Abstract
In light of evidence that receptive language may be a relative weakness for individuals with autism spectrum disorder (ASD), this study characterized receptive vocabulary profiles in boys with ASD using cross-sectional developmental trajectories relative to age, nonverbal cognition, and expressive vocabulary. Participants were 49 boys with ASD (4–11 years) and 80 typically developing boys (2–11 years). Receptive vocabulary, assessed with the Peabody Picture Vocabulary Test, was a weakness for boys with ASD relative to age and nonverbal cognition. Relative to expressive vocabulary, assessed with the Expressive Vocabulary Test, receptive vocabulary increased at a lower rate for boys with ASD. Vocabulary trajectories in ASD are distinguished from typical development; however, nonverbal cognition largely accounts for the patterns observed.
Keywords: Autism, Language development, Comprehension, Production, Vocabulary, Trajectory
Introduction
Characterizing relative strengths and weaknesses in the language abilities of individuals with autism spectrum disorder (ASD) is of interest not only for the purpose of planning effective remediation, but also for delineating the neurocognitive phenotype associated with ASD. The ultimate goal of such a characterization is to understand the ways in which interactions among genes, brain, and behavior unfold over the course of development (Charman et al. 2011). Defining profiles of language impairment also has the potential to discriminate among subgroups of children with ASD, thereby leading to the identification of the psychological factors and cognitive mechanisms underlying ASD phenotypes (Tager-Flusberg and Joseph 2003). To that end, the current study was designed to characterize trajectories of vocabulary in boys with ASD relative to the course of typical development with a focus on the receptive domain, which is thought to be particularly susceptible to impairment in ASD. We utilized the cross-sectional developmental trajectories approach, which emphasizes age-related patterns to characterize specific aspects of delay and allows the formulation of stronger hypotheses to guide future longitudinal research (Thomas et al. 2009).
Evidence of Relative Delays in Receptive Language in ASD
One pattern of strength and weakness in children with ASD that has drawn considerable attention is the reported delay in receptive language relative to expressive language.
Indeed, there is mounting evidence that at least some children with ASD display a profile characterized by a relative advantage of expressive over receptive language (Luyster et al. 2008; Mitchell et al. 2006). This pattern is the converse of that expected in typical development, in which a child’s ability to understand vocabulary and syntax is generally far in advance of the ability to formulate spoken utterances reflecting that same competence. This relative weakness in receptive language is not usually observed in children with other sources of language impairment. For example, Loucas et al. (2008) found that receptive language was less impaired than expressive language in children with specific language impairment (SLI), whereas 9- to 14-year-olds with both ASD and impaired language lacked a receptive language advantage.
Notably, a relative weakness in receptive language has been found even very early in development in children with ASD, including during the preschool and toddler years (Ellis Weismer et al. 2010; Volden et al. 2011). The pattern of receptive language deficits relative to expressive language observed for toddlers with ASD has motivated the suggestion that the profile of language ability associated with ASD is atypical, even taking into account level of cognitive ability. Ellis Weismer et al. (2010) found that toddlers with autism from ages 24 to 36 months had significantly higher expressive language age-equivalent scores than receptive language age-equivalent scores on two direct assessment measures, the Mullen Scales of Early Learning (MSEL; Mullen 1995) and the Sequenced Inventory of Communication Development (Hendrick et al. 1984), although the same pattern was not found for the Vineland Adaptive Behavior Scales (VABS; Sparrow et al. 1984). In contrast, a comparison group of toddlers with developmental delay without ASD displayed the opposite pattern, with significantly higher receptive than expressive age-equivalent scores. Using the fourth edition of the Preschool Language Scales (PLS-4; Zimmerman et al. 2002), Volden et al. (2011) confirmed the advantage of expressive language age-equivalents over receptive language age-equivalents in a large sample of 2- to 4-year-old children with ASD.
Studies specifically examining vocabulary have led to similar conclusions as those utilizing omnibus measures. Using a direct assessment and two parent-report measures, one of which specifically assessed vocabulary, Hudry et al. (2010) found that receptive language was more impaired in preschoolers with ASD than would be expected based on their expressive language abilities. In particular, these preschoolers had significantly higher expressive than receptive language age-equivalent scores on the PLS-3 UK edition and the VABS-II (Sparrow et al. 2005). Compared to typically developing children from a normative sample (Fenson et al. 1994), preschoolers with ASD also have been found to have higher raw expressive vocabulary scores on the MacArthur Communicative Development Inventories (MCDI; Fenson et al. 1993) than expected on the basis of their raw receptive vocabulary scores (Hudry et al. 2010). These findings correspond with those from Charman et al. (2003), who observed that a sample of 134 preschool children with ASD had an overall delay in vocabulary, with receptive vocabulary impaired to a greater extent than expressive vocabulary. Taken together, these studies provide evidence for a profile of impairment in children with ASD involving the comprehension of fewer vocabulary words relative to the number of words produced, although the trajectory of this pattern has yet to be defined over the full course of the childhood years.
Despite the fact that an atypical relationship between language comprehension and production has been reported on average, the extent to which this relationship extends to all individuals with ASD is unclear. For example, results for school-age children are ambiguous. Kjelgaard and Tager-Flusberg (2001) examined language abilities in 89 children with autism between 4 and 14 years of age. Of these children, 44 had language abilities that were sufficient for assessment with the Clinical Evaluation of Language Fundamentals (CELF; Semel et al. 1995) and, for this subgroup, standard scores were higher in the expressive domain than in the receptive language domain. However, standard scores on direct assessments of receptive and expressive vocabulary in the larger sample led the authors to conclude that, on average, children with autism do not display discrepancies in receptive and expressive vocabulary ability.
Furthermore, some studies report that only a subgroup of children with ASD exhibits a relative deficit in receptive language and that the discrepancy between receptive and expressive language abilities varies over the course of development. Hudry et al. (2010) found that approximately 30 % of the children with ASD in their sample had an atypical pattern of receptive and expressive vocabulary on the MCDI. Results are inconsistent in terms of whether such an atypical profile is more likely to occur at earlier developmental levels, when nonverbal cognitive abilities are less fully developed, as reflected by lower age-equivalent scores on standardized tests, (e.g., Volden et al. 2011) or whether such a pattern is more prevalent later in development when nonverbal cognitive abilities are more developmentally advanced and more variability in expressive language abilities is present (e.g., Hudry et al. 2010). Thus, the extent to which a relative deficit in receptive language–and receptive vocabulary, in particular–extends to children with ASD across the entire range of childhood, levels of intellectual functioning, or levels of autism symptom severity remains to be determined. To contribute to characterizations of individual variability and to provide a foundation for identifying subgroups in the future, we examined the relationship between receptive and expressive vocabulary discrepancies and these child characteristics, in addition to addressing the primary research questions regarding developmental trajectories.
Limitations of Previous Research
Besides the conflicting findings noted, previous research on receptive language in children with ASD has had several methodological weaknesses. One limitation is the extent to which specific aspects of receptive or expressive language have been assessed. Studies on the receptive-expressive language profile have sometimes relied upon omnibus measures, such as the MSEL or PLS, which fail to distinguish between important language domains, such as vocabulary and syntax, that could be associated with distinct profiles of receptive and expressive delays (Ellis Weismer et al. 2010; Hudry et al. 2010; Volden et al. 2011).
Another limitation is the emphasis on the use of parent-report measures, including the MCDI and the VABS (e.g., Charman et al. 2003; Hudry et al. 2010; Luyster et al. 2007). Direct assessment may have an advantage over parent report as the latter does not control for the influence of children’s use of contextual cues to support comprehension or whether spoken words are used non-imitatively and meaningfully (i.e., with referential intent). Although echolalia and scripted language may, in fact, serve a communicative function in children with ASD (Prizant and Duchan 1981), stereotyped language might also contribute to parent reports of advanced expressive language ability in children with ASD (Jarrold et al. 1997). Indeed, correlations between direct assessment and parent-report measures have been reported as somewhat lower for receptive language than expressive language in children with ASD (Ellis Weismer et al. 2010; Luyster et al. 2008), further calling into question the utility of parental report for profiling language abilities in children with ASD. In the current study, we directly assessed distinct language domains, namely receptive and expressive vocabulary.
Potentially greater limitations of previous research, however, relate to design and analysis, including the interpretation of age-equivalent scores and the absence of a directly assessed comparison group. Age-equivalent scores are known to have poor psychometric properties and are not conducive to inferential statistics (Mervis and Klein-Tasman 2004; Mervis and Robinson 1999), limiting the conclusions that can be drawn from some studies (e.g., Hudry et al. 2010; Volden et al. 2011). Moreover, the use of age-equivalent scores in characterizing profiles of language abilities may obscure or exaggerate true strengths or weaknesses in receptive language across development (Mervis and Robinson 2003, 2005). In this study, the dependent variables of interest were vocabulary growth scores, which have desirable psychometric properties for statistical comparisons and might be particularly well-suited to research on individuals with ASD because of their sensitivity to small incremental differences in absolute levels of ability.
