Abstract
Purpose
We evaluated treatment outcomes of thymic carcinomas to determine prognostic factors for survival.
Materials and Methods
Between May 1988 and May 2009, 41 patients had pathologic diagnosis of thymic carcinoma in Seoul National University Hospital, Seoul, Korea. Of these, 40 patients were followed up to 188 months after treatment. The mean age of all patients was 58.3 years and male to female ratio was 23 to 17.
Results
Among 30 patients who underwent surgical resection, 26 achieved R0 resection and postoperative radiotherapy (PORT) was performed in 22 patients (73%). Various chemotherapeutic regimens were given with local treatment modalities, surgery and/or radiotherapy, in 12 patients. The 5-year locoregional control (LRC), distant metastasis-free survival, progression-free survival (PFS), and overall survival were 79.4%, 53.0%, 42.6%, and 63.6%, respectively. Patients with Masaoka stage I or II showed excellent prognosis of 5-year PFS around 90%. In advanced stages, invasion of the great vessels or atrium by thymic carcinomas was negative prognostic factor for PFS in univariate analysis. Lymph node involvement was statistically significant factor for LRC and PFS. Local or regional recurrence was infrequent after surgical resection followed by PORT, while distant metastasis was the major component of treatment failure.
Conclusion
Complete resection followed by PORT provided remarkable local control without severe acute toxicities in patients with stage II and favorable stage III thymic carcinoma. Invasion of the great vessels or atrium was statistically significant prognostic factor for PFS.
Keywords: Thymic carcinoma, Prognostic factor, Great vessel invasion, Survival
Introduction
Thymic tumors are the most common tumor arising in the thymus, which include both thymomas and thymic carcinomas. Thymic carcinomas account for less than 1% of thymic malignancies [1]. Thymomas grow very slowly and act like benign tumor. Even after thymomas had become invasive and invaded adjacent organ of thorax, hematogenous or lymphogenous metastasis is still very infrequent [2]. Pleural or pericardial dissemination is a common pattern of progression in this disease [3]. In contrast, thymic carcinomas have more aggressive natures. Thymic carcinomas invade surrounding structures frequently and have early metastasis to intrathoracic lymph nodes and distant organs. Thus, most patients with thymic carcinomas have advanced disease, resulting inferior survival compared with that of thymomas [4].
The pathologic classification of thymic carcinomas had been somewhat confused until World Health Organization (WHO) pathologic classification system was published in 2004, and thus, most of previous studies reporting treatment outcomes of thymic carcinoma had included well-differentiated thymic carcinoma (WHO type B3) and thymic carcinoma (WHO type C) together and regarded them as one disease category, thymic carcinoma. Given that histologic type of the carcinoma is one of the crucial determinants of prognosis, it is necessary to evaluate treatment outcomes of thymic carcinomas separately. Granting its rare incidence, clinical studies of true thymic carcinomas are very few. Therefore, we retrospectively reviewed the clinical and pathologic data of patients with thymic carcinomas to evaluate treatment outcomes and to find prognostic factors for survivals.
Materials and Methods
Between May 1988 and May 2009, 41 patients were diagnosed as thymic carcinoma with tissue confirmation in Seoul National University Hospital, Seoul, Korea. Of these, 40 patients were the subject of the present study excluding one patient with immediate follow-up loss after treatment. The present study was approved by Institutional Review Board.
The stage of thymic carcinoma was classified based on the Masaoka staging system for thymomas and histologic subtypes were determined by findings from microscopy and immunohistochemical staining according to 2004 WHO histologic classification. We only included patients with true thymic carcinoma (type C), excluding well-differentiated thymic carcinoma (type B3), small cell carcinoma, or carcinoid tumors.
All patients were followed up for at least 12 months or until the time of their death. The median follow-up of patients was 51.0 months ranging from 6.7 to 187.5 months. After completing treatment, treatment responses were evaluated through computed tomography (CT) scan and/or positron emission tomography scan with some intervals. Disappearance of all measurable lesions was defined as complete remission (CR) and tumor size decrease less than 30% of initial long diameter as partial remission (PR). Tumor size increase larger than 20% of initial long diameter or new lesions on imaging scans was defined as progressive disease (PD), and other condition that does not meet both PR and PD as stable disease (SD).
