Abstract
Purpose
To analyze the outcome of adjuvant postoperative external beam radiotherapy (EBRT) in well-differentiated thyroid cancer (WDTC).
Materials and Methods
We identified 84 patients treated with EBRT for WDTC from February 1981 to December 2010. Among them, we analyzed 39 patients who received EBRT after initial radical surgery. Twenty-four females and 15 males were included. The median age was 49 years (range, 16 to 72 years). There were 34 papillary thyroid carcinomas and 5 follicular thyroid carcinomas. Most patients showed pathologic T3/T4 stage (54%/26%). Ten patients (25.6%) had gross residual tumors. Five patients (12.8%) had tumor cells at the margin. The median EBRT dose and fraction size were 62.6 Gy and 1.8 to 2.0 Gy, respectively.
Results
The median follow-up was 73 months (range, 21 to 372 months). The five-year overall survival (OS) and locoregional recurrence free survival (LRFS) were 97.4% and 86.9%, respectively. Locoregional failures occurred in 5 and all failure sites were the neck node area. In univariate analysis, OS was significantly influenced by invasion of the trachea (p = 0.016) or esophagus (p = 0.006). LRFS was significantly decreased by male (p = 0.020), gross residuum after resection (p = 0.002), close or positive tumor at surgical margin involvement (p = 0.044), and tracheal invasion (p = 0.040). No significant prognostic factor was identified in the multivariate analysis. No patient experienced the Radiation Therapy Oncology Group grade 3 or more toxicity.
Conclusion
Our locoregional control rate of 87.2% is comparable to historical controls with surgery alone, even though our study had a large proportion of advanced stage. Adjuvant EBRT may an effective and safe treatment option in patients with WDTC.
Keywords: Papillary thyroid cancer, Follicular thyroid cancer, Radiotherapy, Adjuvant
Introduction
Thyroid cancer is the most common newly diagnosed malignancy in Korea, representing approximately 300 cases per 100,000 [1]. In the United States, a high incidence of 37,200 cases was reported in 2009 [2]. And the majority, about 92%, of the histology is well-differentiated thyroid cancer (WDTC) including papillary and follicular type [3]. All around the world, surgery, radioactive iodine (RAI), and thyroid hormone suppression have been accepted as standard treatment for WDTC. But it has not been defined clearly whether RAI is sufficient as a sole adjuvant treatment, particularly in high risk cases.
WDTC has typically prolonged the course. The 10-year survival rates for papillary and follicular thyroid cancer are 93% and 85%, respectively [4]. However, 40-year recurrence rates are reported to be about 35%, of which two-thirds are known to manifest 10 years after initial treatment [5]. In terms of patterns of failure, locoregional failure (LRF) accounts for more than 50% in all risk groups [6]. Therefore clinicians should be cautious about the patients with the following features considered as signs of high risk of LRF; age older than 45 years, male, tumor size larger than 4 cm in diameter, bilateral lobe involvement, extrathyroidal extension (ETE), vascular invasion, and lymph node metastasis [5]. To diminish the rate of LRF in these patients, adjuvant postoperative external beam radiotherapy (EBRT) has been used in many institutions. The American Thyroid Association (ATA) has recommended EBRT in patients over the age of 45 with grossly visible ETE at the time of surgery and a high likelihood of microscopic residual tumor, and for those patients with a gross residual tumor in whom further surgery or RAI would likely be ineffective [7].
However, many difficulties in the building of consensus on indications of EBRT exist. There has been no randomized prospective study on adjuvant RAI or EBRT up to now. And some physicians are reluctant to adopt EBRT. Each institution has different treatment guidelines relied on their experience. Therefore, the present study was conducted to analyze our institutional treatment outcomes for adjuvant EBRT in WDTC.
Materials and Methods
1. Clinical profiles
One hundred and ten patients were treated with EBRT for thyroid cancer from February 1981 to December 2010 in our institution. Of 110 patients, 84 patients were pathologically diagnosed with WDTC. Among them, we analyzed 39 patients who were newly diagnosed and received initial radical surgery followed by adjuvant EBRT for homogeneity. Forty-five patients treated with EBRT for salvage or palliative intent were excluded. This retrospective study was performed with the approval of our Institutional Review Board.
The median follow-up duration was 73 months (range, 21 to 372 months). Patient characteristics are summarized in Table 1. Stage was restaged in accordance with the American Joint Committee on Cancer classification 7th edition [8], based on operation records and pathologic records. M stage was evaluated before EBRT. Thirty-two patients (80%) were T3 or T4, and 24 patients (61.5%) had regional neck node metastasis. All patients had no distant metastasis (DM). Postoperative residuum was evaluated through review of operation records and imaging studies. Two patients (5%) referred from other hospitals after the operation could not be classified as any group due to a lack of records. The surgical margin status was divided into three groups: presence/absence of tumor involvement and close margin. Close margin was defined as the presence of tumor cells within 1 mm of the margin.