When analyzing standard scores is not feasible due to floor effects (e.g., Hudry et al. 2010), a viable strategy for examining relative delays is to utilize a comparison group of typically developing participants. Only studies using the MCDI have attempted to reference the receptive language abilities of children with ASD to those with typical development (e.g., Charman et al. 2003; Hudry et al. 2010). A yet stronger comparison group would be one comprised of a large, directly assessed sample of typically developing children that overlaps completely in terms of both chronological age and nonverbal cognitive abilities with the children with ASD (Thomas et al. 2009). This was the approach taken in the present study.
The Current Study
The purpose of the present study was to characterize profiles of vocabulary development in boys with ASD using directly assessed typically developing children as a point of reference. In contrast to traditional matched-group comparisons, we chose to use the cross-sectional developmental trajectories approach endorsed by Thomas et al. (2009). This continuous approach highlights change over the course of development rather than the characterization of static phenotypes and distinguishes between delayed onset, slowed rates of development, or the presence of both. Such a technique has been fruitful in defining multiple aspects of behavioral phenotypes, including those associated with ASD (e.g., Annaz 2006). Yet, the cross-sectional developmental trajectories approach has not been applied to an examination of language development in boys with ASD with respect to understanding delays in receptive vocabulary relative to other aspects of development.
The cross-sectional developmental trajectories approach allows tests of the extent of delay relative to a carefully chosen comparison group and theoretically chosen constructs of interest (Thomas et al. 2009). This approach leads to tests of between-group differences in the relationships between the dependent measure and chronological age, in addition to other benchmarks, such as nonverbal cognitive ability. In contrast to testing differences in cross-sectional group means, which masks changes associated with age or other foundational cognitive abilities, group differences are evaluated in terms of two coefficients: intercepts and slopes of developmental trajectories. Testing these two parameters of performance provides more information about the nature of the language impairment (i.e., whether it is delayed in onset, has a shallower trajectory, or both) and provides guidance for future longitudinal research to inform an understanding of the emergence of linguistic phenotypes (Thomas et al. 2009).
Our goals were to begin by capturing individual variability in vocabulary abilities, then to allow comparison between the current study and the extant literature by conducting traditional analyses using matched groups, as described below, and finally, and most importantly, to draw conclusions about group differences in trajectory onsets and slopes in receptive vocabulary ability relative to age-, nonverbal cognitive-, and expressive vocabulary-expectations. Thus, we addressed these questions: (1) Do discrepancies between receptive and expressive vocabulary at the individual level relate to child characteristics and to what extent do boys with ASD display a significant weakness in receptive relative to expressive vocabulary?, (2) Does a traditional matched-groups ANOVA analysis of receptive and expressive vocabulary lead to the conclusion that receptive vocabulary is a relative weakness in school-age boys with ASD?, and (3) Is receptive vocabulary delayed in school-age boys with ASD in terms of a lower onset or shallower slope relative to chronological age, nonverbal cognition, and expressive vocabulary? We hypothesized that relative weakness in receptive vocabulary would be related to nonverbal cognition, autism symptom severity, and age. We expected that traditional analyses would replicate the pattern of relative receptive vocabulary weakness in school-age boys with ASD; however, we also expected that cross-sectional developmental trajectories analyses would reveal more nuanced patterns of receptive vocabulary ability, including differences in onsets and slopes relative to age, nonverbal cognition, and expressive vocabulary.
Method
Participants
Participants were boys with ASD and boys with typical development who were drawn from two longitudinal studies on language development and tested at one of two sites: a Midwestern university or a West Coast university. Approval from the respective Institutional Review Boards of the two universities was obtained and informed consent was obtained from parents. Boys with ASD were largely recruited locally with additional outreach through national sources [e.g., the Interactive Autism Network (IAN) at the Kennedy Krieger Institute]; typically developing boys were local to one of the two sites. Local recruitment of boys with ASD at the Midwestern site occurred primarily through community contacts, whereas the West Coast site relied largely on a clinic-recruited sample and Internet contacts. In addition, a larger proportion of typically developing boys, especially those of school age, were recruited at the Midwestern university. Recruitment methods are also described elsewhere in detail (Kover et al. 2012; McDuffie et al. 2012). Participants with ASD overlap with those reported by McDuffie et al. (2012). According to parent report, all participants used spoken English as their primary means of communication and none had uncorrected physical or sensory limitations that would prohibit completion of the tasks of interest. Participants with ASD came into the project with a community or education diagnosis of ASD. Typically developing boys were not receiving special education services at the time of enrollment according to parent report. We chose to recruit only males because of the increased prevalence of ASD in boys and the desire to compare gender-matched groups. Of the total sample of 61 boys with ASD and 85 boys with typical development, only those participants who met all inclusionary criteria and completed all measures of interest were included in the present analyses (i.e., listwise deletion yielded sample sizes of 49 and 80, respectively, as described below).
Additional inclusionary criteria for the boys with ASD were (1) meeting criteria for ASD classification (autism or autism spectrum) on the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 1999), (2) meeting criteria for autism classification on the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al. 2003), (3) negative test results for fragile X syndrome, and (4) according to parent report, comprehension of simple instructions and use of 10 spoken words in the month prior to study enrollment. These inclusionary and exclusionary criteria resulted in the final participant samples: 49 boys with ASD (4–11 years old) and 80 boys with typical development (2–11 years old). Descriptive characteristics of the samples for the present analyses are shown in Table 1.
Table 1.
Participant characteristics and vocabulary ability
| Measure | Autism spectrum disorder (n = 49) |
Typical development (n = 80) |
|||||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Range | Mean | (SD) | Range | ||
| Chronological age | 7.61 | (1.95) | 4–11 | 5.11 | (2.47) | 2–11 | |
| Leiter-R | Brief IQ | 75.92 | (19.86) | 40–117 | 113.24 | (14.90) | 82–145 |
| Age-equivalent | 5.65 | (2.49) | 3–15 | 6.34 | (3.75) | 3–18 | |
| Growth score | 465.71 | (16.92) | 439–510 | 468.24 | (21.97) | 429–517 | |
| PPVT | Standard score | 68.69 | (23.35) | 20–113 | 118.21 | (12.56) | 81–147 |
| Age-equivalent | 4.94 | (2.08) | 2–12 | 6.95 | (4.08) | 2–22 | |
| Growth score | 117.57 | (30.82) | 56–186 | 139.95 | (30.33) | 82–209 | |
| EVT | Standard score | 71.33 | (25.28) | 20–119 | 114.47 | (11.13) | 91–143 |
| Age-equivalent | 5.32 | (2.14) | 2–13 | 6.54 | (3.62) | 3–21 | |
| Growth score | 127.90 | (29.24) | 45–179 | 143.01 | (22.92) | 88–193 | |
| Autism symptom severity | 8.00 | (1.63) | 4–10 | − | − | − | |
PPVT and EVT standard scores are only available for participants over the age of 2;6 and thus, reflect scores from 72 typically developing boys. Due to low raw scores, age-equivalent scores were unavailable for 5 boys with ASD for the PPVT and 6 boys with ASD and 1 boy with typical development for the EVT. Autism symptom severity scores were based on ADOS algorithm scores (Gotham et al. 2009)
PPVT Peabody Picture Vocabulary Test (Dunn and Dunn 1997, 2007), EVT Expressive Vocabulary Test (Williams 1997, 2007)
Materials
ASD Classification
The ADOS and the ADI-R were administered to boys with ASD by research-reliable examiners for the purpose of confirming ASD classification. Cross-site reliability was regularly monitored to ensure fidelity and consensus (see McDuffie et al. (2012) for details). We used the current published classifications provided by the ADOS, which distinguish between autism, autism spectrum, and nonspectrum (Gotham et al. 2007), and the manual for the ADI-R, which distinguishes only autism and not autism (Rutter et al. 2003). Thus, our sample is best described as having autism spectrum disorder, which we use as an umbrella term and not to indicate that participants have met the proposed diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5). Severity scores were calculated based on ADOS algorithm scores (Gotham et al. 2009) and by definition, ranged from 4 to 10. Five boys with ASD had been excluded due to not meeting ADOS and/or ADI-R criteria; two boys with ASD had been excluded because the ADOS and/or ADI-R could not be administered.
