than 1 year after treatment, and the WBRT monotherapy group had a local progression rate of 41.94% (13 cases) within 1 year of treatment.
Survival
The median LPFS, PFS, and OS were: 6.8 months (range, 0–18.7 months), 5.2 months (range, 0–14.7 months) and 8.9 months (range, 4.5–19.7 months), respectively, in the WBRT group; and 10.6 months (range, 4.9–20.7 months), 6.8 months (range, 1.5–20.7 months) and 10.7 months (range, 5.3–29.7 months), respectively, in the combination group. The combination group had significantly higher survival rates at 6 months (91.30% versus 83.87%), 1 year (34.78% versus 6.45%), and 2 years (4.35% versus 0.00%) than the WBRT group. There were statistically significant differences in the LPFS, PFS, and OS between the groups (Figure 1). In the combination group, there were no differences in LPFS, PFS, and OS between the EGFR mutation patients and EGFR wild-type patients (Figure 2).
Figure 1.
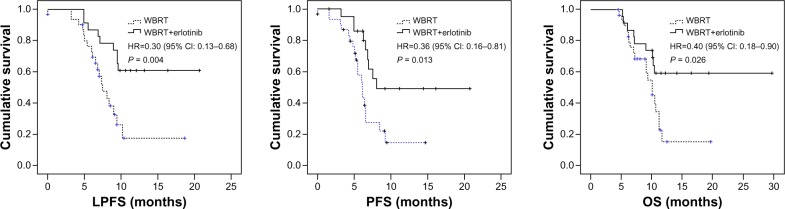
The LPFS, PFS, and OS of WBRT group versus WBRT+erlotinib group.
Notes: The median LPFS, PFS, and OS were 6.8 months (range, 0–18.7 months), 5.2 months (range, 0–14.7 months), and 8.9 months (range, 4.5–19.7 months), respectively, in the WBRT group and 10.6 months (range, 4.9–20.7 months), 6.8 months (range, 1.5–20.7 months), and 10.7 months (range, 5.3–29.7 months), respectively, in the combination group. There were statistically significant differences in the LPFS, PFS, and OS between the groups.
Abbreviations: CI, confidence interval; HR, hazard ratio; LPFS, local progression-free survival; OS, overall survival; PFS, progression-free survival; WBRT, whole brain radiotherapy.
Figure 2.
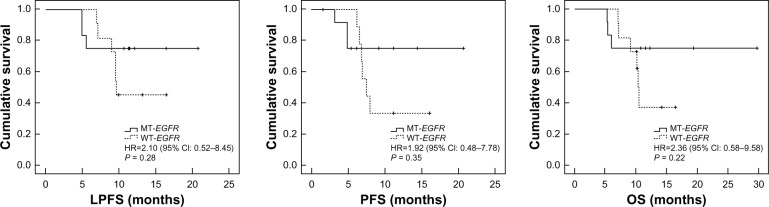
The LPFS, PFS, and OS of EGFR mutation patients versus EGFR wild-type patients in the combination arm.
Notes: In the combination group, 12 patients had no exons mutations; 11 patients had 19/21 exons mutations, and no patients had k-ras mutations. The median LPFS, PFS, and OS were 9.6 months (range, 6.9–16.4 months), 7.5 months (range, 6.2–16.1 months), and 10.2 months (range, 7.1–16.5 months), respectively, in the EGFR wild-type group and 11.2 months (range, 4.9–20.7 months), 6.1 months (range, 3.2–20.7 months), and 11.5 months (range, 5.3–29.7 months), respectively, in the EGFR mutation group. There were no statistically significant differences in the LPFS, PFS, and OS between the two groups.
Abbreviations: CI, confidence interval; HR, hazard ratio; LPFS, local progression-free survival; MT-EGFR, mutation-type EGFR patients; OS, overall survival; PFS, progression-free survival; WT-EGFR, wild-type EGFR patients; EGFR, epidermal growth factor receptor.
Cox regression/analysis of survival factors
Univariate and multivariate analyses were performed to identify the effects of the following factors on LPFS: age, sex, Karnofsky Performance Scale (KPS), the presence or absence of extracranial metastasis or uncontrolled primary lesions, the maximum diameter of the intracranial lesion, the number of intracranial lesions, and the use of tyrosine kinase inhibitors (TKIs) or chemotherapy. Univariate and multivariate analyses were performed to identify the effects of the following factors on PFS and OS: age; sex; KPS; the presence or absence of extracranial metastasis or uncontrolled primary lesions; the presence or absence of intracranial progression; and the use of TKIs or chemotherapy. P < 0.05 in the univariate analysis was the criteria for inclusion in the multivariate analysis. These analyses indicated that erlotinib had a statistically significant effect on LPFS, PFS, and OS, with a greater effect on LPFS than on PFS or OS (the multivariate analysis for LPFS had the highest chi square value) (Table 4).