The radiotherapy-related toxicities were evaluated by Common Terminology Criteria for Adverse Events (CTCAE) ver. 3.0.
1. Statistical analyses
The statistical analysis using the SPSS ver. 18 (SPSS Inc., Chicago, IL, USA) was performed, assuming that p-value less than 0.05 is statistically significant. The survival curves were generated using the Kaplan-Meier method and compared with log-rank test. The Cox proportional hazards model was applied for multivariate analysis and Fisher exact test to calculate the possibility of relationship between prognostic factors. Analysis of variance was adopted to compare the means of age between the Masaoka stages.
Results
Patient characteristics and histologic subtypes according to the stage are summarized in Table 1. The mean age of all patients was 58.3 years ranging from 19.8 to 80.8 years and male to female ratio was 23 to 17. Only 2 patients with routine check-up abnormality in chest CT scan had Eastern Cooperative Group performance status (ECOG PS) 0 and the other patients had ECOG PS 1 or 2. In the stage distribution, patients having stage III or IV were three-fourths of all patients. These patients were younger than those with stage I or II, but there was no statistical difference between each stages (p = 0.875). Squamous cell carcinoma (65.0%) was the most common histologic subtype followed by undifferentiated carcinoma (22.5%), lymphoepithelioma-like carcinoma (7.5%), and basaloid carcinoma (5.0%). Treatment strategies of patients are displayed in Table 2.
Table 1.
Patients characteristics and histopathologic features according to the Masaoka stage
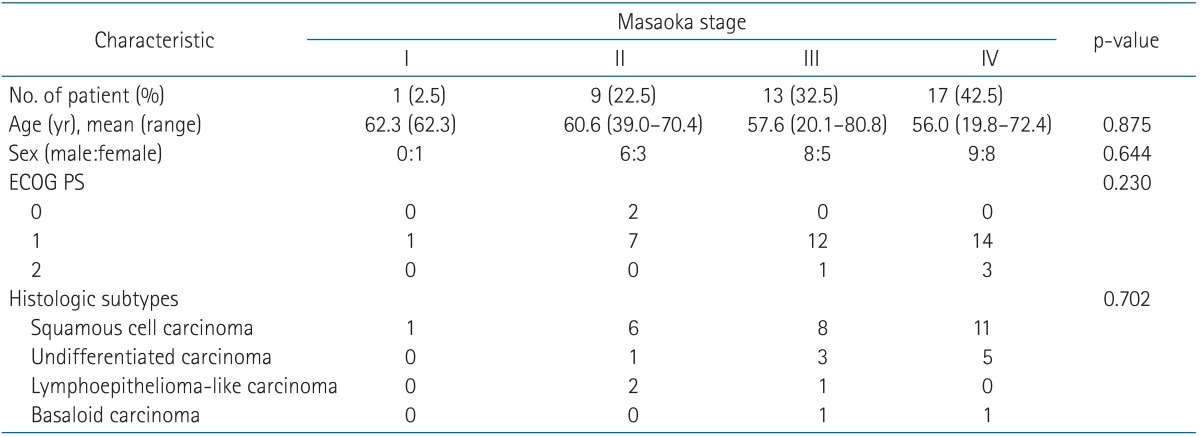
ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 2.