Table 1.
Patients characteristics
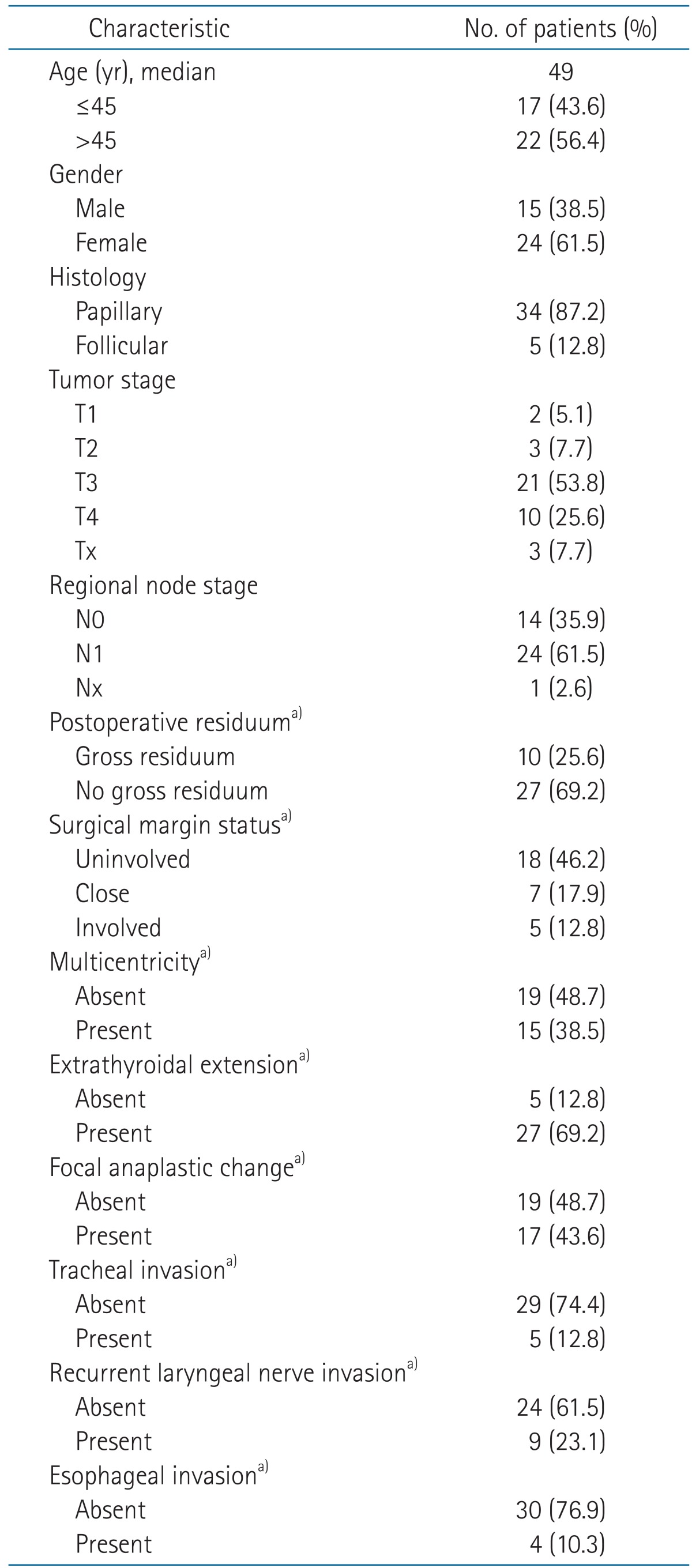
a)Available cases only.