Table 4.
Results of multivariate Cox regression analysis for LPFS, PFS, and OS
| Factor | Regression coefficient | Wald value | P value | RR value | 95% CI |
|---|---|---|---|---|---|
| LPFS | |||||
| Size of largest brain metastasis | 0.764 | 7.566 | 0.006 | 2.146 | 1.245–3.698 |
| Extracranial metastasis or uncontrolled primary tumor | 1.566 | 5.377 | 0.020 | 4.788 | 1.274–17.990 |
| Erlotinib medication | −2.094 | 13.882 | 0.000 | 0.123 | 0.041–0.371 |
| Chemotherapy | −1.123 | 6.001 | 0.014 | 0.325 | 0.132–0.799 |
| PFS | |||||
| Extracranial metastasis or uncontrolled primary tumor | 3.094 | 11.882 | 0.001 | 22.062 | 3.799–128.124 |
| Erlotinib medication | −1.297 | 7.191 | 0.007 | 0.273 | 0.106–0.705 |
| OS | |||||
| Extracranial metastasis or uncontrolled primary tumor | 1.332 | 4.145 | 0.042 | 3.787 | 1.051–13.647 |
| Erlotinib medication | −1.012 | 3.843 | 0.050 | 0.364 | 0.132–1.000 |
| Chemotherapy | −1.296 | 8.605 | 0.003 | 0.274 | 0.115–0.650 |
Abbreviations: LPFS, local progression-free survival; CI, confidence interval; OS, overall survival; PFS, progression-free survival; RR, response rate.
Discussion
This study revealed that erlotinib in combination with WBRT had a tolerable toxicity profile in lung adenocarcinoma patients with multiple brain metastases. Compared with WBRT monotherapy, the combination of erlotinib and WBRT had a significantly greater intracranial efficacy and prolonged PFS and OS in adenocarcinoma patients with multiple brain metastases.
Two aspects regarding the study design should be discussed. No chemotherapy was provided during radiotherapy or for 1 month after radiotherapy in the WBRT monotherapy group for several reasons. First, in patients with multiple brain metastases who are receiving WBRT, concomitant chemotherapy might be poorly tolerated. Second, the second-line cytotoxic drugs (docetaxel or pemetrexed) for lung adenocarcinoma have poor permeability across the blood–brain barrier. During the initial period of radiotherapy, if chemotherapy is used in combination with WBRT, these drugs will not affect the intracranial metastases because the blood–brain barrier is intact. During the later periods of radiotherapy, patients may have serious adverse reactions from the radiotherapy, and concomitant chemotherapy will be poorly tolerated. Third, studies on teniposide and other drugs have demonstrated that patients did not benefit from chemotherapy in combination with WBRT.12–14 Additionally, chemotherapy should be repeated at intervals; therefore, it cannot be combined because erlotinib and radiotherapy are given continuously. In the clinic, patients are evaluated 1 month after WBRT (considering the cell death during the proliferative phase after radiotherapy), and subsequent systemic therapy may be prescribed. This is the current paradigm for treating patients with multiple brain metastases. In the combination group, erlotinib was coadministered with the radiotherapy and continued for 1 month after radiotherapy based on the following considerations. First, LPFS was one of the primary endpoints of the study. Considering the damage WBRT inflicts on the blood–brain barrier and its process of repair as well as the tumor cell death in the proliferative phase after radiotherapy, the periods of time during and for 1 month after radiotherapy are the most critical for coadministering erlotinib. Second, it would be inconsistent with current guidelines, and therefore unethical, to initiate erlotinib treatment at the beginning of WBRT and continue until the disease progressed but give nothing after radiotherapy to patients in the WBRT monotherapy group.