Nonverbal Cognition
The Leiter International Performance Scale-Revised (Leiter-R; Roid and Miller 1997) was the measure of nonverbal cognitive ability. Only the Brief IQ subtests were administered: Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. These subtests yielded raw scores, growth scores, age-equivalents, and standard scores. Boys with ASD had Leiter-R Brief IQ standard scores that spanned from intellectual disability to normative levels (see Table 1). Boys with ASD and those with typical development did not differ on Brief IQ growth scores, t(127) = .69, p = .493, d = .13. Three boys with ASD and four typically developing boys had been excluded due to missing data on the Leiter-R.
Vocabulary Ability
Boys with ASD completed the Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4; Dunn and Dunn 2007) and Expressive Vocabulary Test, Second Edition (EVT-2; Williams 2007). The PPVT is a receptive vocabulary test in which the child points to one of four color pictures on a page that is named by the examiner. The EVT tests expressive vocabulary by requiring the child to provide a label or synonym for each item, presented as a color picture on the page of an easel book. One benefit of these two measures is that they were co-normed, allowing direct comparisons between PPVT and EVT standard scores.
Typically developing boys completed either the PPVT-4 and the EVT-2 (n = 58; ages 2–5; 11) or the previously published versions: PPVT-III (Dunn and Dunn 1997) and EVT (Williams 1997). Typically developing boys who completed the PPVT-III and EVT (n = 22) were ages 6–11. The older editions of those tests differ from the newer revisions in that the pictures are grey scale rather than color. The PPVT-III, PPVT-4, and EVT-2 each have two versions (A and B), which were administered in an alternating manner to participants in the larger studies.
All raw scores were converted to growth scores, which allow valid comparisons across editions (i.e., PPVT-4 and PPVT-III; EVT-2 and EVT) and versions (i.e., A and B) according to the test manuals. Additionally, growth scores have desirable psychometric properties for inferential statistics because they are on an equal-interval scale and they capture absolute level of knowledge for the entire range of ability (Dunn and Dunn 2007). In contrast, floor effects were of concern for standard scores and age-equivalent scores. Of participants with ASD, three were at floor for standard scores (i.e., a score of 20) and five participants received raw scores too low for a valid age-equivalent score on the PPVT; none received the lowest possible growth score on the PPVT. As such, growth scores were the primary dependent variables for trajectory analyses. One additional participant with ASD had been excluded due to missing data for the PPVT; one additional participant with ASD and one participant with typical development had been excluded due to missing data for the EVT.
Procedure
Each participant was tested individually by a trained examiner in a quiet room over the course of multiple sessions. Consistent with standardization procedures, the PPVT preceded the EVT in the experimental protocol, but usually was administered on the same day. Breaks were provided as needed. Note that face-to-face meetings, video review, and regular teleconferences were used to promote consistency of test administration across sites.
Results
Relative Deficits in Receptive Vocabulary at the Individual Level
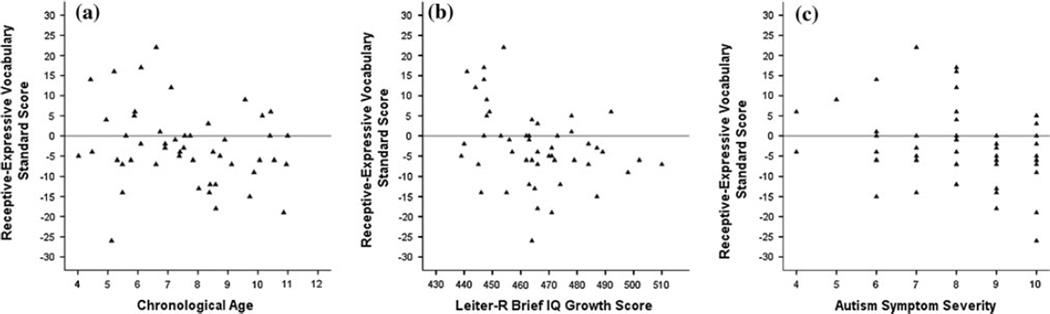
Prior to the primary analyses, we sought to describe the nature of within-sample variability in vocabulary abilities. For these descriptive analyses, we chose to use standard scores because they are directly comparable between the PPVT and EVT and because standard scores are preferred for profiling abilities at the individual level (Mervis and Klein-Tasman 2004). We subtracted EVT standard scores from PPVT standard scores to create a difference score that maps onto the typical pattern of abilities (i.e., higher receptive than expressive abilities). Positive values indicate a relative strength in receptive vocabulary, whereas negative values indicate the reported pattern in ASD, with a relative weakness in receptive vocabulary. Of the 49 boys with ASD, 31 had a negative score. Correlations between the difference score and nonverbal cognition, autism symptom severity, and age were tested using two-tailed p values. For boys with ASD, difference scores were negatively associated with Leiter-R Brief IQ growth scores, r = −.33, p = .020, two-tailed, but not autism symptom severity, r = −25,p = .080, two-tailed, or age, r = −.21, p = .149, two-tailed. See Fig. 1. Thus, greater magnitudes of relative receptive delay were associated with higher nonverbal cognitive ability.
Fig. 1.
Distribution of vocabulary standard score discrepancies of boys with ASD across a age, b nonverbal cognitive ability, and c ADOS autism symptom severity
To further probe within-group variability, we identified those individual boys who had meaningful differences in receptive and expressive vocabulary standard scores according to the definitions provided by the EVT and EVT-2 manuals for statistically significant differences at the .05 level relative to the norming sample (Williams 1997; Williams 2007). We addressed the sensitivity of this profile in terms of the proportion of boys with ASD who met the criteria for the weakness in receptive vocabulary and the specificity of this profile relative to typical development (Mervis and Robinson 2005; and see 1999, 2003). Of the 49 boys with ASD, 9 (18 %) had significantly lower standard scores on the PPVT than the EVT in comparison to 6 of the 72 boys (8 %) with typical development (those boys below age 2;6 were too young for standard scores). The difference between groups in proportion of participants with a relative weakness in receptive vocabulary failed to reach significance, two-sided exact test, p = .158. In contrast, 5 (10 %) of the boys with ASD and 18 (25 %) boys with typical development had significantly higher standard scores on the PPVT than the EVT. The proportion of typically developing boys who demonstrated a significant advantage for receptive over expressive vocabulary was marginally higher than that of the boys with ASD, twosided exact test, p = .058.
Traditional Matched-Groups ANOVA Analyses
Before turning to the trajectory analyses, we assessed average group differences in receptive and expressive vocabulary to allow direct comparisons between our samples and the extant literature. We conducted these traditional ANOVA comparisons for nonverbal cognitive ability-matched groups using growth scores, which also served as the dependent variable of interest for the trajectory analyses. We also conducted the comparisons using standard scores and age-equivalent scores, which are more commonly used in research despite their limitations.
Although the groups did not differ in nonverbal cognitive ability growth scores (p = .49), the samples just failed to meet the matching criteria of p ≥ .50 proposed by Mervis and Robinson (1999). To simulate a matched-groups analysis, we restricted the samples such that no typically developing participant had a Leiter-R growth score lower than that of a participant with ASD and no participants had growth scores >502. This yielded an optimal match in terms of sample size and p value, resulting in 48 boys with ASD and 68 typically developing boys, t(114) = .18, p = .86, d = .03, variance ratio = 0.87 (Kover and Atwood 2013). We then tested group differences for PPVT scores, for EVT scores, and both combined in a 2 (Group: ASD, typical development) × 2 (Task: PPVT, EVT) ANOVA.
Boys with ASD had poorer PPVT and EVT performance than boys with typical development regardless of whether growth scores, standard scores, or age-equivalents were used as the dependent variable, with the exception of EVT age-equivalents (see Table 2). In the repeated-measures analysis for growth scores, there was a main effect of group, F(1,114) = 11.85, p = .001, partial η2 = .09; boys with ASD scored lower on average than boys with typical development. The significant Group × Task interaction, F(1, 114) = 8.57, p = .004, partial η2 = .07, reflected that, on average, boys with ASD had a greater discrepancy in growth scores between tasks than the typically developing boys. These effects, including the Group × Task interaction, F(1, 113) = 9.09, p = .003, partial η 2 = .07, remained significant when including nonverbal cognitive ability growth scores as a covariate. Results for age-equivalent and standard scores were similar to those for growth scores. On average, boys with ASD had lower PPVT than EVT scores, whereas typically developing boys showed the opposite pattern: higher PPVT than EVT scores.
Table 2.