2. Treatment
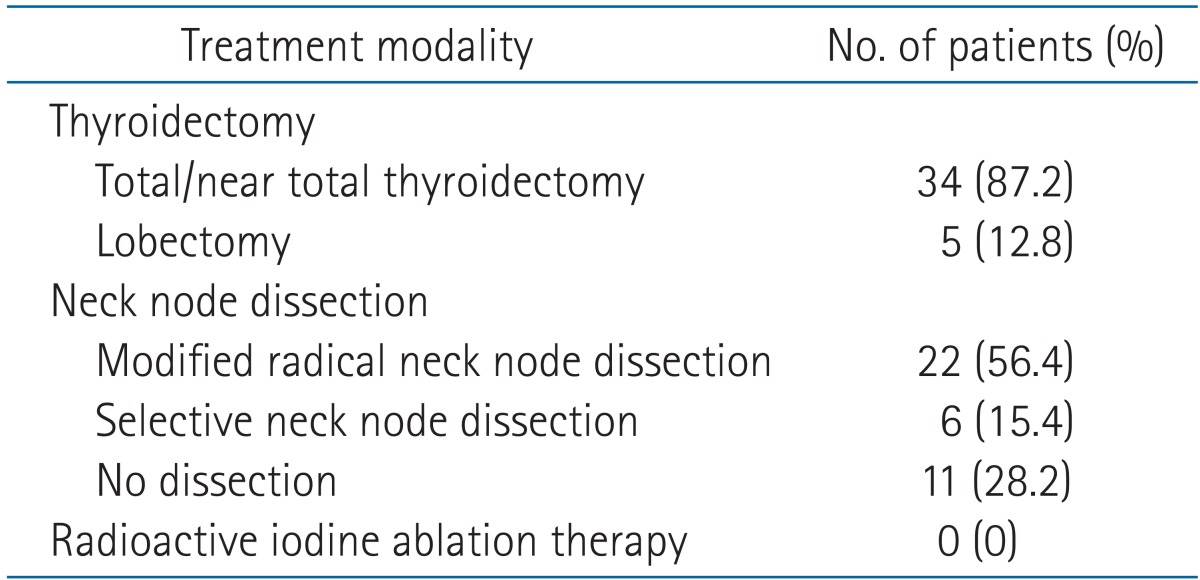
The initial treatment modality before EBRT was listed in Table 2. A total or near total thyroidectomy was performed in 34 patients (87.2%) and a lobectomy in 5 patients (12.8%). Twenty-eight patients (71.8%) underwent a neck node dissection. Of 28 patients, 22 patients underwent a modified radical neck node dissection.
Table 2.
Initial treatment method before external beam radiotherapy
Postoperative EBRT was given to patients with ETE (27 patients, 69.2%), gross residuum (10 patients, 25.6%), suspicious incomplete resection due to adjacent structure invasion (4 patients, 10%), focal anaplastic change (17 patients, 43.6%), and a squamous cell carcinoma component (1 patient, 2.5%). The records of two patients were too old to get enough information. EBRT was most often delivered by 3-dimensional conformal radiotherapy in 28 patients (71.8%). Conventional EBRT and intensity modulated radiotherapy were used in 8 patients (20.5%) and 3 patients (7.7%), respectively. The median total EBRT dose was 62.6 Gy (range, 45 to 70 Gy). Most patients received EBRT using a conventional fraction size, 1.8 to 2.0 Gy (range, 1.6 to 2.25 Gy). The EBRT field included both the thyroid bed and bilateral regional neck node area in 33 patients (84.6%) and the thyroid bed only in 6 patients (15.3%).
No patient received RAI before EBRT. Three patients (7.7%) were treated with RAI after EBRT. The reasons for RAI were as follows: remnant thyroidal uptake on iodine scan, persistently elevated fT4 levels, and LRF after EBRT.
3. Clinical endpoint and statistical analysis
We evaluated the overall survival (OS) and locoregional recurrence free survival (LRFS) rate as the clinical endpoint. OS was calculated from the date of initial surgery to the date of death or the last follow-up. LRFS was defined as the interval between the date of initial surgery to the date of LRF or the last follow-up. We considered the recurrence to be a LRF when a newly suspicious lesion was detected in the thyroid bed or regional neck node area. In the patients with gross residuum after surgery, an increase in the extent of residuum compared with the simulation computed tomography was also considered as LFR. OS and LRFS were estimated by the Kaplan-Meier method. The log-rank test was used for univariate analyses. Multivariate analyses were conducted by the Cox proportional hazard model. Statistical analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
1. Survival and locoregional recurrence free rates
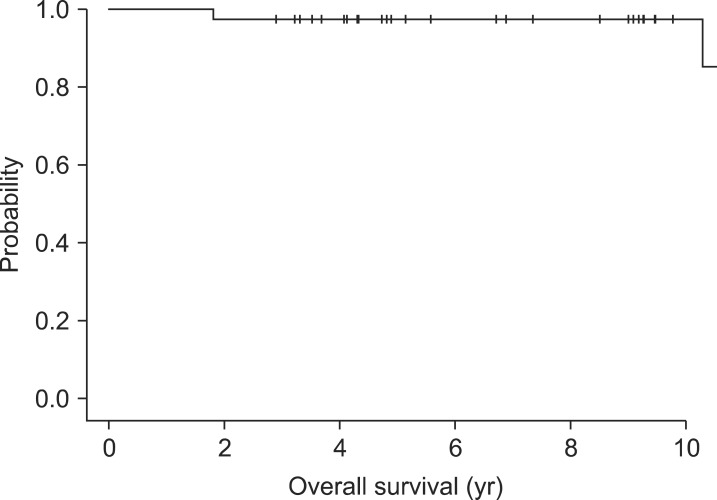
OS and LRFS curves are shown in Figs. 1 and 2. The five-year OS rate was 97.4% and not reached at median value. The LRFS at 5 years was 86.9%. LRF occurred in 5 patients during the follow-up period, thus the crude rate of locoregional control (LRC) was 87.2%.