The efficacy in the combination group might result from the actions of both erlotinib and WBRT. Erlotinib has good permeability across the blood–brain barrier,15,16 and WBRT induces maximal damage to the blood–brain barrier,17–19 which can further increase the intracranial erlotinib concentration. Unlike chemotherapy, erlotinib is dosed daily, which may provide a greater opportunity for success in combination with WBRT. Additionally, erlotinib enhances the effects of radiation, which provides adequate justification for its concomitant use with WBRT. Therefore, the combination of erlotinib and WBRT is reasonable.20,21
This study confirmed that the combination of erlotinib and WBRT is safe, and made a preliminary but beneficial exploration into the difference in efficacy between WBRT monotherapy and the concurrent regimen of erlotinib and WBRT. Based on the ORR and local failure rates, the local efficacy was confirmed in the combination group, and there was a statistically significant difference in the LPFS curve between the groups. The observed toxicities also confirmed the features of erlotinib in combination with WBRT. More necrosis is expected to occur in the combination group. Therefore, there would be more serious reactions to the cerebral edema. The systemic benefits of erlotinib were refected in the PFS and OS curves. There were statistically significant differences between the combination and the WBRT monotherapy groups. There was a statistically significant effect of erlotinib on PFS and OS based on the univariate and multivariate analyses. There was a statistically significant difference in the LPFS. The LPFS was longer than the PFS, but the chi square value for LPFS was greater, potentially because of the TKI-mediated enhancement of the radiation effect in the combination therapy. Most studies have demonstrated that TKIs prolong PFS rather than OS. In this study, however, erlotinib prolonged OS. The prolonged OS might stem from erlotinib itself (an additional treatment compared with the WBRT monotherapy group) and could result from superior intracranial lesion control and the subsequent chemotherapy given to patients in the erlotinib and WBRT combination group (multivariate analysis). Based on our experience and the characteristics of patients with brain metastases, if good intracranial control is achieved, patients are more likely to receive chemotherapy after radiotherapy. If patients have poor intracranial control, poor physical performance, and severe brain metastases, they may not be able to receive subsequent chemotherapy. Chemotherapy is sometimes given to patients with intracranial disease progression, but it is not standardized and cannot be continuously administered. Additionally, second-line erlotinib treatment and single-agent chemotherapy have similar efficacy.22 If erlotinib is administered from the beginning of radiotherapy until systemic progression occurs, rather than for only 1 month after radiotherapy, erlotinib may further improve PFS and OS in patients ineligible for chemotherapy because of poor physical performance. Although it is clear that EGFR mutation has influence on the effect of erlotinib, in this study, the EGFR status had no influence on the survival in combination groups. This result might be due to the drug administration time and the small sample of the study.
Further research is needed on the influence of EGFR mutations on the combination of erlotinib with radiotherapy. The impact of mutations on the effect of radiotherapy, and the impact of radiotherapy on mutations, is unclear. Radiotherapy has an important effect on the chromosomes, and it is an important mutagenic factor. Only a few trials have focused on this topic; the sample sizes of the trials studying WBRT combined with erlotinib were small, and some were case reports. This question warrants further research.
Compared with previous studies,6,23 this was a prospective single-arm study that compared erlotinib and WBRT combination therapy with WBRT monotherapy in patients with lung adenocarcinoma. This study determined the advantages of erlotinib/WBRT combination therapy compared with WBRT monotherapy in managing intracranial lesions, enhancing the local efficacy of erlotinib and its effect on overall survival, and on the KPS, extracranial metastases, chemotherapy, and other factors that influence survival. These results are relevant to the management of lung adenocarcinoma patients with multiple brain metastases. There are many second-line therapeutic options for lung adenocarcinoma, but if patients progress and have multiple brain metastases after first-line treatment, this study suggests that erlotinib may be superior when combined with WBRT for local lesion management. This strategy may provide specific guidelines for the second-line treatment of lung adenocarcinoma and therefore is clinically significant. A previous paper24 has demonstrated that erlotinib had a good objective response rate and survival time in patients with brain metastases of lung adenocarcinoma. However, the patients treated with erlotinib alone for brain metastasis often had either none or mild symptoms of metastasis. If a brain metastasis patient had serious symptoms, he/she was typically treated with WBRT. The differences are often obvious between the samples in different trials; therefore, the survival time of the patients may have obvious differences between different clinical trials.
In summary, erlotinib/WBRT combination therapy for the treatment of lung adenocarcinoma patients with multiple brain metastases has a reasonable theoretical basis. This study demonstrated that erlotinib in combination with WBRT significantly improved intracranial lesion control and prolonged LPFS, PFS, and OS compared with WBRT monotherapy. The combination of erlotinib and WBRT provides a superior option for the treatment of lung adenocarcinoma patients with multiple brain metastases. We believe that the combination of erlotinib and WBRT has a promising future for the treatment of patients with metastatic lung cancer in the brain.