Traditional matched-groups analysis of PPVT and EVT scores
| Variable | Group mean difference |
Standard error of difference |
t | df | P | d | |
|---|---|---|---|---|---|---|---|
| PPVT | Standard score | 49.52 | 3.53 | 14.02 | 107 | <001 | 2.73 |
| Age-equivalent | 1.40 | .52 | 2.66 | 109 | .009 | .52 | |
| Growth score | 19.88 | 5.34 | 3.72 | 114 | <001 | .71 | |
| EVT | Standard score | 43.45 | 3.63 | 11.98 | 107 | <001 | 2.33 |
| Age-equivalent | .65 | .47 | 1.39 | 107 | .167 | .28 | |
| Growth score | 13.37 | 4.54 | 2.95 | 114 | .004 | .56 |
Traditional matched-group t-tests were conducted on matched subsets of the larger samples: 48 boys with ASD and 68 typically developing boys. PPVT Peabody Picture Vocabulary Test (Dunn and Dunn 1997, 2007). EVT Expressive Vocabulary Test (Williams 1997, 2007). Only 61 of the 68 typically developing boys were old enough to receive standard scores. Only 43 boys with ASD had raw scores high enough to receive PPVT age-equivalent scores; only 42 boys with ASD and 67 typically developing boys had raw scores high enough for EVT age-equivalent scores. Two-tailed p values are reported here
Cross-Sectional Developmental Trajectories
For the primary analyses, trajectories were calculated for receptive vocabulary growth scores relative to age, nonverbal cognition growth scores, and expressive vocabulary growth scores. Compatible with a regression framework, but conceptually similar to ANCOVA, these analyses were implemented by predicting receptive vocabulary growth scores from each of the predictors of interest separately along with a dummy variable for group and the interaction term between group and the slope between vocabulary and the respective predictor. Thus, F tests of the main effects of group, main effects of the continuous predictor (i.e., age, nonverbal cognition, expressive vocabulary), and interactions between group and slope were of interest. As a check against violating assumptions associated with the trajectory analyses, we examined scatter plots of standardized residuals for receptive vocabulary by chronological age, Leiter-R growth scores, and expressive vocabulary growth scores. These residual plots revealed no systematic relationships. Although we analyzed linear trajectories here, researchers analyzing data from other, larger samples should examine the possibility that nonlinear trends might characterize observed patterns of data.
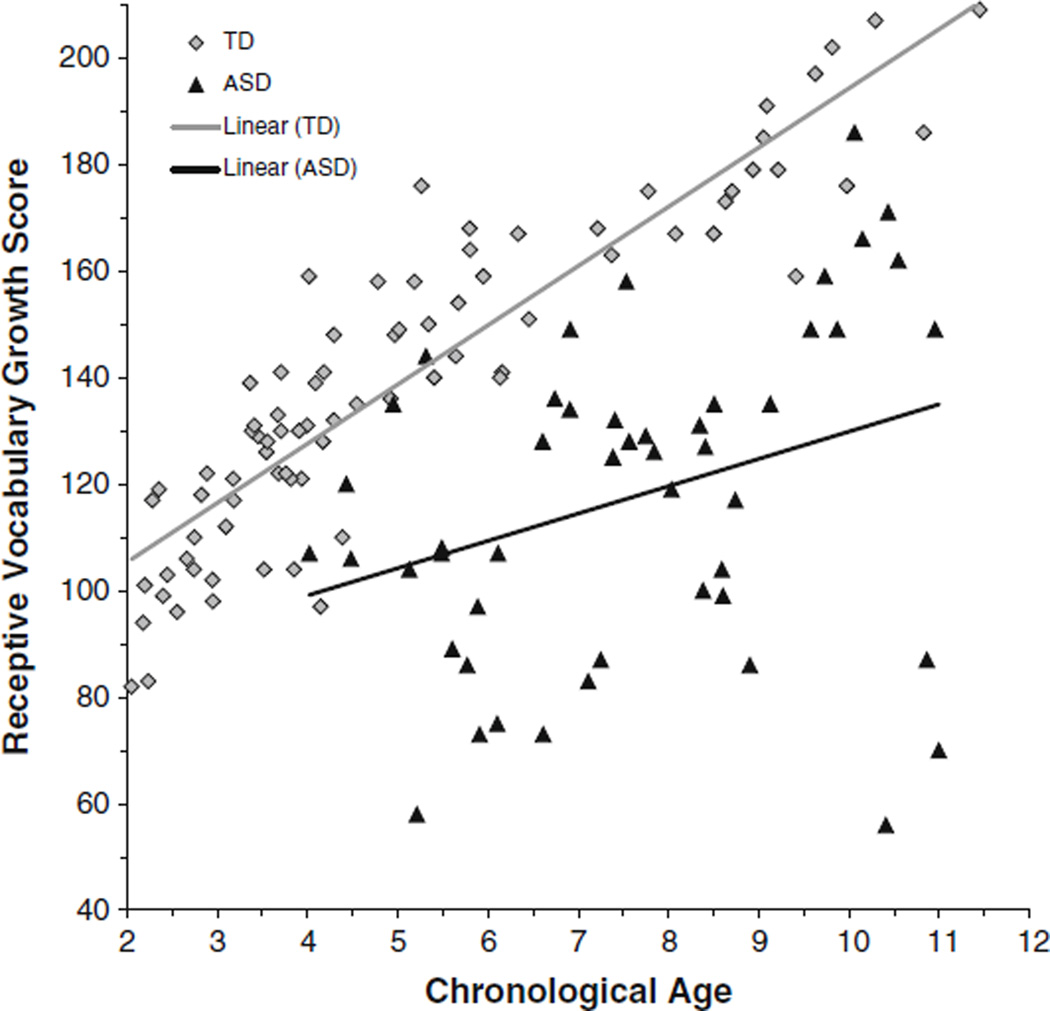
First, receptive vocabulary was evaluated relative to age expectations by examining the trajectory onset and within-group trajectory slopes between PPVT growth scores and chronological age. The difference in onset between groups was evaluated at the earliest overlap in chronological age between groups (i.e., 4 years) to avoid extrapolation. The onset of the trajectory for receptive vocabulary growth scores relative to age was lower for boys with ASD than boys with typical development (i.e., vocabulary was delayed relative to chronological age), F(1, 125) = 17.55, p < .001, partial η2 = .12. The relationship between receptive vocabulary and age was strongly positive on average, F(1, 125) = 80.47, p < .001, partial η2 = .39. However, the positive relationship between receptive vocabulary and age differed between groups, resulting in a significant interaction, F(1, 125) = 10.93, p = .001, partial η2 = .08. Inspection of Fig. 2 reveals that the trajectory of receptive vocabulary growth was steeper for typically developing boys than for boys with ASD.
Fig. 2.