Fig. 1.
Kaplan-Meier curve for overall survival.
Fig. 2.
Kaplan-Meier curve for locoregional recurrence free survival.
In the subset analysis stratified by high risk features, the five-year OS in pT4, lymph node involvement, and postoperative gross residuum group were 90%, 95.8%, and 100%, respectively. The 5-year OS in patients with ETE or invasion of the trachea was 96.3% or 80.0%.
LRFS at 5 years was observed at 75.0% in pT4, and 78.6% in N1. Of patients with gross residual tumor or ETE, 57.9% and 83.1% maintained a recurrence free-state at 5 years, respectively. In the case of tracheal invasion, the 5-year LRFS was 50% (Table 3).
Table 3.
Univariate analysis of the prognostic factors
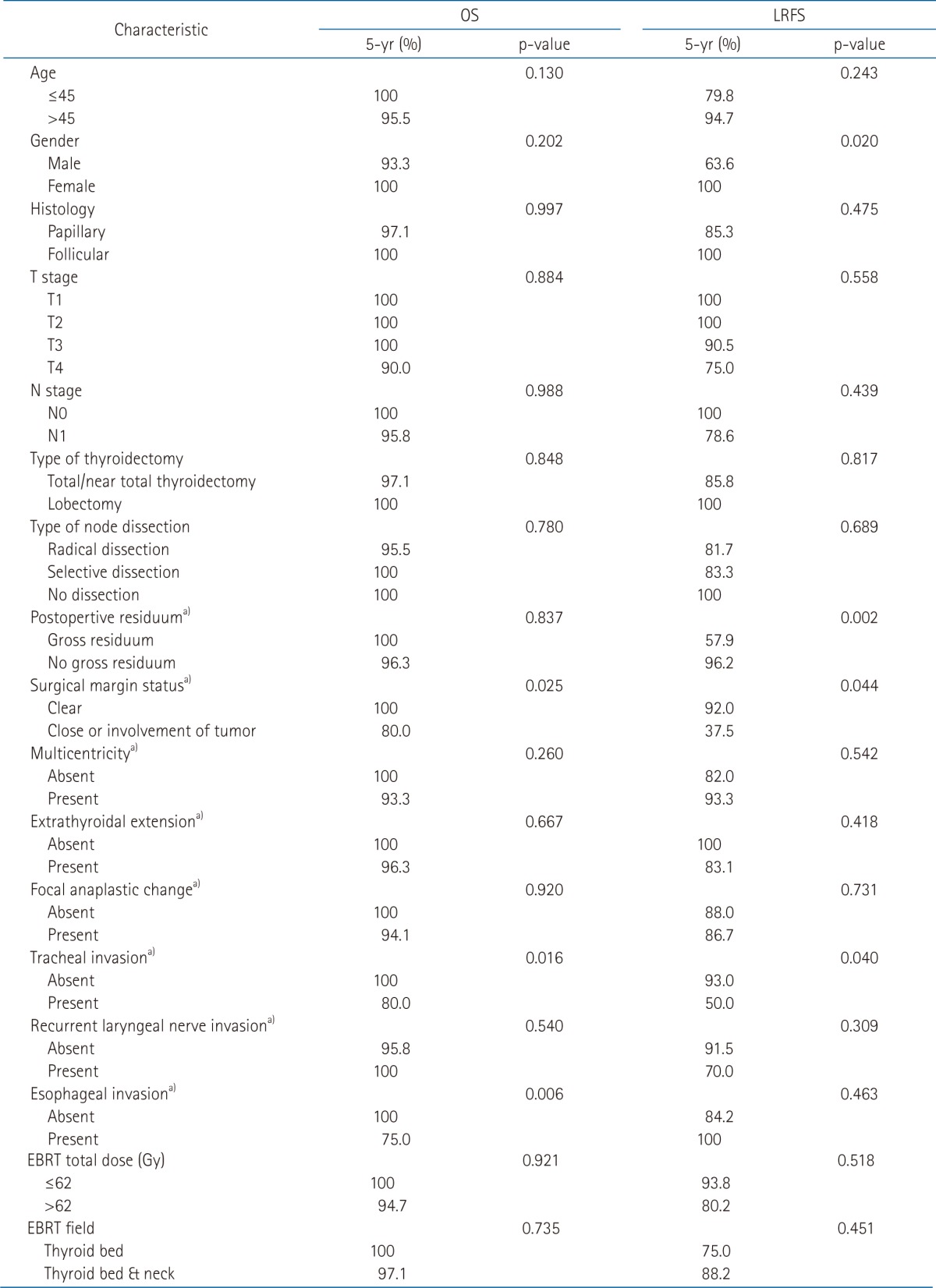
p-value by log-rank test.