Distribution of the patients according to treatment methods
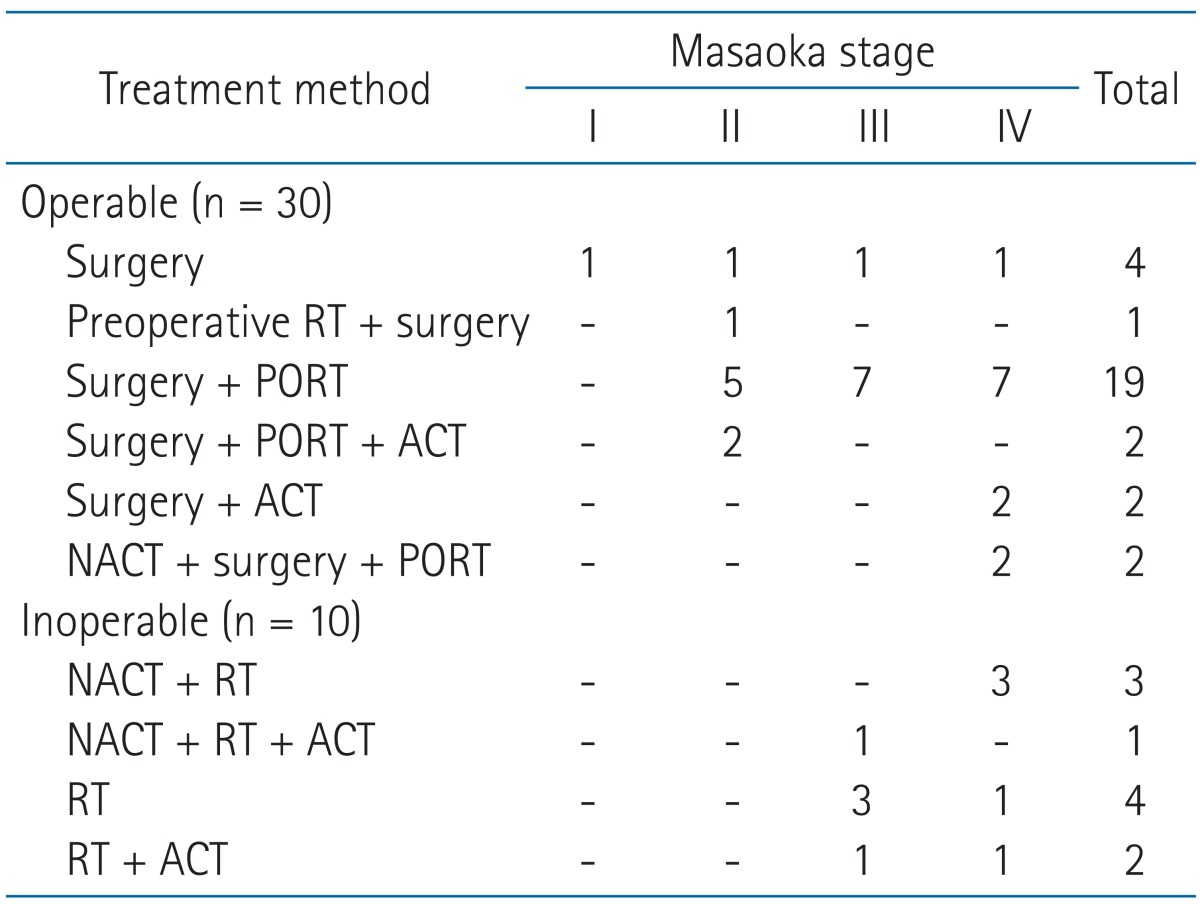
RT, radiation therapy; PORT, postoperative radiation therapy; ACT, adjuvant chemotherapy; NACT, neoadjuvant chemotherapy.
1. Biopsy and surgical resection
Ten patients were inoperable because of their medical problem in 2 patients, the great vessel encasement or invasion in 4, and distant metastatic disease in 4, respectively. They underwent mediastinoscopic or open biopsy. Surgical resection was generally avoided in patients with pleural implants or distant metastases. Twenty-eight of 30 (93.3%) operable patients underwent curative resection and the other 2 with pleural drop metastases did thymectomy and metastasectomy for tumor debulking.
Total thymectomy with en bloc resection of tumor was most commonly performed; wedge resection of involved lung was also performed when indicated. In 7 patients with innominate vein and/or superior vena cava (SVC) invasion, reconstruction using Gore-Tex was performed after partial resection of invaded structures. Chest wall reconstruction was done in one patient after en bloc resection of invaded chest wall. Resection margin status was as follows: R0 resection in 26 patients, R1 in 2, and R2 in 2, respectively.
2. Chemotherapy
A total of 12 patients underwent chemotherapy (Table 2). The regimens, cycles, and schedules of chemotherapy were variable. The most common was the combination of cyclophosphamide, adriamycin, and cisplatin (CAP) regimen in 1 to 6 cycles (n = 6). The combination of taxol and cisplatin was the second common regimen (n = 3). The overall response to neoadjuvant chemotherapy was PR in 2 (33.3%), SD in 3 (50.0%), and PD in 1 (16.7%), respectively. In patients with advanced stages, there was a trend of performing chemotherapy.