Trajectory of receptive vocabulary relative to chronological age for boys with typical development (TD) and boys with ASD
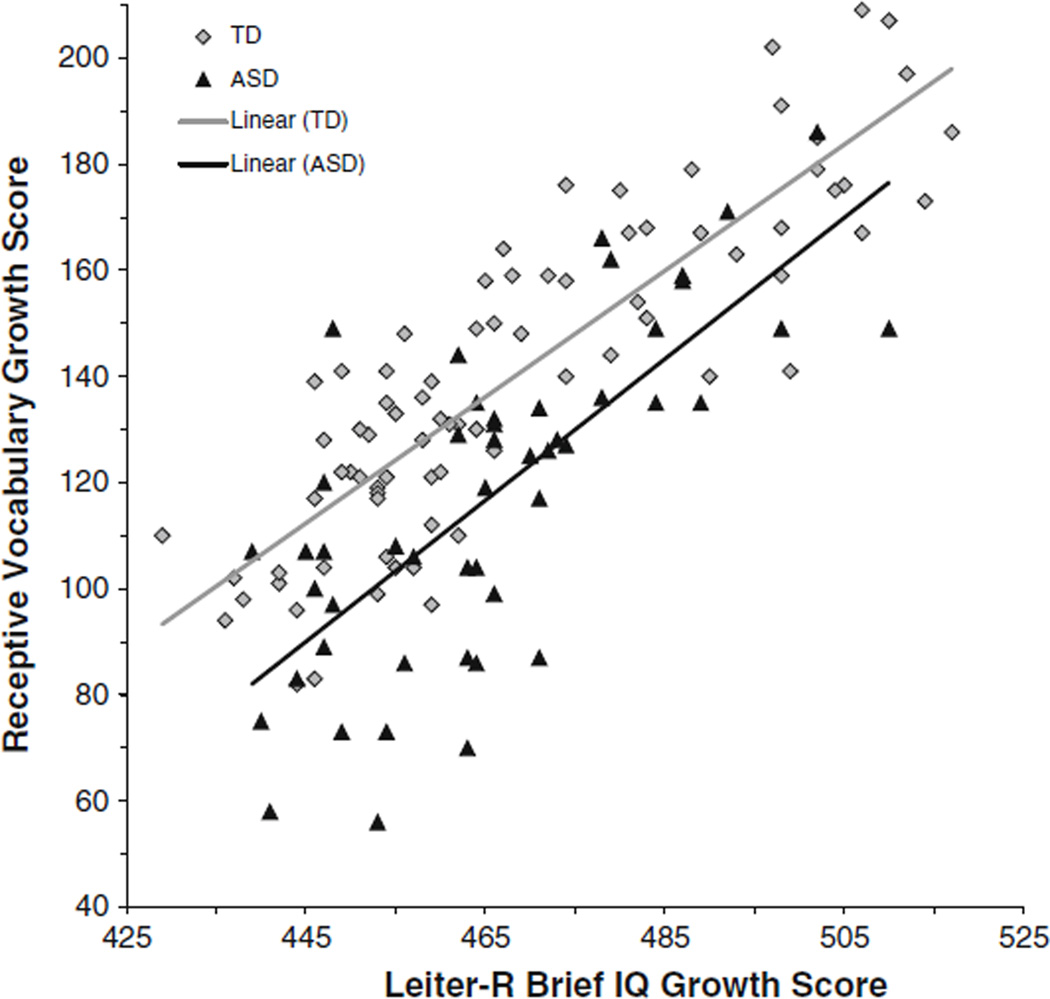
Next, receptive vocabulary was evaluated against nonverbal cognitive ability expectations in terms of onset at the lowest point of overlap between the groups (i.e., a Leiter-R Brief IQ growth score of 439) and in terms of the within-group slopes. A growth score of 439 on the Leiter-R can be interpreted as a cognitive-developmental level somewhat below that of a typical 3-year-old. The onset of the receptive vocabulary trajectory was lower for boys with ASD than boys with typical development (i.e., vocabulary comprehension was delayed relative to nonverbal cognition), F(1, 125) = 15.62, p < .001, partial η2 = .11. The relationship between receptive vocabulary and nonverbal cognition was strong and positive on average, F(1, 125) = 199.73, p < .001, partial η2 = .62. The interaction between group and nonverbal cognition was not significant, F(1, 125) = .64, p = .426, partial η2 = .01. As seen in Fig. 3, the relationship between receptive vocabulary and nonverbal cognition appears relatively comparable between groups, although the variability in receptive vocabulary at low levels of nonverbal cognitive ability for boys with ASD should be noted.
Fig. 3.
Trajectory of receptive vocabulary relative to nonverbal cognitive ability for boys with typical development (TD) and boys with ASD
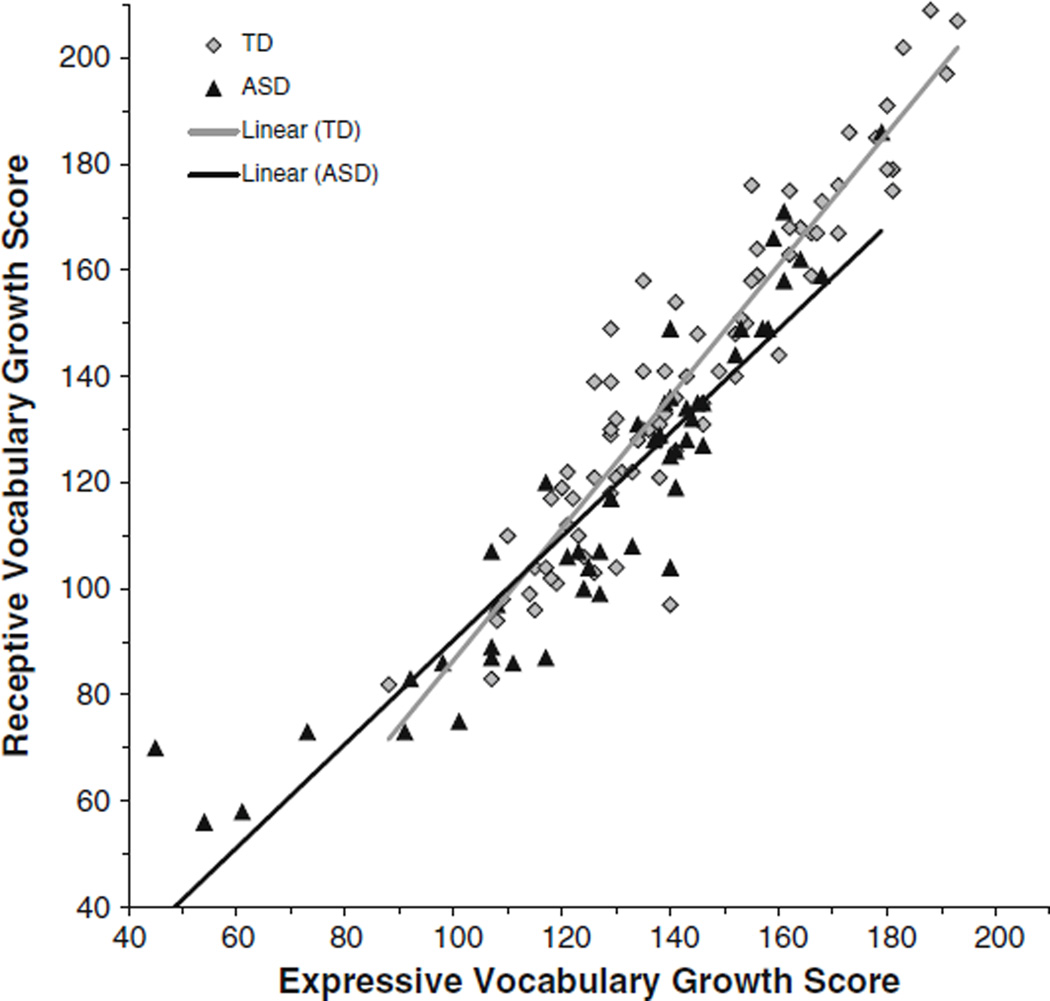
Receptive vocabulary was then evaluated against expressive vocabulary to assess the possibility of an atypical profile of relative weakness in comprehension relative to production. The group difference in onset was tested at the lowest point of overlap between the groups (i.e., an EVT growth score of 88) and the within-group slopes between receptive and expressive vocabulary were compared. A growth score of 88 on the EVT can be interpreted as indicating an expressive vocabulary level somewhat below that of a typical 2-year-old. The onset of the receptive vocabulary trajectory did not differ between groups, F(1, 125) = 2.78, p = .098, partial η2 = .02. Thus, at the earliest stage of language learning that was examined, receptive vocabulary levels were not significantly delayed beyond expressive vocabulary levels in boys with ASD on average. The positive relationship between receptive and expressive vocabulary was significant, F(1, 125) = 835.70, p < .001, partial η2 = .87; however, this relationship differed between groups, yielding a significant interaction, F(1,125) = 11.98, p = .001, partial η2 = .09. The slope was shallower for boys with ASD, meaning that receptive vocabulary was less advanced relative to expressive vocabulary as it increased across participants. For boys with ASD, receptive vocabulary increased at a rate of 20 % less than that for boys with typical development relative to expressive vocabulary, leading to lower predicted receptive vocabulary at advanced levels of expressive vocabulary. The difference in slopes can be seen in Fig. 4.
Fig. 4.
