Abstract
Background
Stress-related disorders (e.g., depression) are associated with hypothalamic-pituitary-adrenocortical axis dysregulation and prefrontal cortex (PFC) dysfunction, suggesting a functional link between aberrant prefrontal corticosteroid signaling and mood regulation.
Methods
We used a virally mediated knockdown strategy (short hairpin RNA targeting the glucocorticoid receptor [GR]) to attenuate PFC GR signaling in the rat PFC. Adult male rats received bilateral microinjections of vector control or short hairpin RNA targeting the GR into the prelimbic (n = 44) or infralimbic (n = 52) cortices. Half of the animals from each injection group underwent chronic variable stress, and all were subjected to novel restraint. The first 2 days of chronic variable stress were used to assess depression- and anxiety-like behavior in the forced swim test and open field.
Results
The GR knockdown confined to the infralimbic PFC caused acute stress hyper-responsiveness, sensitization of stress responses after chronic variable stress, and induced depression-like behavior (increased immobility in the forced swim test). Knockdown of GR in the neighboring prelimbic PFC increased hypothalamic-pituitary-adrenocortical axis responses to acute stress and caused hyper-locomotion in the open field, but did not affect stress sensitization or helplessness behavior.
Conclusions
The data indicate a marked functional heterogeneity of glucocorticoid action in the PFC and highlight a prominent role for the infralimbic GR in appropriate stress adaptation, emotional control, and mood regulation.
Keywords: Depression-like behavior, glucocorticoid receptor, HPA axis, prefrontal cortex, rat, stress
The prefrontal cortex plays a primary role in translating stressful emotional information into action. In human, the ventral prefrontal cortex is linked to multiple forms of stress-related psychopathologies, including major depressive disorder (MDD) and posttraumatic stress disorder. For example, the subgenual cingulate cortex (Brodmann area 25) is metabolically hyperactive in MDD, and deep brain stimulation in treatment-resistant patients is capable of quieting this area and alleviating depressive symptoms (e.g., feelings of helplessness and anhedonia) (1). In rodent, the ventral prefrontal cortex (comprising of prelimbic [plPFC] and infralimbic [ilPFC] subdivisions) has analogous functions, processing memories of negative life events and controlling the magnitude of physiologic responses to adversity, including secretion of glucocorticoid stress hormones. The plPFC is linked to the nucleus accumbens and basolateral amygdala (BLA) and plays a major role in control of stress response inhibition and reward. The ilPFC is connected to visceral/emotional effector systems (central amygdaloid nucleus, hindbrain cardiovascular regulatory pathways) and is important for control of emotional responses to fear as well as activation of stress effector pathways (2). Thus, in both human and rodent, the prefrontal cortical region is well-positioned to participate in neural mechanisms underlying stress adaptation and pathology.
The prefrontal cortex is directly targeted by stress hormones via resident glucocorticoid and mineralocorticoid receptors (GR and MR, respectively). Prefrontal GRs read stress levels of glucocorticoids and are implicated in feedback control of hypothalamic-pituitary-adrenocortical (HPA) axis activity (3–7). Pathological activation of prefrontal cortical GR by chronic stress negatively impacts GR expression and causes dendritic atrophy and spine loss, suggesting both a loss of prefrontal feedback control and altered neuronal excitability (8–12). Glucocorticoid dyshomeostasis (elevated basal hormone secretion and feedback resistance) is known to occur in stress-related diseases such as MDD, raising the possibility of a link between excessive GR signaling and PFC dysfunction (13,14).
In the current study, we test the role of PFC glucocorticoid signaling on behavior and stress reactivity, with virally mediated GR knockdown (short hairpin RNA targeting the glucocorticoid receptor [shRNA-GR]) in the ilPFC and plPFC. Our data provide evidence for a pronounced anatomical heterogeneity of prefrontal GR actions on behavior and stress responses and define a critical role of GR signaling in the ilPFC in control of depression-related behavior and stress adaptation. Given the link between area 25 and depression, our data provide new evidence for a dedicated PFC circuit responsible for glucocorticoid control of emotionality.
Methods and Materials
Lentiviral Constructs
Three different lentiviral constructs, each containing unique double-stranded, shRNA targeting a different position in the GR gene (shRNA-GR) (constructs 468, 469, and 470 targeting positions + 187, +1690, and +2245 in exon 1 of the GR gene) were obtained from America Pharma Source (Gaithersburg, Maryland). The shRNA sequences were as follows: 5’- AATTCCAAAAAGCAGCAGAGGATTCTCCTTGACTCTTGATCAAGGAGAATCCTCTGCTGCTG-3’ (468), 5’- AATTCCAAAAAGGTGTTGTATGCAGGATATGACTCTTGATCATATCCTGCATACAACACCTG-3’ (469), and 5’- AATTC-CAAAAAGGTGGTTGAGAATCTCCTTACCTCTTGAGTAAGGAGATTCTCAACCACCTG (470). Nucleotide sequences specific to GR messenger RNA (mRNA) are displayed in boldface type. We also obtained a vector control (lacking a shRNA insert) and a scrambled-sequence control virus (shRNA-Sc; proprietary sequence). All constructs contained a human U6 promoter to drive shRNA expression and contained a green fluorescent protein (GFP) cassette. For in vitro and in vivo studies, titers of 1 × 106 infection units (IU)/mL and of 1 × 109 IU/mL were used, respectively. All experimental procedures were approved by the University of Cincinnati Institutional BioSafety Committee.
In Vitro
Cell Culture and Transfection
The GR-expressing 4B cells (Dr. Toni Pak, Loyola University, Chicago, Illinois) were seeded in HyClone Dulbecco's modified Eagle's medium (DMEM)/high glucose media (with L-glutamine and L-glucose; Thermo Scientific, Waltham, Massachusetts) and 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, Georgia). Cells were treated with trypsin (Invitrogen, Carlsbad, California) and subcultured and transferred to 6-well tissue-cultured plate such that they would reach 70% confluency overnight. Media was removed and replaced with 8 × 105 plaque forming units (PFUs) (or 800 µl of 1 × 106 IU/mL), shRNA-GR 468,8 × 105 PFUs shRNA-GR 469,8 × 105 PFUs shRNA-GR 470, 800 µL media, or 2 mL media (to control for transfection volume). After 16 hours, the contents of the wells were aspirated and media was replaced (2 mL). Cells were harvested 5 days later for quantification of GR mRNA.