OS, overall survival; LRFS, locoregional recurrence free survival; EBRT, external beam radiation.
a)Available cases only.
2. Analysis of prognostic factors
We analyzed clinical and pathologic factors which could impact the course of the disease. Those included age, gender, stage, extent of surgery, EBRT method including dose and field, postoperative residuum, surgical margin status, and presence of several pathologic factors; multicentricity, ETE, focal anaplastic change, and invasion of the trachea, recurrent laryngeal nerve or esophagus. Results of the univariate and multivariate analysis were summarized in Tables 3 and 4.
Table 4.
Multivariate analysis of prognostic factors for locoregional recurrence free survival (LRFS)
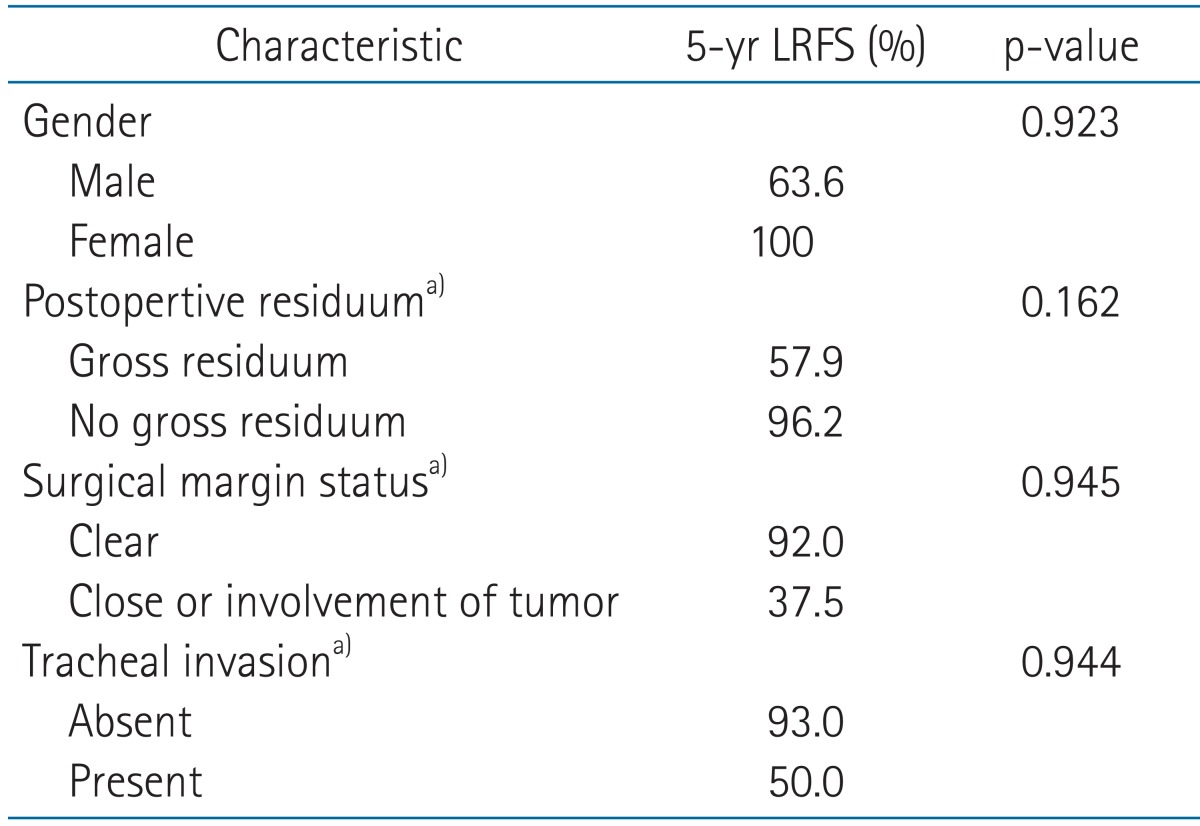
p-value by Cox proportional hazards model results.
a)Available cases only.