Trajectory of receptive vocabulary relative to expressive vocabulary for boys with typical development (TD) and boys with ASD
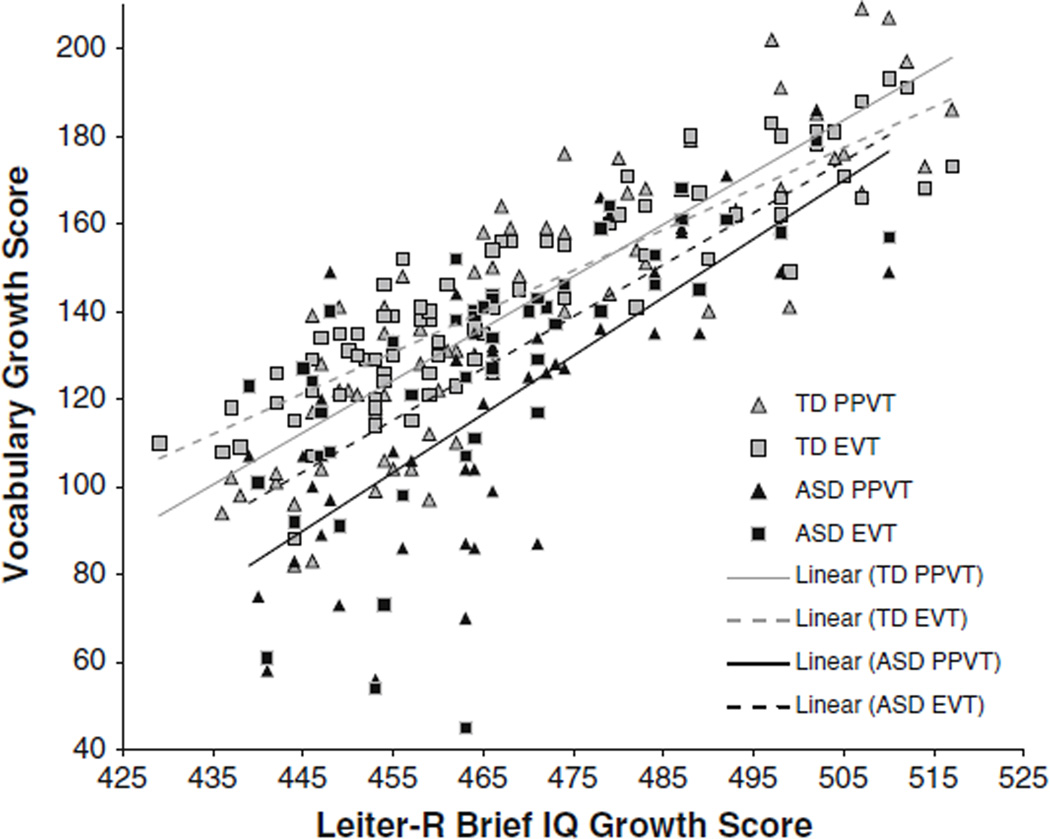
Finally, we calculated trajectories for receptive and expressive vocabulary simultaneously relative to nonverbal cognitive ability as a way to evaluate the relationship between receptive and expressive vocabulary while taking into account nonverbal cognitive ability, given the documented association between language and nonverbal cognition in children with ASD (e.g., Luyster et al. 2008). As expected, the positive relationship between nonverbal cognitive ability and vocabulary was significant, F(1, 125) = 215.78, p < .001, partial η2 = .63; see Fig. 5. The effect of group was also significant, with higher performance on these measures by the boys with typical development, F(1, 125) = 16.83, p < .001, partial η2 = .12.
Fig. 5.
Trajectories of receptive and expressive vocabulary relative to nonverbal cognition for boys with typical development (TD) and boys with ASD
In this repeated-measures analysis, the Group (ASD, typical development) × Task (PPVT, EVT) interaction was not significant, F(1, 125) = 1.12, p = .291, partial η2 = .01, whereas a Task × Nonverbal Cognition interaction indicated a difference in rate of development in the two tasks on average, F(1, 125) = 13.88, p < .001, partial η2 = .10. Neither the Group × Nonverbal Cognition interaction, F(1, 125) = 1.54, p = .217, partial η2 = .012, nor the Group × Task × Nonverbal Cognition interaction, F(1, 125) = .96, p = .329, partial η2 = .008, was significant. Thus, there was no evidence that the developmental relationship among tasks differed between groups. These findings suggest that, although vocabulary is delayed relative to nonverbal cognitive ability, the relationship between receptive vocabulary, expressive vocabulary, and nonverbal cognition does not differ between groups. This analysis provides a caveat to the trajectories examining receptive vocabulary separately with individual predictors (i.e., age, nonverbal cognition, expressive vocabulary) in that the interaction between vocabulary domain and group does not hold when taking into account nonverbal cognitive ability.
Discussion
The purpose of the present study was to characterize the vocabulary ability of boys with ASD with respect to the relative weakness in receptive language that has been ascribed to children with ASD, using co-normed direct assessment measures of receptive and expressive vocabulary and a strong comparison group of typically developing boys. We took a more nuanced approach to the characterization of the relationships among vocabulary and cognition in ASD and avoided many of the methodological limitations of previous studies on this topic. Nevertheless, the current results converge with previous research demonstrating a receptive language weakness in ASD that emerges at higher ability levels.
Cross-Sectional Trajectories of Receptive Vocabulary
Based on a cross-sectional developmental trajectories approach (Thomas et al. 2009), we concluded that receptive vocabulary ability of school-age boys with ASD is, on average, delayed in terms of level (onset) and rate (trajectory slope) relative to chronological age, delayed in onset relative to nonverbal cognition, and delayed in trajectory slope relative to expressive vocabulary. A principal advantage of this analysis approach over traditional matched-group designs is the estimate of differences between groups in the continuous relationship between the dependent measure and other theoretically chosen constructs, allowing these conclusions to be drawn.
The current results replicated previous findings that receptive vocabulary is delayed relative to chronological age and nonverbal cognitive ability expectations in boys with ASD (Charman et al. 2003; Ellis Weismer et al. 2010). In and of itself, the finding that vocabulary comprehension lags behind age expectations in boys with ASD is not surprising given the diagnostic criteria for ASD, previous research on language ability in children with ASD (Luyster et al. 2007), and the relationship between nonverbal cognition and language development in children with ASD (Bopp et al. 2009; Thurm et al. 2007). Nonetheless, as in previous research on younger children with ASD, we found that receptive vocabulary was significantly associated with age, nonverbal cognition, and expressive vocabulary (Hudry et al. 2010; Luyster et al. 2007; Volden et al. 2011).
However, in the current sample, the relationships between receptive vocabulary and both age and expressive vocabulary were significantly weaker in boys with ASD than boys with typical development. This difference between groups suggests not only that receptive vocabulary falls further behind across childhood in boys with ASD, but also that as expressive vocabulary develops, receptive vocabulary is unlikely to keep pace with the rate observed for typically developing boys. Although level of receptive vocabulary ability was not delayed for boys with ASD at the trajectory onset, we estimated the discrepancy in the receptive-expressive vocabulary trajectory slope for boys with ASD as approximately 20 % shallower than for boys with typical development. The finding that expected receptive vocabulary abilities are lower for boys with ASD than typical development as expressive vocabulary abilities increase corresponds with previous studies on language in ASD (Hudry et al. 2010), but extends these findings to a diverse sample of 4- to 11-year-old boys using an improved methodological approach.
Although we found that receptive vocabulary ability did not advance at the rate expected given expressive vocabulary, this result was not significant when controlling for nonverbal cognitive ability. In that repeated-measures analysis, no interactions with group were significant, suggesting that the relationship between receptive and expressive vocabulary is not different for boys with ASD and typical development after taking nonverbal cognitive ability into account. Thus, on average, vocabulary development for boys with ASD does not fall outside of developmental-level expectations. This conclusion differs from that of Hudry et al. (2010) and Volden et al. (2011), who failed to statistically account for nonverbal cognitive abilities in testing differences between receptive and expressive language and who examined an earlier developmental period (i.e., preschool children). Indeed, a shallower trajectory of receptive relative to expressive vocabulary might be accounted for by nonverbal cognitive ability, a topic that needs to be addressed in longitudinal research.
An alternative explanation for the discrepancy between the current results and studies of preschool children relates to the language domains assessed. It is possible that, when examining vocabulary ability in isolation, weaknesses in receptive ability are accounted for largely by nonverbal cognitive ability, but not when assessing receptive language broadly defined, as with an omnibus test. The results reported by Kjelgaard and Tager-FIusberg (2001) lend some support for this explanation in that a relative receptive weakness was present using the CELF, but not the PPVT and EVT, although the comparison of findings for omnibus language and vocabulary measures is ambiguous for this study because only a subgroup of participants completed the CELF. Future research will benefit from examining multiple distinct domains of language with respect to receptive and expressive skills in individuals with ASD.
Traditional Matched-Group Comparisons
Despite some evidence of floor effects for standard scores and age-equivalents, the traditional group-matching analyses revealed a weakness in receptive vocabulary regardless of dependent measure: growth score, standard score, or age-equivalent. Similarly, Hudry et al. (2010) and Volden et al. (2011) found that PLS receptive age-equivalent scores were lower than expressive age-equivalent scores in preschoolers with ASD. Although results from the cross-sectional developmental trajectories approach were in general agreement with traditional matched-group analyses, the former approach offers additional theoretical advantages, not only providing a compelling justification for longitudinal studies but also a scaffold for well-developed hypotheses about the nature and course of vocabulary development in children with ASD. Results between analysis strategies contrasted in that the interaction between group and vocabulary task remained significant in the traditional analysis, but not in the trajectory analysis, when controlling for nonverbal cognitive ability. Minimizing the role of development, including change with age or other dimensions of ability, could lead to spurious conclusions regarding phenotypic profiles (Karmiloff-Smith 1998).