Real Time Quantitative Polymerase Chain Reaction
The RNA was isolated with an RNeasy kit, according to manufacturer protocol (Qiagen, Valencia, California). The RNA quantity and quality were determined with a NanoVue Plus spectrophotometer (General Electric Healthcare, Piscataway, New Jersey). The RNA was treated with Turbo DNA-free to remove genomic DNA (Ambion, Foster City, California) and reverse transcribed with an iScript complementary DNA synthesis kit according to manufacturer protocol (Bio-Rad, Hercules, California). Real time quantitative polymerase chain reaction (RT qPCR) analysis was performed in an iCycler iQ Multi-Color Real Time PCR Detection System (Bio-Rad). Primers for GR mRNA (10µmol/L) (forward: 5’- CCACTGCAGGAGTCTCACAA-3’; and reverse: 5’-ACTGCTGCAATCACTTGACG-3’) and the house-keeping gene L-32 (forward: 5’-CATCGTAGAAAGAGCAGCAC-3’; and reverse: 5’-GCACACAAGCCATCTATTCAT-3’) were used (Integrated DNA Technologies, Coralville, Iowa). Quantification of complementary DNA was determined with iQ SYBR Green Supermix (Bio-Rad). Values were calculated with L-32 as an internal standard, and GR mRNA expression is presented as a percentage of control GR expression. Threshold cycle readings for each of the unknown samples were used, and the results were calculated with the ΔΔCt method (15). Negative RT samples were included to rule out genomic DNA contamination.
In Vivo
Subjects
Male Sprague Dawley rats from Harlan (Indianapolis, Indiana) weighing 250–275 g upon arrival were singly housed throughout the experiment in a temperature/humidity-controlled room on a 12-hour/12-hour light/dark cycle. Food (Teklad; Harlan) and water were available ad libitum. All experimental procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Stereotaxic Surgery
After 1 week of habituation, animals were anesthetized (90 mg/kg ketamine, 10 mg/kg xylazine), and preemptive analgesia (butorphanol) and antibiotic (gentamicin) were administered. Animals received 1-µL bilateral microinjections into the ilPFC (anterior-posterior [AP] = +3.0, medial-lateral [ML] ± .6, and dorsal-ventral [DV] = −4.3, Paxinos and Watson [16] coordinates) of shRNA-Sc (n = 21) or shRNA-GR (n = 31) or 2-µL bilateral microinjections into the plPFC (AP = +3.0, ML ± .7, and DV = −3.3) of vehicle control (high glucose DMEM media with 4.5 g/L glucose, L-glutamine, and pyruvate [Mediatech, Manassas, Virginia]) and 10% heat-inactivated fetal calf serum (Invitrogen; n = 21), empty vector control (n = 22), or shRNA-GR (targeting position 1690 in the GR gene; n = 22) with a 25-gauge, 2-µL Hamilton syringe (Reno, Nevada). To reduce tissue damage, each injection took place over 20 min. After the needle remained in place for 5 min, the virus was infused over 10 min with a microdriver (Model 5001; Kopf, Tujunga, California) and remained in place for 5 min to allow for complete diffusion. Animals recovered for at least 5–6 weeks before any experiments.
Chronic Variable Stress
Approximately half of the animals underwent chronic variable stress (CVS) for 14 days (n = 11–16 from each microinjection group). The CVS was comprised of twice daily (am and pm) repeated and unpredictable stressors, including cold swims (10 min, 16°–18°C), warm swims (20 min, 30°–32°C), cold room exposure (1 hour, 4°C), shaker stress (1 hour, 100 rpm), and hypoxia (30 min, 8% oxygen). Only animals undergoing CVS were used in the forced swim test (FST) and open field, and these tests were treated as morning stressors for the first 2 days of CVS.
FST
Approximately half of the animals from each microinjection group went through the modified FST, as described previously (17,18), to assess depression-like behavior. Animals were placed in a cylindrical container (46 cm in height × 20 cm in diameter) filled with 30 cm of 29° ± 2°C water for 10 min. Behavior was video recorded and scored every 5 sec for 10 min. Scoring was done by an observer blinded to the experimental condition. Mobility (swimming, climbing, headshakes, and diving) versus immobility was scored as previously described (18). Animals were not exposed to any swims before the FST, because the modified FST is a single exposure test.
Open Field Test
Animals were exposed to a novel open field to assess anxiety-like behavior and locomotor activity. Animals were placed in a 1-meter × 1-meter black opaque acrylic glass box with 30.48-cm-tall white opaque walls surrounding each side for 5 min. A video recording of the behavior of the animal was scored and analyzed with Clever TopScan Software (CleverSys, Reston, Virginia). Time spent in the center versus the periphery of the open field was used as a measure of anxiety-like behavior (19).
Acute Restraint and Blood Collection
The morning after completion of CVS (at least 16 hours after last stress exposure), all animals were exposed to a novel 30-min restraint. Blood samples (approximately 250 µL) were collected in tubes containing 10 µL 100 mmol/L ethylenediamine tetraacetate by tail clip before (0 min) and 30,60, and 120 min after onset of 30-min restraint and immediately placed on ice. Samples were collected in under 3 min before any rise in adrenocorticotropic hormone (ACTH) or corticosterone levels due to sampling (20). Blood samples were centrifuged at 3000× g for 15 min at 4°C, and plasma was stored at −20°C until time of radioimmunoassays (RIAs).
Tissue Collection
Animals were given an overdose of sodium pentobarbital and transcardialy perfused with .9% saline followed by 4% sodium phosphate-buffered paraformaldehyde. Brains were postfixed in 4% sodium phosphate-buffered paraformaldehyde for 24 hours, then stored in 30% sucrose in diethylpyrocarbonate-treated water at 4°C. Brains were sectioned on a mictotome in 30-µm coronal sections (Leica, Buffalo Grove, Illinois). Thymus and adrenal glands were dissected and weighed.
Immunohistochemistry
Sections were immunolabeled with primary antibodies against GR (M-20) (1:1000; Santa Cruz Biotech, Santa Cruz, California), neuronal nuclei (NeuN) (1:200; Millipore, Billerica, Massachusetts), MR (ID-5) (1:200 and 1:500; provided by Dr. Elise Gomez-Sanchez from University of Mississippi, Jackson, Mississippi) (21), or glial fibrillary acidic protein (1:2000; Dako, Carpinteria, California) with standard immunohistochemical procedures. For additional detail, see Supplement 1.
RIA
Plasma ACTH was determined by a RIA that used a specific antiserum (1:120,000 dilution; donated by Dr. William Engeland University of Minnesota, Minneapolis, Minnesota) with 125I ACTH (Amersham Biosciences, Piscataway, New Jersey) as labeled tracer. All samples were run in duplicate (when sample was sufficient) in the same assay. Plasma corticosterone levels were measured with an 125I RIA kit (MP Biomedicals, Solon, Ohio). All samples were run in duplicate and each time point was run in the same assay. For additional detail, see Supplement 1.