3. Radiation therapy
Among all patients, 32 underwent radiation therapy. Curative radiotherapy was performed in 5 patients with stage III and in one patient having lymph node metastasis on initial CT scan, respectively. Palliative radiotherapy was given to another 5 patients with stage IV. Preoperative and postoperative radiotherapy were performed in one and 23 patients, respectively. The patient with stage I was not candidate for PORT. Among 9 patients with stage II, 8 underwent PORT except one patient who was not fit for PORT owing to severe postoperative complication. Among 8 patients with stage III, who underwent surgical resection, PORT was delivered in 7. In stage IV, 9 of 12 patients underwent PORT, including one patient treated with total thymectomy and metastasectomy.
Megavoltage machines were utilized in treating patients. Two-dimensional radiotherapy planning system was used until 2003 and three-dimensional conformal radiotherapy was adopted thereafter. The median dose to tumor, tumor bed, and the mediastinum was 55.8 Gy (range, 54 to 61.2 Gy), 54.0 Gy (range, 45 to 60 Gy), and 45.0 Gy (range, 25.2 to 54 Gy), respectively. As for the response of radiotherapy, CR was achieved in 3 patients (21.4%) and PR in 8 (64.3%), respectively. Disease progression during radiotherapy was observed in 2 patients (14.3%).
4. Patterns of failure and survival
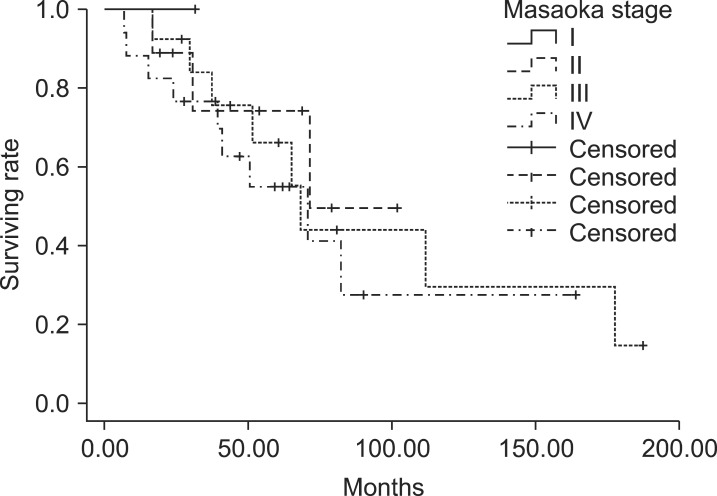
For all patients, 5-year locoregional control (LRC), distant metastasis-free survival (DMFS), progression-free survival (DFS), and overall survival (OS) were 79.4%, 53.0%, 42.6%, and 63.5%, respectively. The OS curves according to the Masaoka stage were displayed in Fig. 1.
Fig. 1.
The Kaplan-Meier overall survival curves were displayed according to the Masaoka stage. There was no difference between the Masaoka stages.
The patient with stage I was disease-free at last follow-up. Among 9 patients with stage II, one distant failure had developed at 3.2 months after treatment. The 5-year LRC, DMFS, and PFS for stage II patients were 100%, 88.9%, and 88.9%, respectively.
Locoregional failure after surgical resection and PORT was not observed in patients (n = 7) with stage III; 2 local recurrences were found in patients (n = 5) who underwent definitive radiotherapy. Seven patients developed distant metastases, which were the main cause of failure in stage III. The 5-year LRC, DMFS, and DFS for stage III patients were 82.1%, 46.2%, and 38.5%, respectively.
The survivals of 17 patients with stage IV were very disappointing. All patients except 4 developed regional and/or distant metastases, which were manifested mostly within 24 months. The 5-year LRC, DMFS, and DFS for stage IV patients were 67.1%, 42.9%, and 23.5%, respectively.