A further limitation of the traditional matched-groups comparison was the loss of power due to excluding participants to obtain a “better” match, accompanied by truncation of the range of ability of the groups, limiting the generalizability of conclusions. Additionally, the likelihood of obtaining floor effects for standard and age-equivalent scores when assessing children with neurodevelopmental disorders further supports the use of growth scores, paired with statistical techniques that emphasize development and capture the full range of the ASD phenotype. Growth scores will continue to be useful for assessing levels of ability and change in individuals with ASD as research moves beyond the descriptive to explanatory, longitudinal studies.
Individual Variability and Discrepancies in Vocabulary Ability
The heterogeneity in receptive vocabulary among boys with ASD across age, nonverbal cognition, and expressive language ability is apparent in Figs. 2, 3, 4 and 5. This extensive variability among individuals extends to expressive vocabulary as well (Smith et al. 2007). For the school-age boys in the current sample, difference scores between receptive and expressive abilities for individual participants were correlated with nonverbal cognition. This suggests that delays in receptive vocabulary relative to expressive vocabulary are likely to be observed as nonverbal cognitive ability increases. This finding also converges with the trajectory analysis relative to expressive vocabulary, in which the slope, but not the onset, was lower for boys with ASD than typical development. Although contrasting with Volden et al. (2011), the current results extend those from Hudry et al. (2010) regarding the relationship between advanced cognition and relative receptive deficits to school-age children with ASD. However, Hudry et al. also found this profile to be associated with lower total ADOS scores rather than higher ADOS severity scores as in the current sample, although this result did not reach statistical significance in the present study. In general, the observed effects align with the notion that increased ability levels might allow greater detection of a relative weakness in receptive vocabulary.
One way to bridge from conclusions drawn about phenotypic averages to individual patterns of ability will be to identify meaningful subgroups in the ASD phenotype. This strategy has been advocated by Tager-Flusberg and Joseph (2003) and applied to other populations with language impairments, such as SLI. A recent study of school-age children with a history of SLI identified seven subgroups, which were differentiated in terms of level of language impairment (Conti-Ramsden et al. 2012). Of note is that two of these subgroups, which were among the most impaired, displayed the atypical profile of weaker receptive than expressive language that has been ascribed to ASD. In addition to highlighting the utility of describing phenotypic subgroups when sample sizes allow (Charman et al. 2011), these findings suggest that relative receptive weaknesses may not be a unique marker of language impairment in ASD per se and/or that subgroups of children with SLI and ASD share common aspects of ability profiles, as has been suggested by others (Tager-Flusberg 2006; Tomblin 2011). In the current study, few boys with ASD showed the typical profile of a relative receptive advantage; however, relative deficits in receptive vocabulary–although not always large–were observed across the range of age, nonverbal cognitive ability, and autism symptom severity, which might lead one to believe that all the relevant dimensions for discriminating phenotypic ASD subgroups have yet to be identified.
Limitations and Future Directions
Several limitations temper our conclusions. First, our focus was on vocabulary, but we were unable to distinguish among types of vocabulary, such as abstract, relational, or mental state words, using the PPVT and EVT. It is possible that such lexical categories are associated with different developmental trajectories. Second, many typically developing boys had statistically higher receptive than expressive vocabulary standard scores, which could have led to the appearance of relative receptive deficits in boys with ASD. In this sense, it should be acknowledged that the current sample of typically developing boys did not perform within the expectations of the samples used to norm the PPVT and EVT, perhaps because of selection bias related to participation in a longitudinal study or the restricted demographics of the locales from which they were sampled. The fact that nonverbal cognitive ability at least partially accounted for the differential patterns of receptive relative to expressive vocabulary across groups does not diminish this concern, given the above-average performance of the typically developing boys on the Leiter-R. Variability among the participants with ASD on the Leiter-R was a strength of the study in that a large range of the ASD phenotype was captured, including those with above-average cognitive abilities and those with intellectual disability; however, replication of these findings will be necessary to fully understand the role of nonverbal cognition in the relationship between receptive and expressive vocabulary across development in ASD. In addition, our samples included only boys and the current findings might not extend to girls.
In terms of the abilities of children with ASD, measures of receptive language rely heavily on compliance and it can often be unclear whether the demands of the task or comprehension of the target forms provide the greater challenge to any given child with ASD. Our expressive vocabulary measure required only a verbal label of a picture, whereas our receptive vocabulary measure required coordination of a fine motor response and inhibition of that response prior to comparison of all four stimulus plates on a page of the test book. These performance differences, which are inherent in standardized tests, make it difficult to equate nonlinguistic processing demands across modalities or to precisely determine the source of poor performance. In fact, the task demands associated with standardized language testing have led many to assess language comprehension in children with ASD using paradigms designed for infants and toddlers that require only eye gaze as a response (e.g., Swensen et al. 2007). These preferential looking paradigms avoid some of the shortcomings of standardized behavioral testing, but have limitations themselves including the need for specialized equipment, resources for stimuli creation and eye gaze coding, and the lack of norms for accuracy or speed of processing for vocabulary comprehension. Future research on receptive and expressive vocabulary in individuals with ASD or other neurodevelopmental disorders will be shaped by these issues.
If a relative weakness in receptive vocabulary is not an artifact of measurement and continues to be replicated across multiple assessment techniques, developmental explanations for a relative receptive vocabulary deficit and its potential downstream effects on later learning will be important areas for future research. One theory on cognitive ability in ASD posits that declarative memory is impaired in some individuals with ASD, whereas procedural (i.e., implicit) learning is intact (Boucher 2012; Boucher et al. 2008). It will be of great interest to examine whether such a theory can account for receptive vocabulary deficits, accompanied by relatively intact syntactic ability (but see Eigsti et al. 2007), in the ASD phenotype or subgroups thereof. Whether nonverbal cognitive ability–perhaps mediated by aspects of memory–acts as a limiting factor for receptive vocabulary development in children with ASD and whether this extends to other neurodevelopmental disorders remains to be seen (Boucher et al. 2008). Longitudinal studies with cross-syndrome comparisons will be crucial for disentangling these factors.
Summary and Conclusions
To characterize receptive vocabulary ability in boys with ASD, this study estimated cross-sectional developmental trajectories using a large comparison group of typically developing boys with overlapping age and nonverbal cognitive ability, a requirement and advantage of the approach. Findings suggest that, on average, boys with ASD experience delays in receptive vocabulary relative to age and nonverbal cognition. A shallower trajectory between receptive and expressive vocabulary was also detected in boys with ASD. Although receptive vocabulary was found to lag behind as expressive vocabulary levels increased, this conclusion no longer held after accounting for nonverbal cognition. The factors that contribute to vocabulary development in children with ASD are complex and this cross-sectional study provides a basis for exploring mechanisms of vocabulary acquisition in future longitudinal work.
Acknowledgments
This research was supported by NIH grants R01HD024356, R01HD054764, T32 DC005359, and P30HD03352, as well as F31DC010959, which was awarded to the first author. Preliminary results were presented at the 2012 Symposium on Research in Child Language Disorders in Madison, Wisconsin. We offer our gratitude to the families who participated in this study. We would like to thank those individuals who contributed to the collection of data: Beth Goodlin-Jones, Susan Harris, David Benjamin, Susen Schroeder, Sara Armson, Eileen Haebig, Ashley Oakes, and Cecilia Compton.
Footnotes
Conflict of interest
Sara T. Kover, Andrea S. McDuffie and Leonard Abbeduto have no conflicts of interest. Randi J. Hagerman has received funding from Novartis, Roche, and Seaside Therapeutics.
Contributor Information
Sara T. Kover, Email: kover@wisc.edu, Waisman Center, University of Wisconsin-Madison, Room 475, 1500 Highland Avenue, Madison, WI 53705, USA.
Andrea S. McDuffie, Email: andrea.mcduffie@ucdmc.ucdavis.edu, MIND Institute, University of California, Davis, Davis, CA, USA; Department of Psychiatry and Behavioral Sciences, University of California, Davis, Davis, CA, USA.
Randi J. Hagerman, Email: randi.hagerman@ucdmc.ucdavis.edu, MIND Institute, University of California, Davis, Davis, CA, USA; Department of Pediatrics, University of California, Davis, Davis, CA, USA.
Leonard Abbeduto, Email: leonard.abbeduto@ucdmc.ucdavis.edu, MIND Institute, University of California, Davis, Davis, CA, USA; Department of Psychiatry and Behavioral Sciences, University of California, Davis, Davis, CA, USA.