Cell Counting
For analysis of GR-, NeuN-, or MR-positive immunoreactive nuclei, digital images of each side of the plPFC or ilPFC, as defined by the rat stereotaxic brain atlas of Paxinos and Watson, were captured at 5× or 10× magnification with a Carl Zeiss Imager Z.1 (Carl Zeiss Microimaging, Thornwood, New York). Quantitative analysis of cell counts was performed with the Automatic Measurement Program, Axiovision 4.4 (Carl Zeiss Microimaging). Images were captured on the same day with the same settings, and a uniform threshold was applied to all images in a given brain region.
Statistical Analysis
Data are expressed as mean ± SEM. Behavioral data, body weight before CVS, and immunoreactive counts were analyzed with one-way analysis of variance (ANOVA). Body weight (after CVS), organ weights, and baseline corticosterone levels were analyzed with a two-way ANOVA (microinjection [vector control or shRNA-GR]) × stress (acute stress or CVS). Fisher’s least significant difference post hoc analyses were conducted. Hormonal data were analyzed with two-way repeated measures ANOVA (microinjection × time [0, 15, 30, 60 or 120 min]) or three-way repeated measures ANOVA (microinjection × stress × time [0, 30, 60 or 120 min]), time being the repeated measure. Fisher’s least significant difference was used for a priori planned comparisons across microinjection and stress at each time point. Data were analyzed with GBStat (version 6.5.4) software (Dynamic Microsystems, Silver Spring, Maryland), and statistical significance was set at p ≤ .05. Where appropriate, behavioral data failing Levene’s F, Hartley’s F-max, Cochran’s C, and Barlett’s χ2 homogeneity of variance tests were log transformed. Outliers were removed as outlined previously (22). Animals with unilateral or no GFP expression or injections outside the ilPFC or plPFC were excluded (n = 21 or 6, respectively). For simplicity of presentation, results are graphed by acute stress only (No CVS) or chronic stress (CVS), although data were part of the same statistical analysis. Experiments targeting the plPFC or the ilPFC were conducted separately, and therefore statistical comparisons across experiments were not analyzed.
Results
shRNA Validation
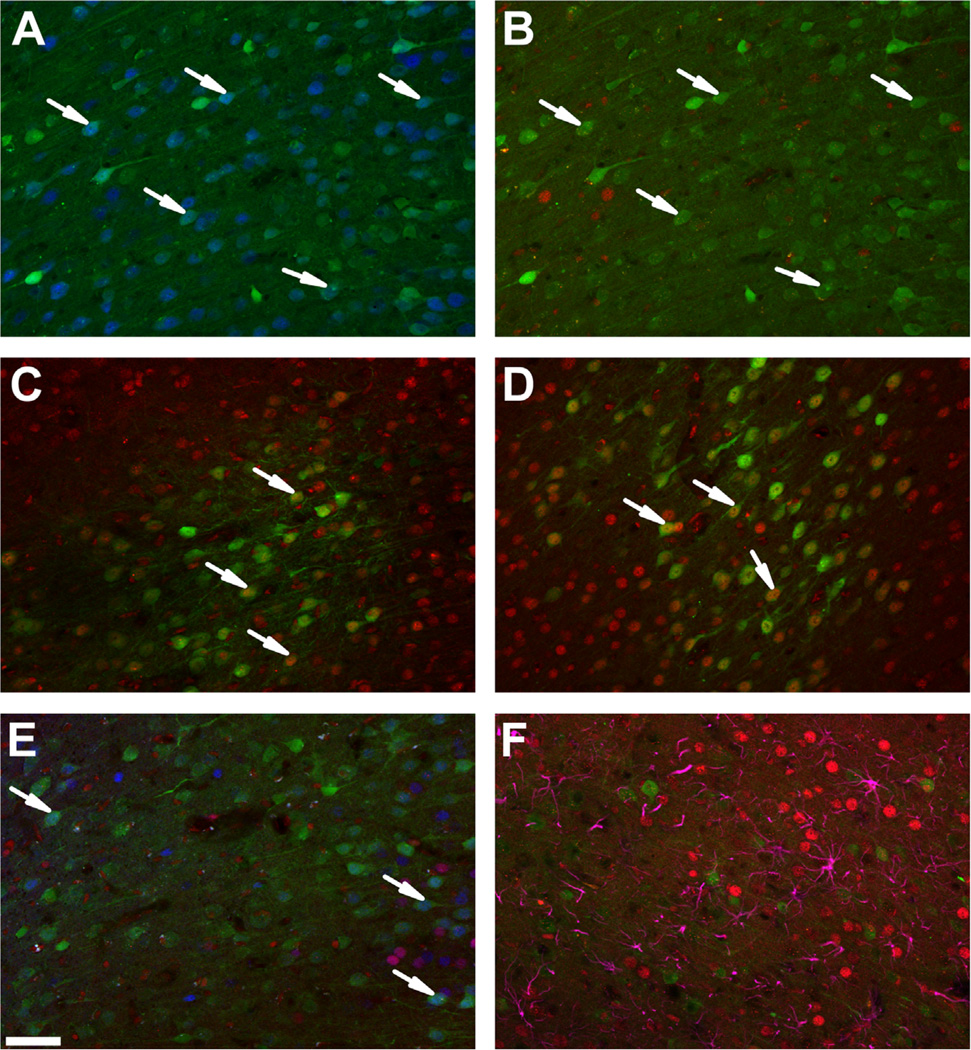
We first performed in vitro studies to identify an shRNA sequence that can specifically knockdown GR expression. We transfected immortalized, GR-expressing hypothalamic 4B cells with several different lentiviral-packaged shRNAs predicted to target GR mRNA (shRNA-GR) (23). As determined by RT qPCR, GR mRNA expression was reduced after transfection with shRNA-GR 469 (99.8% reduction as shown by results from one PCR experiment) (Figure S1 in Supplement 1). We next validated the ability of this shRNA to knockdown expression in vivo. Immunofluorescence analysis revealed reduced GR immunoreactivity at the site of injection in animals that received shRNA-GR, without loss of neuronal viability (NeuN immunolabeling is intact in GFP-positive neurons) (Figure 1A). Reduced GR was observed as a loss of GR immunoreactivity in GFP-positive (i.e., virus-infected) neurons (Figure 1B). No reduction in GR was observed in animals that received a scrambled-sequence control (shRNA-Sc) (Figure 1C), empty vector control (Figure 1D), or vehicle control (data not shown). The MR immunoreactivity was also intact in transduced neurons that lack GR, demonstrating that the shRNA-GR does not downregulate expression of a closely related protein (Figure 1E). Furthermore, shRNA-GR microinjection did not produce recruitment of astrocytes to the region beyond that of a control injection site. Importantly, astrocytes did not seem to incorporate the shRNA-GR (no co-localization of glial fibrillary acidic protein with GFP), and expression of the astrocytic GR was intact (Figure 1F).