Isolated local recurrence was observed in one patient who underwent definitive radiotherapy alone at 41 months of follow-up. The site of regional nodal recurrence was pulmonary lymph node outside of radiation field (n = 1), supraclavicular lymph node at the margin of radiation field (n = 1), and neck lymph node in whom not undergoing radiotherapy (n = 1), respectively. Simultaneous recurrences of local, regional, and/or distant metastasis developed in 3 patients. Locoregional recurrence rate of radiotherapy alone was 18.2% (2 of 11) and that of PORT was 8.7% (2 of 23), respectively. The most common site of metastasis was the lungs (44.4%) followed by pleura (33.3%), and liver (27.8%).
Acute side effects of radiotherapy were observed in 21 patients (61.8%); 19 in grade 1 and 2 in grade 2, respectively. Late radiation fibrosis was found in 6 patients and radiation pneumonitis in 2.
5. Prognostic factors
When comparing clinical and pathologic factors of all patients, under the age of 60 years old, atrium invasion by thymic carcinomas, lymph node involvement were statistically significant factors predicting PFS (Table 3). The Masaoka stage did not predict PFS or OS of the patients. Clear resection margin and invasion of the great vessels (IOGVs) including the aorta, SVC and/or the innominate vein affected DMFS. Lymph node metastasis had an adverse effect on LRC and PFS.
Table 3.
Prognostic factors for LRC, DMFS, and PFS of all patients
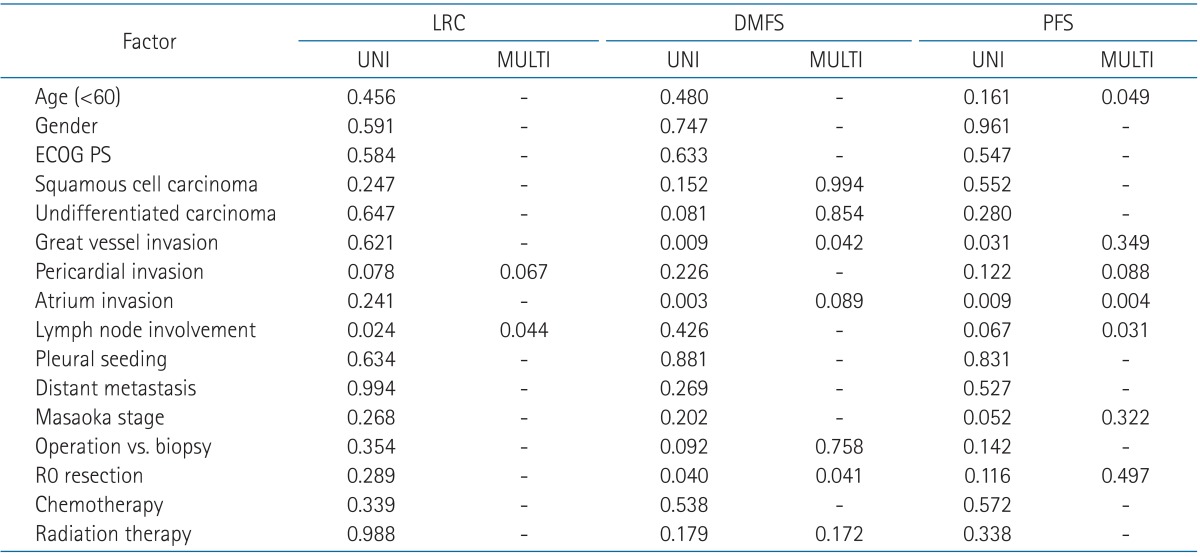
LRC, locoregional control; DMFS, distant metastasis-free survival; PFS, progression-free survival; UNI, univariate analysis; MULTI, multivariate analysis; ECOG PS, Eastern Cooperative Oncology Group performance status.