In the univariate analysis for OS, there was a significant difference in the case of tracheal invasion (100% vs. 80.0%, p = 0.016) and esophageal invasion (100% vs. 75.0%, p = 0.006). The extent of surgery and the EBRT method did not influence OS.
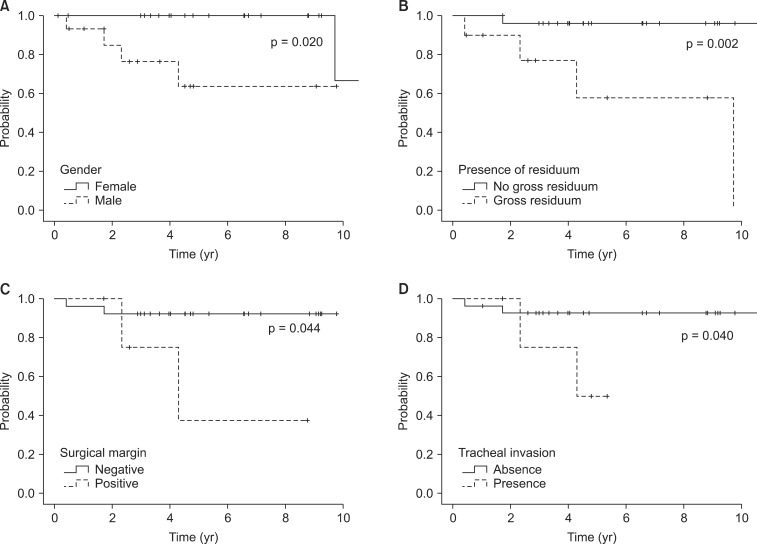
LRFS was significantly decreased by male (63.6% vs. 100%, p = 0.020; Fig. 3A), gross residuum after resection (57.9% vs. 96.2%, p = 0.002; Fig. 3B), close or positive tumor at surgical margin involvement (37.5% vs. 92.0%, p = 0.044; Fig. 3C), and tracheal invasion (93% vs. 50.0%, p = 0.040; Fig. 3D). The extent of surgery and EBRT method did not show a relationship with recurrence.
Fig. 3.
Prognostic factors for locoregional recurrence free survival (LRFS). LRFS according to gender (A), presence of residuum (B), surgical margin (C), and tracheal invasion (D).
Multivariate analysis for OS could not be conducted due to insufficient occurrence of death. And there was no factor which could significantly influence LRFS in the multivariate analysis.
3. Patterns of failure
Table 5 shows patterns of failure. During the follow-up period, seven patients experienced failures. Of these, three patients experienced both LRF and DM after EBRT. Isolated LRF and DM occurred in 2 patients, respectively. Median time to LRF and DM was 38.6 months (range, 5 to 115 months) and 41.8 months (range, 16.2 to 115 months), respectively.
Table 5.
Patterns of failure and salvage treatment
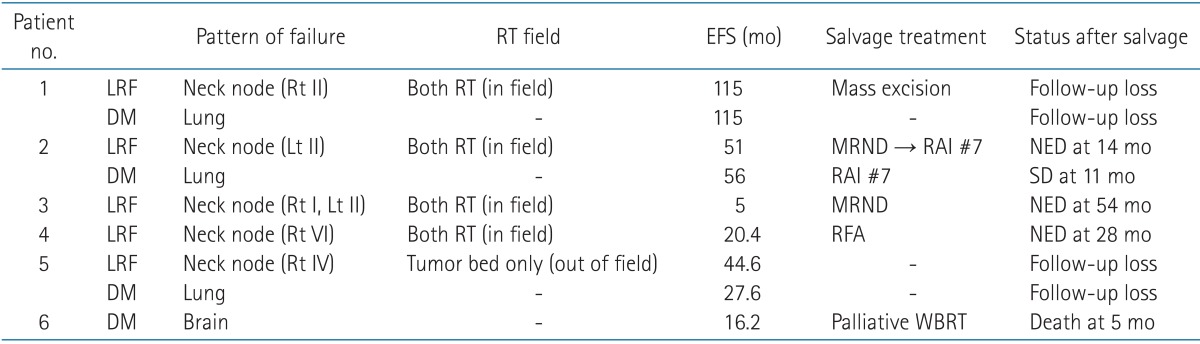
RT, radiotherapy; EFS, event free survival; LRF, locoregional failure; DM, distant metastasis; MRND, modified radical neck node dissection; RAI, radioactive iodine ablation therapy; NED, no evidence of disease; SD, stable disease; RFA, radiofrequency ablation; WBRT, whole brain radiotherapy, Rt, right; Lt, left.