Acknowledgments
This research was funded by the Tianjin Health Bureau Science and Technology Development Fund (2012KZ066).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tsao MN, Lloyd N, Wong R, Chow E, Rakovitch E, Laperriere N. Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database Syst Rev. 2006;19(3):CD003869. doi: 10.1002/14651858.CD003869.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Kawabe T, Phi JH, Yamamoto M, Kim DG, Barfod BE, Urakawa Y. Treatment of brain metastasis from lung cancer. Prog Neurol Surg. 2012;25:148–155. doi: 10.1159/000331188. [DOI] [PubMed] [Google Scholar]
- 3.Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65(3):351–354. doi: 10.1016/j.lungcan.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Gounant V, Wislez M, Poulot V, et al. Subsequent brain metastasis responses to epidermal growth factor receptor tyrosine kinase inhibitors in a patient with non-small-cell lung cancer. Lung Cancer. 2007;58(3):425–428. doi: 10.1016/j.lungcan.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Olmez I, Donahue BR, Butler JS, Huang Y, Rubin P, Xu Y. Clinical outcomes in extracranial tumor sites and unusual toxicities with concurrent whole brain radiation (WBRT) and Erlotinib treatment in patients with non-small cell lung cancer (NSCLC) with brain metastasis. Lung Cancer. 2010;70(2):174–179. doi: 10.1016/j.lungcan.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Lind JS, Lagerwaard FJ, Smit EF, Senan S. Phase I study of concurrent whole brain radiotherapy and erlotinib for multiple brain metastases from non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1391–1396. doi: 10.1016/j.ijrobp.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Yi HG, Kim HJ, Kim YJ, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009;65(1):80–84. doi: 10.1016/j.lungcan.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 9.Palazzi M, Tomatis S, Orlandi E, et al. Effects of treatment intensification on acute local toxicity during radiotherapy for head and neck cancer: prospective observational study validating CTCAE, version 3.0, scoring system. Int J Radiat Oncol Biol Phys. 2008;70(2):330–337. doi: 10.1016/j.ijrobp.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Sun JM, Ahn MJ, Park MJ, et al. Accuracy of RECIST 1.1 for non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors. Lung Cancer. 2010;69(1):105–109. doi: 10.1016/j.lungcan.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Robinet G, Thomas P, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):59–67. doi: 10.1023/a:1008338312647. [DOI] [PubMed] [Google Scholar]
- 13.Cortot AB, Gerinière L, Robinet G, et al. Groupe Lyon-Saint-Etienne d’Oncologie Thoracique. Groupe Français de Pneumo-Cancérologie Phase II trial of temozolomide and cisplatin followed by whole brain radiotherapy in non-small-cell lung cancer patients with brain metastases: a GLOT-GFPC study. Ann Oncol. 2006;17(9):1412–1417. doi: 10.1093/annonc/mdl146. [DOI] [PubMed] [Google Scholar]
- 14.Guerrieri M, Wong K, Ryan G, Millward M, Quong G, Ball DL. A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lung. Lung Cancer. 2004;46(1):107–111. doi: 10.1016/j.lungcan.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5(7):950–955. doi: 10.1097/JTO.0b013e3181e2138b. [DOI] [PubMed] [Google Scholar]
- 16.Broniscer A, Panetta JC, O’Shaughnessy M, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420. Clin Cancer Res. 2007;13(5):1511–1515. doi: 10.1158/1078-0432.CCR-06-2372. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor MM, Mayberg MR. Effects of radiation on cerebral vasculature: a review. J Neurosurgery. 2000;46(1):138–149. doi: 10.1093/neurosurgery/46.1.138. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson F. Molecular biology of mutagenesis of mammalian cells by ionizing radiation. Semin Cancer Biol. 1993;4(2):85–92. [PubMed] [Google Scholar]
- 19.Harms-Ringdahl M, Nicotera P, Radford IR. Radiation induced apoptosis. Mutat Res. 1996;366(2):171–179. doi: 10.1016/s0165-1110(96)90038-x. [DOI] [PubMed] [Google Scholar]
- 20.Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer Res. 2005;65(8):3328–3335. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 21.Halatsch ME, Löw S, Mursch K, et al. Candidate genes for sensitivity and resistance of human glioblastoma multiforme cell lines to erlotinib. Laboratory investigation. J Neurosurg. 2009;111(2):211–218. doi: 10.3171/2008.9.JNS08551. [DOI] [PubMed] [Google Scholar]
- 22.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13(3):300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 23.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37(3):624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]