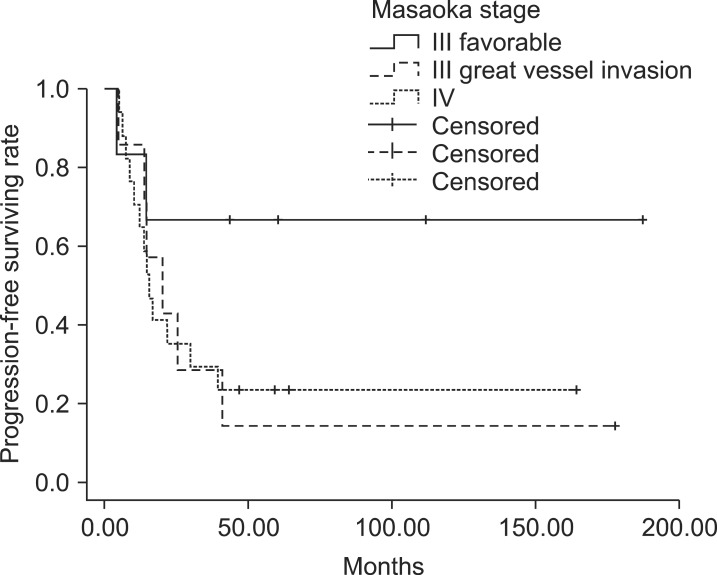
In patients with stage III, IOGVs by thymic carcinoma was detrimental to PFS (p = 0.035) in univariate analysis, but the others were not statistically significant (Table 4). Among 8 patients with IOGVs, 6 developed distant metastases in lung (n = 2), pleura (n = 2), lung and pleura (n = 1), and liver (n = 1). The PFS of patients with IOGVs was rather similar to that of patients with stage IV (Fig. 2). Atrial invasion by thymic carcinoma was also negative prognostic factor for LRC (p = 0.001) and histologic subtype of squamous cell carcinoma was related to better DMFS (p = 0.036).
Table 4.
Prognostic factors for LRC, DMFS, and PFS in patients with Masaoka stage III
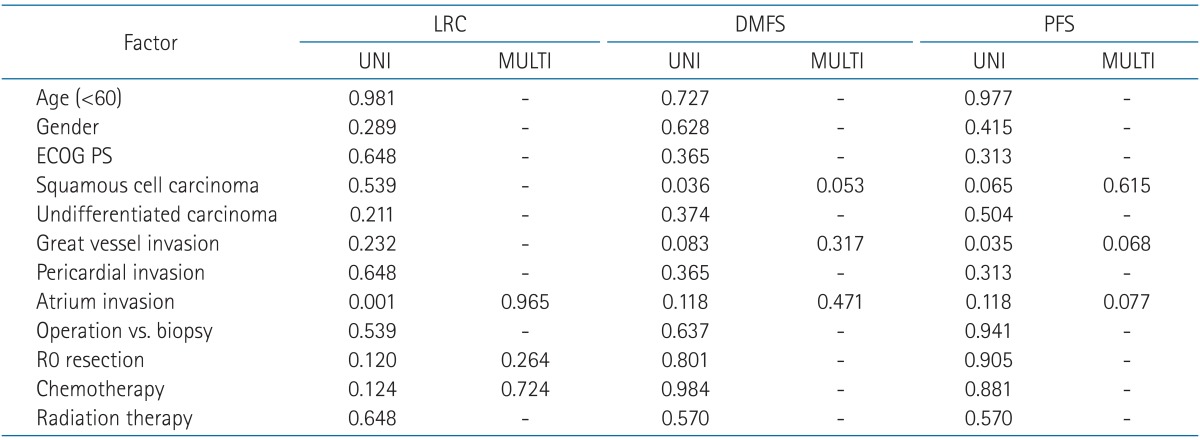
LRC, locoregional control; DMFS, distant metastasis-free survival; PFS, progression-free survival; UNI, univariate analysis; MULTI, multivariate analysis; ECOG PS, Eastern Cooperative Oncology Group performance status.
Fig. 2.
The progression-free survival curve of stage III with great vessel invasion and/or atrial invasion was not different from that of stage IV (p = 0.957).
Discussion and Conclusion
The status of resection is one of the widely accepted prognostic factors for survival [4-9]. Blumberg at al. [5] demonstrated that when surgical resection was incomplete, main pattern of failure was local recurrence. Huang et al. [4], in a similar context, reported in their study, in which 21 of 23 patients with thymic carcinoma underwent PORT, that incomplete resection was associated with local relapse and early distant metastasis. Patterns of failure in the present study is similar to the study of Huang et al. [4] in that local or regional recurrence had developed infrequently, but failure at distant sites exceeded three-fourths of all failures. In the present study, R0 resection was associated with DMFS (p = 0.040) but not LRC (p = 0.289). These results may be explained by the small number of event regarding locoregional failure that statistical power to detect the difference cannot be achieved.