Figure 1.
Verification and specificity of a short hairpin RNA targeting the glucocorticoid receptor (shRNA-GR). (A) Representative NeuN (blue) immunolabeled sections after microinjection with shRNA-GR (green). After microinjection of shRNA-GR, NeuN immunoreactivity remained intact in transduced neurons, indicating that neuronal viability was not affected (see arrows). (B) Representative GR (red) immunolabeled sections after microinjection with shRNA-GR (green), (C) shRNA-scrambled control (green), and (D) empty vector control (green). The shRNA-GR reduced GR in green fluorescent protein co-localized cells (B) relative to animals that received microinjections of shRNA-scrambled control (C) or empty vector control (D) (see arrows). (E) Representative dual GR (red) and mineralocorticoid receptor (MR) (blue) immunolabeled sections after intracranial microinjection with shRNA-GR. Mineralocorticoid receptor expression is intact in cells in which GR immunoreactivity is knocked down (as demonstrated in the superficial layers ll/lll on the left of the image and deep layers V/VI of the prelimbic prefrontal cortex on the right of the image, where MR is typically expressed. The agranular layer between layers III and V typically has little MR expression) (see arrows). (F) Representative glial fibrillary acidic protein (purple) and GR (red) immunolabeled sections after intracranial injection with shRNA-GR. The shRNA-GR did not transduce astrocytes (no green fluorescent protein and glial fibrillary acidic protein co-localization) and did not seem to knockdown astrocytic GR. Scale bar = 50 µm.
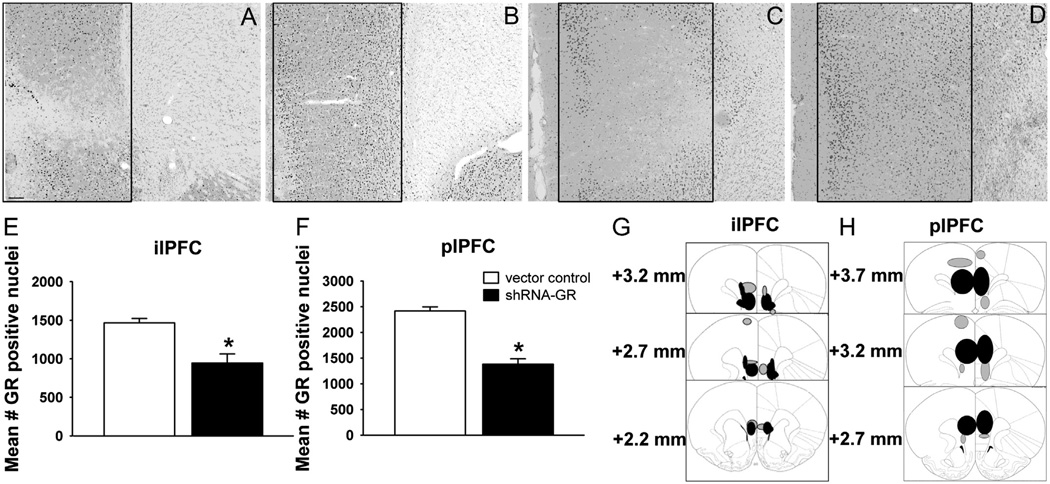
To assess the extent of GR knockdown in the ilPFC and plPFC, we quantified the number of GR-positive immunoreactive nuclei in the area of injection. The GR expression was selectively knocked down in shRNA-GR-microinjected animals in the ilPFC [F1,13 = 20.33, p = .0006] (Figure 2A,E) and in the plPFC [F1,10 = 59.48, p < .0001] (Figure 2C,F) relative to vector control-microinjected animals (Figures 2B,D–F), without affecting the number of NeuN [F1,10 = 1.06, p = .33] or MR [F1,11 = .168, p = .69] immunoreactive cells at the site of injection (as quantified in the plPFC) (Figure S2 in Supplement 1). Knockdown of GR expression is mostly confined to the ilPFC or the plPFC (with minimal spread to adjacent areas) (Figure 2G,H).
Figure 2.
Selective decreases in glucocorticoid receptor (GR) immunoreactive neurons in the infralimbic prefrontal cortex (ilPFC) and prelimbic prefrontal cortex (plPFC) after short hairpin RNA targeting the GR (shRNA-GR) microinjection. Representative GR-immunolabeled sections from vector control-microinjected animals in (B) the ilPFC and the (D) plPFC and shRNA-GR-microinjected animals in (A) the ilPFC and (C) the plPFC (representative areas of quantification are outlined in panels A–D). (E) Quantified GR expression from vector control-microinjected animals (n = 10) and shRNA-GR-microinjected animals (n = 5) in the ilPFC and (F) vector control-microinjected animals (n = 6) and shRNA-GR-microinjected animals (n = 6) in the plPFC. The GR immunoreactivity was significantly reduced in animals that received shRNA-GR relative to vector control-microinjected animals (p < .05). (G) Extent of GR knockdown in the ilPFC of all shRNA-GR-microinjected animals that were considered “hits” (n = 10) or (H) in the plPFC of all shRNA-GR-microinjected animals that were considered “hits” (n = 16) (reprinted from Paxinos and Watson [16] with permission from Elsevier, copyright 1998). Green fluorescent protein (GFP) expression throughout the plPFC in each animal was traced onto stereotaxic images and compiled into one visual representation. Black circles indicate where GFP expression was most prominent (n ≥ 4 in the ilPFC and n ≥ 8 in the plPFC), whereas gray circles represent areas where GFP was less prominent in animals that received shRNA-GR and were considered “hits” (n ≤ 3 in the ilPFC or n ≤ 7 in the plPFC). Immunoreactive counts are mean ± SEM. Scale bar = 100 µm. *p < .05 vs. vector control-microinjected animals.