All LRF sites were in the neck node area and also in the previous EBRT field, except for one patient who received EBRT to the thyroid tumor bed only. Of 5 patients, apart from 2 patients who were lost during follow-up, 3 patients have maintained their status with no evidence of disease after salvage treatment, such as modified neck node dissection or radiofrequency ablation of recurred neck nodal lesion.
The most frequent site of DM is the lung (n = 4). One patient with brain metastasis died after 5.5 months, another 2 patients were lost during follow-up, and the others who had lung metastasis were sustained in the stable disease state after RAI.
4. Toxicity
EBRT-induced toxicity was graded using the Radiation Therapy Oncology Group grading system (Table 6). Acute complication was defined as symptoms that occurred during the EBRT. Skin reaction or esophagitis less than grade 3 were commonly reported as 84.6% and 97.5%, respectively. More than half of the patients complained of symptoms of laryngeal irritation or hoarseness less than a grade 3. There was no grade 3 or more toxicity. Late complications were defined as the presence of symptoms occurred one year after completion of EBRT. Most skin reaction, esophagitis, and laryngeal irritation were resolved during follow-up. Hoarseness and xerostomia were sustained in a quarter of patients, but those were grade 1 or 2.
Table 6.
Toxicity induced radiotherapy (the Radiation Therapy Oncology Group grade)
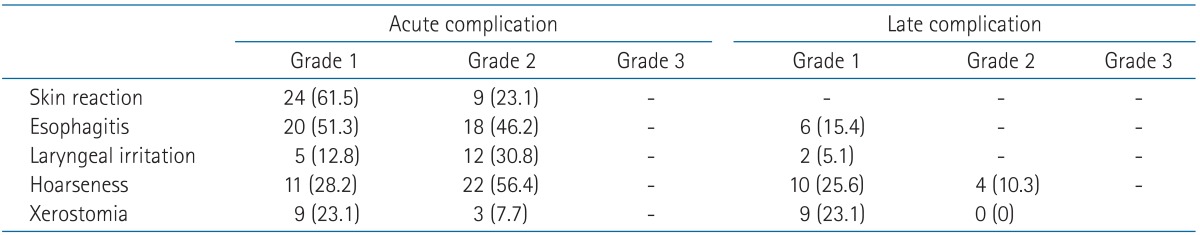
Values are presented as number (%).
Discussion and Conclusion
This study shows that 5-year OS and LRFS were 97.4% and 86.9%, respectively. During the follow-up period (median, 74 months), the crude rate of LRC was 87.2%. These results are similar with those of the previous literature. Chow et al. [9], including 842 patients with papillary thyroid cancer, reported that the LRFS at 5 years was 84% and confirmed the efficacy of EBRT in reducing the risk of LRF to 0.35. Especially, the benefit was more definite in patients with a gross residual tumor after surgery as improvement in the 10-year LRFS from 24% to 56.2% was shown. In our cohort, LRFS at 5 years was lower in patients with gross residuum at 57.9% than patients without gross residuum, 96.2%. But it was not significant in the multivariate analysis. We could not analyze the impact of the microscopic residuum because of the small subgroup size. According to Tsang et al. [10], there was a beneficial effect of EBRT in patients with papillary tumors and microscopic residuum. They showed superior 10-year cancer specific survival (CSS, 100%) and LRFS (93%) in the irradiated group compared with the non-irradiated group (CSS 95%, p = 0.038; LRFS 78%, p = 0.01).
It has been considered that the patients with ETE or lymph node metastases have a poor prognosis. Adjuvant EBRT may provide a chance to compensate for this adverse feature. Keum et al. [11] observed that EBRT significantly lowers the LRF in patients with papillary thyroid cancer invading the trachea in spite of a higher frequency of microscopic or macroscopic residual tumor in the irradiated group (51% for no-EBRT vs. 8% for EBRT group, p < 0.01). Hu et al. [12] reviewed 55 patients with stage III WDTC and the presence of ETE, and showed no significant difference in CSS and OS according to the type of ETE. But patients with macroscopic ETE who did not receive EBRT showed a marginally significant decrease in CSS (p = 0.07) than those who received EBRT. They also demonstrated that EBRT was a significant predictor of CSS (p = 0.02) on multivariate analysis. In the study by Kim et al. [13], analyzing 91 papillary thyroid cancer cases with ETE or lymph node involvement, the LRFS at 5 years was significantly improved at 95.2% with EBRT vs. 67.5% without EBRT (p = 0.0408). Farahati et al. [14] found that EBRT was a predictive factor for improvement of both LRF and DM (p = 0.0003 and p = 0.0001). They also confirmed the efficacy of EBRT in lymph node positive patients older than age 40 years with invasive papillary thyroid carcinoma (p = 0.01). In the current study, even though patients had adjacent structure invasion (i.e., trachea, esophagus, or recurrent laryngeal nerve) or regional lymph node metastases, their outcome was not significantly different from patients who did not in multivariate analysis (Table 4). It would seem that EBRT countervail negative effect of these risk factors, albeit it is not conclusive because our study had a relatively small number of patients and included only patients who received EBRT.