Although surgical resection remains the mainstay in the treatment of thymic carcinoma, it is unclear which combination of treatment modalities is best for improving the survival of patients with advanced disease. Moreover, the role of PORT in thymic carcinomas is still controversial. In patients with R1 or R2 resection margin, PORT serves additional local control [10,11]. However, the high failure rate at distant sites raises a question regarding actual survival benefit of PORT in thymic carcinoma. In the largest retrospective study performed by Kondo and Monden [7], there was no difference of survival between surgery only and surgery plus PORT in patients with stage III and IV thymic carcinoma. Conversely, some authors argued routine use of PORT as a part of standard treatment for thymic carcinoma [12,13]. In the present study, the role of PORT cannot be assessed because most of the patients underwent PORT. However, it is of note that excellent locoregional control rate over 90% was achieved by PORT without acute grade 3 or 4 toxicities. The role of PORT, not only in patients with incomplete resection but also in complete resection, should further be investigated.
The IOGVs by thymic carcinomas is a poor prognostic factor for survival, often indicating unresectable disease. Blumberg et al. [5] showed that survival of patients was dependent on innominate vessel invasion, and Tseng et al. [3] also reported that invasion of the SVC, pulmonary vessels, or aorta was a significant predictor for poor prognosis. In the present study, DMFS and PFS were affected by IOGVs by thymic carcinomas as well: in 8 patients with IOGVs in this study, 6 had eventually developed distant metastases. However, in some selected patients with IOGVs by thymic carcinomas, surgical resection should be carefully considered as a part of treatment. Tseng et al. [3] suggested that if the tumor invading the SVC could be en-bloc resected, one could achieve long-term survival. In the present study, among 7 patients who underwent en-bloc resection of the SVC or innominate vein, early distant metastases within 4 months after surgery were found in 2 patients. Among the others, 3 patients had experienced distant metastasis at 12, 20, and 25 months of follow-up and 2 were disease-free at the last follow-up.
The prognosis of patients with locally advanced disease is dismal that to improve therapeutic outcomes, several studies addressed the potential role of neoadjuvant chemotherapy. In the present study, 6 patients underwent neoadjuvant chemotherapy and obtained clinical response of 33.3% which is similar to other studies [5,14]. However, Lucchi et al. [15] reported excellent response rate of thymic carcinoma to neoadjuvant chemotherapy based on cisplatin regimen. Moreover, a prospective phase II study enrolling patients with Masaoka stage III/IV thymic epithelial tumors adopted neoadjuvant doublet chemotherapy consisted of docetaxel and cisplatin. A promising result of PR 63% and SD 37% was shown, leading to complete resection of disease in 55.6% of patients [16]. In this regard, clinical trials evaluating the role of neoadjuvant chemotherapy in locally advanced thymic carcinomas should be conducted in a multi-institutional setting, considering the rarity of this disease.
The staging system of thymic carcinoma is a point of dispute. Although Masaoka staging system is being widely used, the prognostic value of this system has not been proved. Some authors [7] have insisted the usefulness of Masaoka staging system, but others [5,9] have not become convinced of its value. In the present study, the Masaoka stage was not a significant predictor for PFS in multivariate analysis (p = 0.322), but instead IOGVs or atrial invasion was more powerful factor predicting PFS. Especially, PFS of patients with unfavorable factors such as IOGVs and/or atrial invasion by thymic carcinomas did not show distinct difference from that of patients with stage IV. Given that IOGVs or atrial invasion by thymic carcinomas is associated with unresectability and early distant metastasis, it might be reasonable to regard such patients with unfavorable factors as stage IV. When those factors were regarded as stage IVA, the predictive value of modified Masaoka staging system was much improved compared with original system (p = 0.052 vs. p = 0.006 in univariate analysis; p = 0.697 vs. p = 0.046 in multivariate analysis). Furthermore, in the modified Masakoka system, a marginal OS difference was shown between stage I-III and IV (84.8% vs 53.0% at 5 years, p = 0.055).