Behavioral Testing
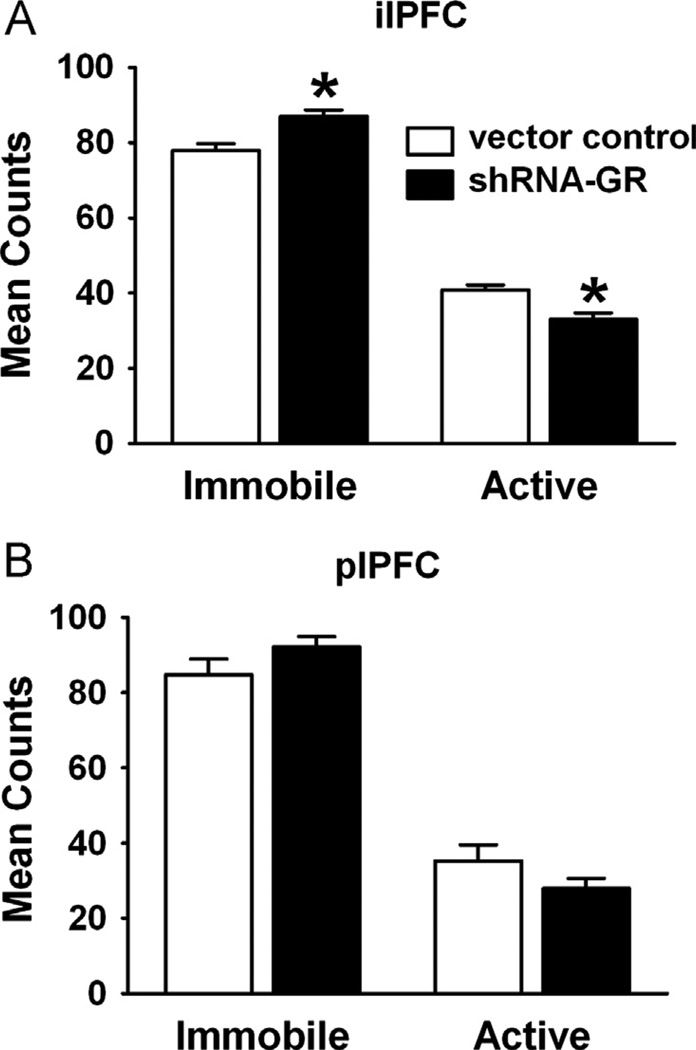
The medial PFC is thought to be an important mediator of depression-like behavior (24,25). To test the effect of GR knockdown on depression-like behavior, we first examined performance in the FST, commonly used as an assay for behavioral helplessness. Animals that received microinjection with shRNA-GR in the ilPFC had significantly increased immobility in the FST compared with animals receiving vector control (F1,13 = 9.67, p = .008), suggestive of a depression-like phenotype (Figure 3A). However, there were no significant differences in scored individual active behaviors (e.g., swimming [F1,13 = .46, p = .51], climbing [F1,14 = .75, p = .40], diving [F1,14 = .23, p = .64], or headshakes [F1,14 = .55, p = .47]). In contrast, knockdown of plPFC GR did not affect immobility (F1,17 = 1.81, p = .20) or individually scored activities (e.g., swimming [F1,17 = 3.18, p = .09], climbing [F1,17 = .04, p = .85], diving [F1,18 = .39, p = .54], or headshakes [F1,16 = .88, p = .36]) (Table S1 in Supplement 1) in the FST (Figure 3B).
Figure 3.
Increased helplessness behavior after GR knockdown in the ilPFC. (A) Immobility vs. activity in the modified forced swim test after vector control- or shRNA-GR-microinjection in the ilPFC (n = 10 or 5, respectively) or (B) in the plPFC (n = 11 or 8, respectively). Animals receiving shRNA-GR in the ilPFC but not the plPFC, exhibited increased immobility in the forced swim test relative to vector controls (p < .05). Data are mean ± SEM. *p < .05 vs. vector control-microinjected animals. Abbreviations as in Figure 2.
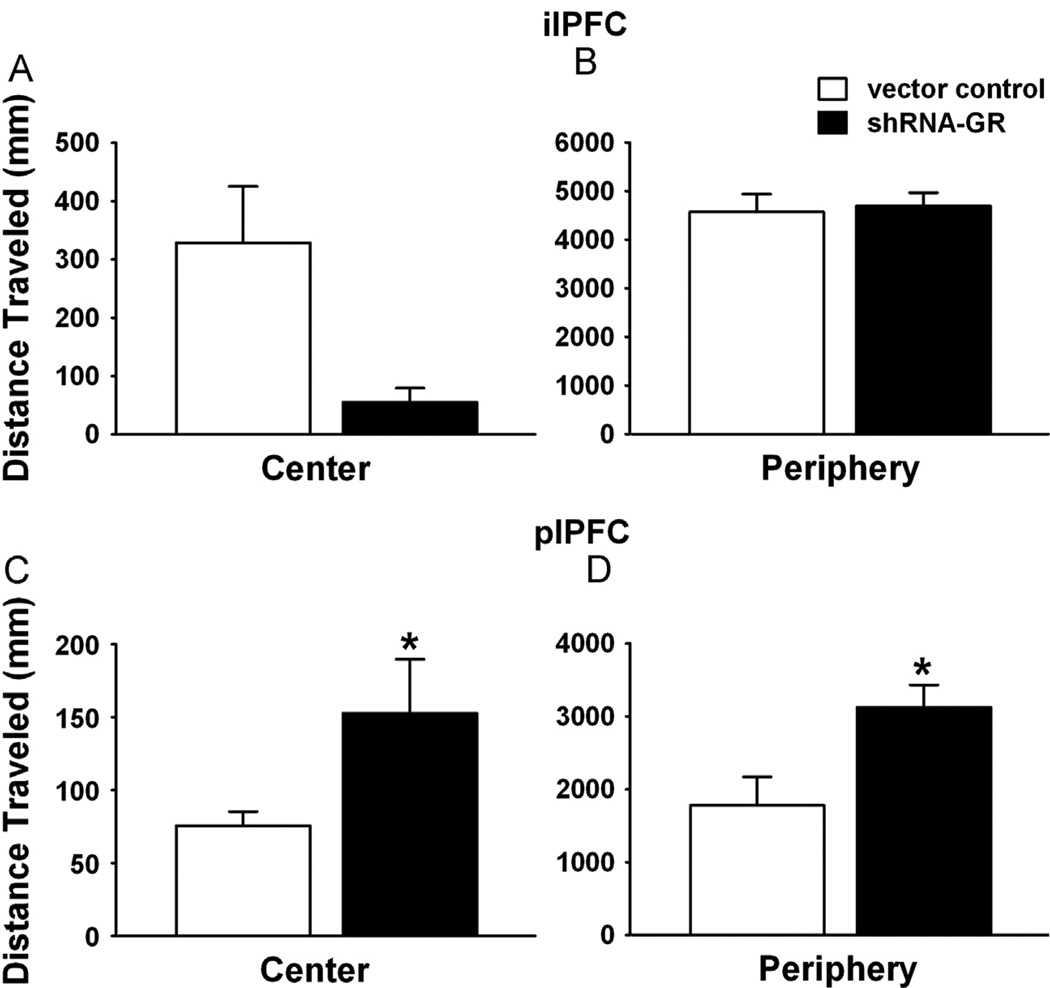
Previous studies indicate that electrolytic lesions of the ilPFC or the plPFC decrease time spent in the center of the open field (26). Therefore, we tested anxiety-related behavior and locomotion in the open field test, with the same cohorts of animals used in the FST. Microinjection of shRNA-GR in the ilPFC did not precipitate an anxiety-like phenotype (no main effect of microinjection on time spent in the center of the open field) (F1,11 = 1.69 p = .22) (Table S2 in Supplement 1). Furthermore, there were no significant differences in overall locomotor activity (F1,11 = .60, p = .46) or locomotor activity in the center (F1,11 = 2.98, p = .11) (Figure 4A) and the periphery (F1,16 = .04, p = .86) (Figure 4B) relative to vector control-microinjected animals. Similarly, injection of shRNA-GR in the plPFC was without effect on anxiety-related open field behavior (F1,16 = .23, p = .64) (Table S2 in Supplement 1) but did cause a substantial increase in total locomotor activity (F1,17 = 7.59, p = .01), distance traveled in the center (F1,16 = 103.15, p < .0001) (Figure 4C), and in the periphery (F1,17 = 6.42, p = .02) (Figure 4D) of the open field and a significant increase in rearing (F1,17 = 7.69, p = .01) (Table S2 in Supplement 1).