References
- Annaz D. The development of visuospatial processing in children with autism, Down syndrome, and Williams syndrome. London: University of London; 2006. [Google Scholar]
- Bopp KD, Mirenda P, Zumbo BD. Behavior predictors of language development over 2 years in children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2009;52(5):1106–1120. doi: 10.1044/1092-4388(2009/07-0262). [DOI] [PubMed] [Google Scholar]
- Boucher J. Research review: structural language in autistic spectrum disorder—Characteristics and causes. Journal of Child Psychology and Psychiatry. 2012;55(3):219–233. doi: 10.1111/j.1469-7610.2011.02508.x. [DOI] [PubMed] [Google Scholar]
- Boucher J, Bigham S, Mayes A, Muskett T. Recognition and language in low functioning autism. Journal of Autism and Develompental Disorders. 2008a;38(7):1259–1269. doi: 10.1007/s10803-007-0508-8. [DOI] [PubMed] [Google Scholar]
- Boucher J, Mayes A, Bigham S. Memory, language, and intellectual ability in low-functining autism. In: Boucher J, Bowler DM, editors. Memory in autism. Cambridge: Cambridge University Press; 2008b. pp. 268–289. [Google Scholar]
- Charman T, Drew A, Baird C, Baird G. Measuring early language development in preschool children with autism spectrum disorder using the MacArthur communicative development inventory (Infant Form) Journal of Child Language. 2003;30(1):213–236. doi: 10.1017/s0305000902005482. [DOI] [PubMed] [Google Scholar]
- Charman T, Jones CR, Pickles A, Simonoff E, Baird G, Happe F. Defining the cognitive phenotype of autism. Brain Research. 2011;1380:10–21. doi: 10.1016/j.brainres.2010.10.075. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, St Clair MC, Pickles A, Durkin K. Developmental trajectories of verbal and nonverbal skills in individuals with a history of SLI: From childhood to adolescence. Journal of Speech, Language, and Hearing Research. 2012;55:1716–1735. doi: 10.1044/1092-4388(2012/10-0182). [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test. 3rd ed. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Dunn LM, Dunn DM. Peabody picture vocabulary test. 4th ed. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Eigsti I-M, Bennetto L, Dadlani MB. Beyond pragmatics: Morphosyntactic development in autism. Journal of Autism and Developmental Disorders. 2007;37:1007–1023. doi: 10.1007/s10803-006-0239-2. [DOI] [PubMed] [Google Scholar]
- Ellis Weismer S, Lord C, Esler A. Early language patterns of toddlers on the autism spectrum compared to toddlers with developmental delay. Journal of Autism and Develompental Disorders. 2010;40(10):1259–1273. doi: 10.1007/s10803-010-0983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Bates E, Thai DJ, Pethick SJ. Variability in early communicative development. Monographs of the Society for Research in Child Development. 1994;59(5):1–185. [PubMed] [Google Scholar]
- Fenson L, Dale P, Reznick JS, Thai D, Bates E, Hartung J, et al. The MacArthur communicative development inventory: User’s guide and technical manual. San Diego, CA: Singular Publishing Group; 1993. [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Hendrick D, Prather E, Tobin A. Sequenced inventory of communication development. Seattle, WA: University of Washington Press; 1984. (Vol. Revised) [Google Scholar]
- Hudry K, Leadbitter K, Temple K, Slonims V, McConachie H, Aldred C, et al. Preschoolers with autism show greater impairment in receptive compared with expressive language abilities. International Journal of Language and Communication Disorders. 2010;45(6):681–690. doi: 10.3109/13682820903461493. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Boucher J, Russell J. Language profiles in children with autism: Theoretical and methodological implications. Autism. 1997;1(1):57–76. [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Science. 1998;2(10):390–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kjelgaard M, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16(2–3):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, Atwood AK. Establishing equivalence: Methodological progress in group-matching design and analysis. American Journal on Intellectual and Developmental Disabilities. 2013;118(1):3–15. doi: 10.1352/1944-7558-118.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, McDuffie A, Abbeduto L, Brown WT. Effects of sampling context on spontaneous expressive language in males with fragile X syndrome or Down syndrome. Journal of Speech, Language, and Hearing Research. 2012;55(4):1022–1038. doi: 10.1044/1092-4388(2011/11-0075). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autiam diagnostic observation schedule—Generic. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Loucas T, Charman T, Pickles A, Chandler S, Meldrum D, Baird G. Autistic symptomatology and language ability in autism spectrum disorder and specific language impairment. Journal of Child Psychology and Psychiatry. 2008;49(11):1184–1192. doi: 10.1111/j.1469-7610.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- Luyster RJ, Kadlec MB, Carter A, Tager-Flusberg H. Language assessment and development in toddlers with autism spectrum disorders. Journal of Autism and Develompental Disorders. 2008;38(8):1426–1438. doi: 10.1007/s10803-007-0510-1. [DOI] [PubMed] [Google Scholar]
- Luyster R, Lopez K, Lord C. Characterizing communicative development in children referred for autism spectrum disorders using the Mac Arthur-Bates communicative development inventory (CDI) Journal of Child Language. 2007;34(3):623–654. doi: 10.1017/s0305000907008094. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Kover ST, Hagerman R, Abbeduto L. Investigating word learning in fragile X syndrome: A fast- mapping study. Journal of Autism and Develompental Disorders. 2012 doi: 10.1007/s10803-012-1717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Methodological Issues in group-matching designs: a levels for control variable comparisons and measurement characteristics of control and target variables. Journal of Autism and Developmental Disorders. 2004;34(1):7–17. doi: 10.1023/b:jadd.0000018069.69562.b8. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF. Methodological issues in cross-syndrome comparisons: Matching procedures, sensitivity (Se) and specificity (Sp) Monographs of the Society for Research in Child Development. 1999;64(1):115–130. doi: 10.1111/1540-5834.00011. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF. Methodological issues in cross-group comparisons of language and/or cognitive development. In: Levy Y, Schaeffer J, editors. Language competence across populations: Toward a definition of specific language impairment. Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 233–258. [Google Scholar]
- Mervis CB, Robinson BF. Designing measures for profiling and genotype/phenotype studies of individuals with genetic syndromes or developmental language disorders. Applied Psycholinguistics. 2005;26:41–64. [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, et al. Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics. 2006;27(2 Suppl):S69–S78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Mullen EM. In: Mullen scales of early learning. AGS, editor. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Prizant BM, Duchan JF. The functions of immediate echolalia in autistic children. Journal of Speech and Hearing Disorders. 1981;46(3):241–249. doi: 10.1044/jshd.4603.241. [DOI] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter international performance scale—revised. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism diagnostic interview—revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 3rd ed. San Antonio, TX: Psychological Corporation; 1995. [Google Scholar]
- Smith V, Mirenda P, Zaidman-Zait A. Predictors of expressive vocabulary growth in children with autism. Journal of Speech, Language, and Hearing Research. 2007;50(1):149–160. doi: 10.1044/1092-4388(2007/013). [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla D, Cicchetti D. Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. Vineland-II adaptive behavior scales. Circle Pines, MN: American Guidance Services, Inc; 2005. [Google Scholar]
- Swensen LD, Kelley E, Fein D, Naigles LR. Processes of language acquisition in children with autism: Evidence from preferential looking. Child Development. 2007;78(2):542–557. doi: 10.1111/j.1467-8624.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Defining language phenotypes in autism. Clinical Neuroscience Research. 2006;6(3–41):219–224. [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society of London. 2003;558(1430):303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MS, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. Journal of Speech, Language, and Hearing Research. 2009;52(2):336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- Thurm A, Lord C, Lee LC, Newschaffer C. Predictors of language acquisition in preschool children with autism spectrum disorders. Journal of Autism and Develompental Disorders. 2007;37(9):1721–1734. doi: 10.1007/s10803-006-0300-1. [DOI] [PubMed] [Google Scholar]
- Tomblin B. Co-morbidity of autism and SLI: kinds, kin and complexity. International Journal of Language and Communication Disorders. 2011;46(2):127–137. doi: 10.1111/j.1460-6984.2011.00017.x. [DOI] [PubMed] [Google Scholar]
- Volden J, Smith IM, Szatmari P, Bryson S, Fombonne E, Mirenda P, et al. Using the preschool language scale, fourth edition to characterize language in preschoolers with autism spectrum disorders. American Journal of Speech-Language Pathology. 2011;20(3):200–208. doi: 10.1044/1058-0360(2011/10-0035). [DOI] [PubMed] [Google Scholar]
- Williams K. Expressive vocabulary test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Williams KT. Expressive vocabulary test. 2nd ed. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Zimmerman I, Steiner V, Pond R. Preschool language scale. 4th ed. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]