There are some limitations of the present study. Besides of its retrospective nature, the heterogeneity of chemotherapeutic agents and schedules made it difficult to evaluate the appropriate efficacy of chemotherapy. Small number of patients, which may result in a few events, is another critical limitation of the present study.
In conclusion, complete resection followed by PORT provided remarkable local control without severe toxicity in stage II and favorable stage III thymic carcinoma. IOGVs and/or atrial invasion by thymic carcinomas were significant negative predictive factors for PFS. In patients with IOGVs and/or atrial invasion, multidisciplinary approach such as neoadjvuant chemotherapy followed by local treatment, surgery and/or radiotherapy, should be considered, given the high incidence of distant failure.
Acknowledgements
This work was supported by a grant No. 04-2012-0440 from the Seoul National University Hospital Research Fund, a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111098 and A120313), and National R&D Program through the Dongnam Institute of Radiological & Medical Sciences funded by the Ministry of Education, Science and Technology (50595-2012).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Masaoka A. Staging system of thymoma. J Thorac Oncol. 2010;5(10 Suppl 4):S304–S312. doi: 10.1097/JTO.0b013e3181f20c05. [DOI] [PubMed] [Google Scholar]
- 3.Tseng YL, Wang ST, Wu MH, Lin MY, Lai WW, Cheng FF. Thymic carcinoma: involvement of great vessels indicates poor prognosis. Ann Thorac Surg. 2003;76:1041–1045. doi: 10.1016/s0003-4975(03)00831-2. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg. 2009;138:26–31. doi: 10.1016/j.jtcvs.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg D, Burt ME, Bains MS, et al. Thymic carcinoma: current staging does not predict prognosis. J Thorac Cardiovasc Surg. 1998;115:303–308. doi: 10.1016/S0022-5223(98)70273-9. [DOI] [PubMed] [Google Scholar]
- 6.Mayer R, Beham-Schmid C, Groell R, et al. Radiotherapy for invasive thymoma and thymic carcinoma. Strahlenther Onkol. 1999;175:271–278. doi: 10.1007/BF02743578. [DOI] [PubMed] [Google Scholar]
- 7.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878–884. doi: 10.1016/s0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 8.Strobel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004;22:1501–1509. doi: 10.1200/JCO.2004.10.113. [DOI] [PubMed] [Google Scholar]
- 9.Bedini AV, Andreani SM, Tavecchio L, et al. Proposal of a novel system for the staging of thymic epithelial tumors. Ann Thorac Surg. 2005;80:1994–2000. doi: 10.1016/j.athoracsur.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Chang HK, Wang CH, Liaw CC, et al. Prognosis of thymic carcinoma: analysis of 16 cases. J Formos Med Assoc. 1992;91:764–769. [PubMed] [Google Scholar]
- 11.Hsu CP, Chen CY, Chen CL, et al. Thymic carcinoma: ten years' experience in twenty patients. J Thorac Cardiovasc Surg. 1994;107:615–620. [PubMed] [Google Scholar]
- 12.Yano T, Hara N, Ichinose Y, Asoh H, Yokoyama H, Ohta M. Treatment and prognosis of primary thymic carcinoma. J Surg Oncol. 1993;52:255–258. doi: 10.1002/jso.2930520412. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa K, Toita T, Uno T, et al. Treatment and prognosis of thymic carcinoma: a retrospective analysis of 40 cases. Cancer. 2002;94:3115–3119. doi: 10.1002/cncr.10588. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Kunitoh H, Kubota K, et al. Platinum-based chemotherapy with or without thoracic radiation therapy in patients with unresectable thymic carcinoma. Jpn J Clin Oncol. 2000;30:385–388. doi: 10.1093/jjco/hyd108. [DOI] [PubMed] [Google Scholar]
- 15.Lucchi M, Mussi A, Ambrogi M, et al. Thymic carcinoma: a report of 13 cases. Eur J Surg Oncol. 2001;27:636–640. doi: 10.1053/ejso.2001.1197. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Ahn MJ, Ahn JS, et al. A prospective phase II trial of induction chemotherapy with docetaxel/cisplatin for Masaoka stage III/IV thymic epithelial tumors. J Thorac Oncol. 2013;8:959–966. doi: 10.1097/JTO.0b013e318292c41e. [DOI] [PubMed] [Google Scholar]