Figure 4.
Increased locomotor activity after GR knockdown in the plPFC relative to vector control-microinjected animals. (A) Locomotor activity in the center and (B) periphery after vector control- or shRNA-GR microinjections in the ilPFC (n = 9–10 or 4, respectively). (C) Locomotor activity in the center and (D) periphery after microinjections of vector control or shRNA-GR in the plPFC (n = 11 or 7–8, respectively). Animals receiving shRNA-GR in the plPFC traveled significantly more throughout the center (C) and periphery (D) than vector controls (p < .05). Data are mean ± SEM. *p < .05 vs. vector control-microinjected animals. Abbreviations as in Figure 2.
Body/Organ Weights
Rats were exposed to a 2-week CVS regimen to test the impact of GR signaling in prefrontal regions on physiological reactivity to prolonged adversity. As documented previously, attenuated weight gain, adrenal hypertrophy, and thymic involution are consistent attributes of chronically stressed rats (27). There was no main effect of microinjection on body weight gained before CVS in either the ilPFC (F1,30 = .13, p = .72) or plPFC (F1,35 = .99, p = .33) of shRNA-GR-microinjected animals, indicating that PFC GR knockdown did not affect body weight. Gross somatic effects of chronic stress on adrenal hypertrophy were not affected by GR knockdown in either PFC subregion. However, thymic involution was selectively enhanced in the CVS-ilPFC group, consistent with greater cumulative exposure to glucocorticoids over the stress regimen (main effect of stress [F1,26 = 6.54,p = .02]) (Table S3 in Supplement 1).
Hormonal Responses
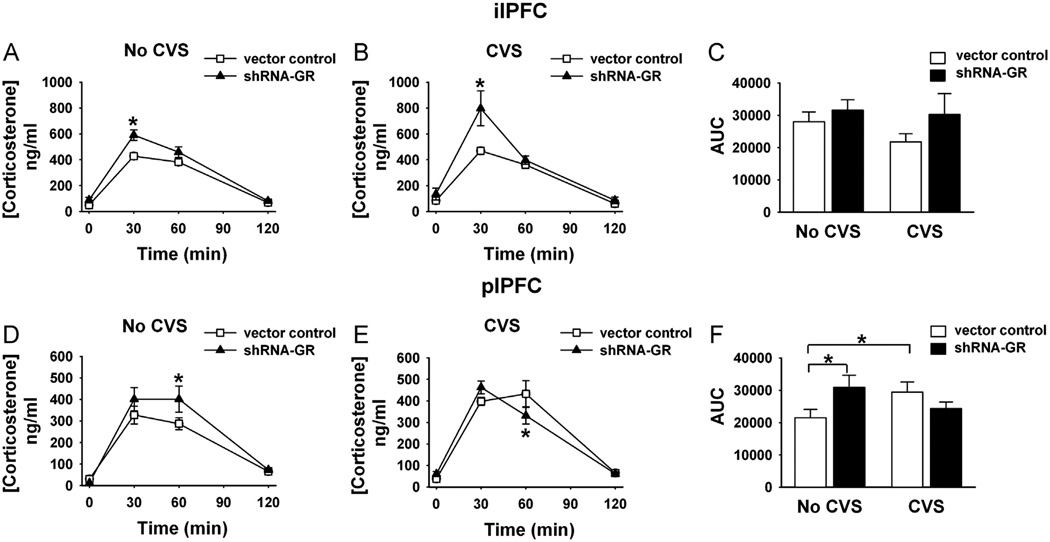
We next tested the role of ilPFC and plPFC in control of HPA axis responses to acute restraint stress. Both ilPFC and plPFC injections of shRNA-GR enhanced acute activation of the HPA axis (increased peak corticosterone release; at 30 or 60 min post-stress time points, respectively) (Figure 5). Enhanced corticosterone release was accompanied by increased ACTH release 15 min after acute restraint (measured in a separate study after plPFC GR knockdown [F4,40 = 6.80, p = .0003]) (Figure S3 in Supplement 1). After chronic stress, ilPFC GR knockdown potentiated the corticosterone response to a novel stressor, consistent with hyper-sensitization of the HPA axis (F3,75 = 3.42, p = .02). Knockdown of GR in the plPFC did not affect the post-CVS peak HPA axis response to a novel stressor, and in fact corticosterone levels were significantly lower 60 min after restraint (F3,102 = 4.43, p = .006). Together, the data suggest differential roles of the ilPFC and plPFC in chronic stress processing.
Figure 5.
Differential impact of GR knockdown in the ilPFC vs. plPFC on hypothalamic-pituitary-adrenal axis reactivity after acute and chronic stress. (A) Corticosterone responses after acute novel restraint in unstressed (no chronic variable stress [CVS]) and (B) CVS animals that received microinjections of vector control (n = 9–11/unstressed or stressed group) or shRNA-GR (n = 5/unstressed or stressed group) in the ilPFC. (C) Integrated area under the curve (AUC) for corticosterone responses (not including baseline values) after vector control-microinjections (n = 9–11/group) or shRNA-GR (n = 5/group) in the ilPFC. (D) Corticosterone responses after acute novel restraint in unstressed (No CVS) and (E) CVS animals that received microinjections of vector control (n = 10–11/unstressed or stress group, respectively) or shRNA-GR (n = 7–9/unstressed or stressed group) in the plPFC. (F) Integrated AUC for corticosterone responses (not including baseline values) after vector control-microinjections (n = 10–11/group) or shRNA-GR (n = 7–9/group) or in the plPFC. After ilPFC microinjection of shRNA-GR, acute stress caused a significant elevation in corticosterone at 30 min compared with vector controls, an effect that is exacerbated in chronically stressed animals relative to acutely stressed shRNA-GR-microinjected animals and controls (p < .05). After microinjection of shRNA-GR in the plPFC, animals acutely stressed in the absence of CVS have significantly elevated corticosterone levels at 60 min compared with vector controls, whereas chronically stressed animals have significantly lower corticosterone responses at 60 min compared with vector control-microinjected animals (p < .05). Data are mean ± SEM. *p < .05 vs. vector control-microinjected animals or between groups indicated by the brackets. Abbreviations as in Figure 2.
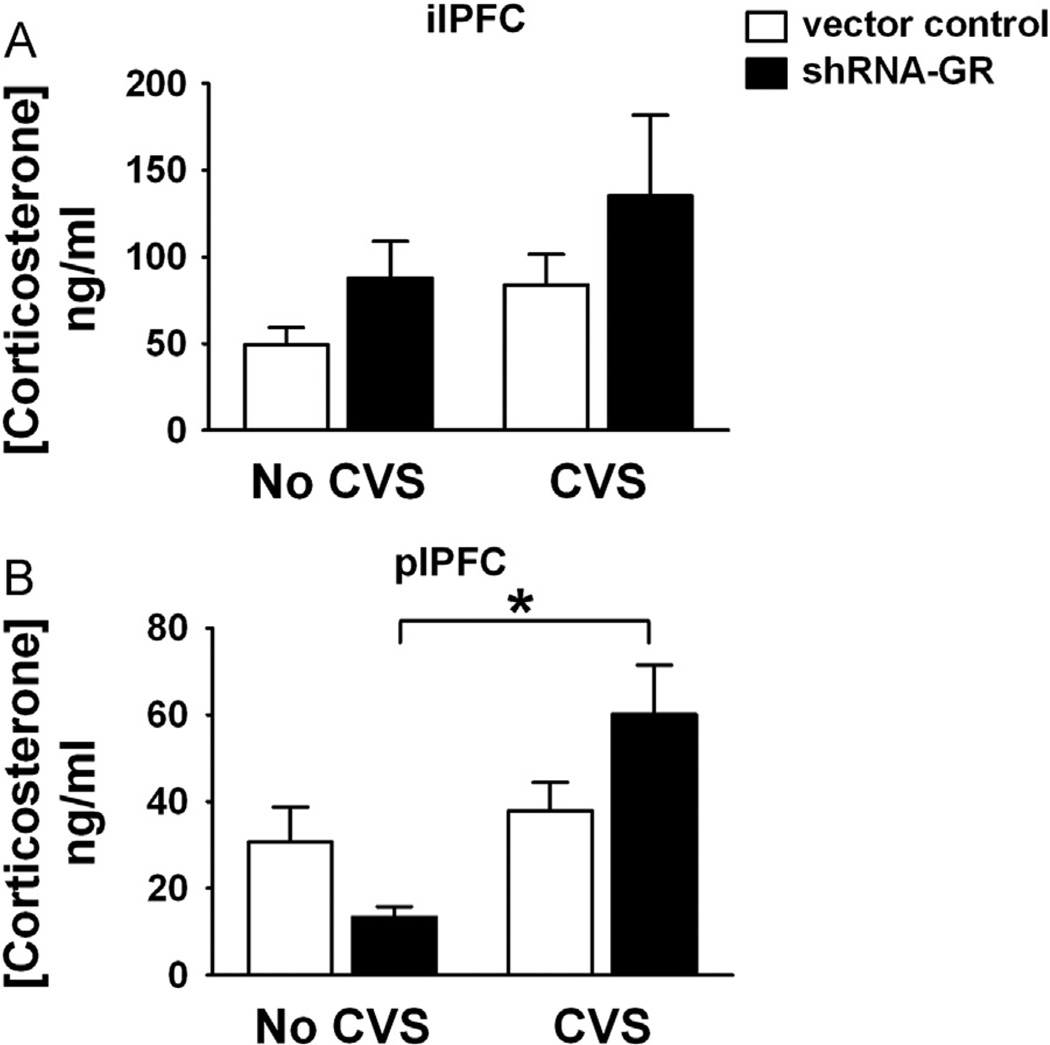
Finally, we determined the impact of plPFC GR and ilPFC GR knockdown on baseline levels of stress hormones in unstressed animals or animals exposed to chronic stress. The shRNA-GR microinjection in the plPFC increased baseline levels of corticosterone in chronically stressed animals, reflected in a microinjection × stress interaction (F1,31 = 6.05, p = .02) (Figure 6B), suggesting selective involvement in control of basal glucocorticoid homeostasis under chronic stress.
Figure 6.
The GR knockdown in the plPFC but not NPFC of chronically stressed animals significantly increased baseline corticosterone levels. (A) Baseline corticosterone levels in unstressed and chronically stressed animals receiving vector control (n = 10 or 11/group, respectively) or shRNA-GR (n = 5/group) in the ilPFC and (B) in unstressed and chronically stressed animals receiving vector-control (n = 10 or 11, respectively) or shRNA-GR (n = 7 or 9, respectively) in the plPFC. Baseline corticosterone levels were significantly different in chronically stressed animals receiving shRNA-GR in the plPFC only (relative to acutely stressed animals that received shRNA-GR) (p < .05). Data are mean ± SEM. *p < .05 vs. acutely stressed shRNA-GR-microinjected animals or between groups indicated by the brackets. Abbreviations as in Figures 2 and 5.
Discussion
Our study indicates that glucocorticoid control of stress responsiveness and emotional reactivity is mediated by distinct prefrontal cortical mechanisms, with the ilPFC particularly important for mediating chronic stress adaptation and emotional reactivity to stress. Loss of infralimbic GR caused increased helplessness behavior and hormonal hypersensitivity to chronic stress, consistent with a role in integrating glucocorticoid signals into appropriate behavioral and physiological responses to prolonged challenge. Importantly, in human, area 25 (ilPFC homolog) is linked to depression, a disease that is characterized by helplessness behavior and reduced central sensitivity to glucocorticoids (1,13,14). The current data suggest that local glucocorticoid signaling in this confined prefrontal locus might be critical for appropriate control of mood.
The prelimbic cortex seems to play a very different role in chronic stress adaptation. Like the ilPFC, the plPFC participates in control of HPA responses to an acute stressful event. Our data suggest that, unlike the ilPFC, the plPFC GR does not seem to be involved in either regulation of HPA axis reactivity to chronic stress or control of initial emotional responses to stressors. However, plPFC GR knockdown induces a significant increment in basal morning corticosterone release during chronic stress, suggesting that this region participates in setting the basal tone of the HPA axis under chronic stress. Thus, the prelimbic and infralimbic cortices, anatomical neighbors in the prefrontal region, are both involved in processing glucocorticoid information with regard to prolonged adversity but play very different roles in regulating behavioral and physiological responses.
Previous studies have used multiple techniques to assess the role of the PFC in regulation of the HPA axis in rats, including ibotenic lesions, acute activation, and corticosterone implants (6,7,28–31). The shRNA-mediated technique offers many advantages, because it allows for anatomical and molecular specificity through long-term knockdown confined to the region of shRNA expression. The shRNA-GR is packaged in a lentiviral construct and is therefore useful for targeting nondividing cells (i.e., neurons) without eliciting an immune response (32). Our data indicate that lentiviral delivery of shRNA-GR can effectively reduce GR immunoreactivity in virally transduced neurons locally in the region of the ilPFC or the plPFC, affording the ability to use this method to query the role of the GR in defined neural populations. Our data suggest that the lentiviral knockdown spares medial PFC astrocytes, which play a role in depression-like behavior (33). Additional studies are required to determine whether the glial GR is also involved in modulation of mood.
In patients with MDD, area 25 is hyperactive, and deep brain stimulation ameliorates depressive symptoms in patients with treatment-resistant MDD (34). In rodent, the infralimbic cortex projects to regions implicated in visceral/autonomic control (e.g., the nucleus of the solitary tract, lateral septum, bed nucleus of the stria terminalis, central amygdaloid nucleus, and posterior hypothalamus) (2), consistent with a role in mediating physical and emotional responses to chronic drive. Moreover, glucocorticoids are thought to inhibit limbic neuronal responses by reducing neural excitability and retracting dendritic trees (35–37). We hypothesize that the loss of a GR-mediated “brake” in the infralimbic cortex might allow prolonged activation of downstream targets, thereby promoting aberrant physical and emotional responses, as observed in the FST.
Despite its anatomic proximity to the infralimbic cortex, the prelimbic cortex has a markedly different efferent output, sending heavy projections to regions involved in limbic/cognitive processing, such as the nucleus accumbens, BLA, and raphe nuclei (2). These sites have polysynaptic input to stress regulatory systems, such as the paraventricular nucleus, and might buffer the impact of altered plPFC GR signaling on HPA axis drive. For example, chronic stress causes morphological and functional neuroadaptation in regions such as the BLA, which might be sufficient to diminish the overall impact of plPFC knockdown on HPA stress reactivity (38). The effect of plPFC on basal morning corticosterone might either reflect modulation of processes relating to stress habituation (e.g., by the paraventricular thalamus) or energetic demands associated with stress-induced hyperactivity, as observed in the open field test (39,40).
Our work is consistent with dedicated roles of the GR within specific neurocircuits. Not surprisingly, total forebrain GR deletion (in mouse) has features in common with the lentiviral knockdown approach. Forebrain GR deletion (encompassing neocortex, hippocampus, and basolateral/cortical amygdala) produces glucocorticoid stress hyperresponsiveness, in response to acute restraint stress, characteristic of plPFC and ilPFC GR knockdown in the rat (41). However, forebrain GR knockout does not cause chronic stress sensitization (like the plPFC but not ilPFC knockdown). The latter data suggest that GR signaling in other forebrain regions (such as the hippocampus) might negate or magnify effects of local changes in the prefrontal cortex.
The present study indicates a distinct role of ilPFC versus plPFC GR in acute versus chronic stress regulation. Human genetic studies link the GR to depression or posttraumatic stress disorder, either directly or via interactions with other proteins (e.g., FK506 binding protein 5, a chaperone protein that modulates GR function) (42). Given the overriding importance of area 25 (human ilPFC-equivalent) in affective disease states, our data suggest that stress-related GR signaling might be uniquely important in regulating resistance or resilience, with loss of function driving pathological behavioral and endocrine reactivity to situational adversity.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants MH049698 (JPH), MH069860 (JPH), NS007453 (JMM), and MH097430 (JMM); Udall Parkinson’s Disease Center of Excellence Grant NS058830 (KBS, JPH); a Gardner Family Center for Parkinson’s Disease and Movement Disorders grant (KBS); and a University Research Council Fellowship grant (JMM).
We would like to thank Renu Sah, Ph.D., Kenny Jones, Benjamin Packard, Eduardo Carvalho Netto, Ph.D., Sriparna Ghosal, Annette de Kloet, Ph.D., Eric Krause, Ph.D., Lauren Larke Vollmer, and Yvonne Ulrich-Lai, Ph.D., for technical assistance. JMM, BM, JNF, MBS, KBS, and JPH designed research; JMM, BM, MBS, JNF, and JB performed research; JMM analyzed data; JMM and JPH wrote the paper; and JPH supervised the project.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.03.024.
References
- 1.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 3.Reul JM, De Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986;24:269–272. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- 4.Fuxe K, Wikstrom AC, Okret S, Agnati LF, Harfstrand A, Yu ZY, et al. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985;117:1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- 5.Meaney MJ, Sapolsky RM, Aitken DH, McEwen BS. [3H] dexamethasone binding in the limbic brain of the fetal rat. Brain Res. 1985;355:297–300. doi: 10.1016/0165-3806(85)90054-9. [DOI] [PubMed] [Google Scholar]
- 6.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: Involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 9.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 10.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 14.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego, California: Academic Press; 1998. [Google Scholar]
- 17.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 20.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez CE, De Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, et al. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147:1343–1348. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- 22.McClave JT, Dietrich FH. Statistics. 6th edition. Englewood, NJ: Macmillan College Publishing Company, Inc.; 1994. [Google Scholar]
- 23.Przybycien-Szymanska MM, Mott NN, Pak TR. Alcohol dysregulates corticotropin-releasing-hormone (CRH) promoter activity by interfering with the negative glucocorticoid response element (nGRE) PLoS One. 2011;6:e26647. doi: 10.1371/journal.pone.0026647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Scopinho AA, Scopinho M, Lisboa SF, Correa FM, Guimaraes FS, Joca SR. Acute reversible inactivation of the ventral medial prefrontal cortex induces antidepressant-like effects in rats. Behav Brain Res. 2010;214:437–442. doi: 10.1016/j.bbr.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Jinks AL, McGregor IS. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- 27.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinal. 2001;13:625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 30.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 31.Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104:266–271. doi: 10.1016/j.physbeh.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sliva K, Schnierle BS. Selective gene silencing by viral delivery of short hairpin RNA. Virol J. 2010;7:248. doi: 10.1186/1743-422X-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 35.Joels M, Karst H. Corticosteroid effects on calcium signaling in limbic neurons. Cell Calcium. 2012;51:277–283. doi: 10.1016/j.ceca.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippo-campal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinal. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- 40.Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- 41.Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.