On the other hand, in the 1990s, some studies failed to demonstrate the advantage of EBRT in reducing recurrence or death. However, these results could not exclude the effect of selection bias of the irradiated group. The study of Lin et al. [15] compared 72 patients who received EBRT with 625 patients without EBRT and showed that OS and disease-free survival were not improved by EBRT. But the main reason was probably due to the fact that the irradiated group included a higher proportion of advanced stage patients than the non-irradiated group did. Samaan et al. [16] reviewed 1,599 cases of WDTC. They reported that there was no benefit in 113 patients treated with EBRT. It should not be overlooked that patients in the irradiated group were significantly older, and more likely to have more extensive disease, and treated with less surgical intervention than those in the non-irradiated group.
Most clinicians have preferred RAI to EBRT for adjuvant treatment although there was no randomized study to compare the efficacy between the two modalities. While many studies showed that the benefit from EBRT was limited to reducing the risk of LRF only, it seems that RAI could provide not only a reduction of LRF rate but also the benefit of cause-specific survival. In addition, RAI gives an advantage in terms of surveillance with whole-body iodine scans. ATA proposed the indication of RAI including all patients with DM, gross ETE, and tumor size > 4 cm and selected patients with tumor size 1-4 cm who have high risk features (cervical lymph node metastases, vascular invasion or more aggressive histologies) [7]. However, RAI and EBRT are not alternative options. Several institutions also applied EBRT to high risk patients with RAI per their protocols to lower the risk of LRF [9-11,13].
More recently, Sun et al. [17] proposed a scoring system for WDTC including classical prognostic factors and histology as follows: age, gender, extensive extracapsular nodal spread, extrathyroidal disease, extent of residual disease after surgery, histological variants, recurrent disease, and tumors with radioiodine fixation. This system scores 0, 1, 2 or 4 points for each item, and if the score is 4 or more, EBRT should be discussed; and if 6 or more, EBRT should be recommended. This scoring system could not be applied completely to our cohort due to a lack of some records. However, more than 60% of our patients were scored at least 4 despite of some kind of missing values. The efficacy of the scoring system needs to be validated through multiple and large studies.
Our retrospective analysis of 39 cases, even though all patients had at least one advanced pathologic feature, showed comparable results with the previous literature which demonstrated a superior LRFS of the irradiated group. Furthermore, we could find considerably good outcomes in patients with each high risk feature, such as gross residual tumor, ETE, T4, or N1 stage, which were not significantly different in patients without those features.
The present study has some limitations. In addition to the retrospective design of the study, patients who did not receive EBRT were not included. Another limitation stems from its small sample size. But, it suggests that the addition of EBRT to surgery could improve LRFS even in patients with a worse prognosis, compared with the historical results of non-irradiated groups.
Even though WDTC shows a high survival rate, it is necessary to try to reduce the rate of LRF when considering patterns of failure. Given EBRT properly, the LRF rate could be decreased in patients with high risk features including gross residual tumor, ETE, T4 or N1 stage. To develop guidelines of adjuvant EBRT of thyroid cancer, well-designed prospective studies are required.
Acknowledgments
This work was supported by a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111098 and A120313), and National R&D program through the Dongnam Institute of Radiological & Medical Sciences funded by the Ministry of Education, Science and Technology (50595-2013).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma: a population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 6.Shaha AR, Shah JP, Loree TR. Patterns of failure in differentiated carcinoma of the thyroid based on risk groups. Head Neck. 1998;20:26–30. doi: 10.1002/(sici)1097-0347(199801)20:1<26::aid-hed5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 9.Chow SM, Law SC, Mendenhall WM, et al. Papillary thyroid carcinoma: prognostic factors and the role of radioiodine and external radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:784–795. doi: 10.1016/s0360-3016(01)02686-4. [DOI] [PubMed] [Google Scholar]
- 10.Tsang RW, Brierley JD, Simpson WJ, Panzarella T, Gospodarowicz MK, Sutcliffe SB. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375–388. [PubMed] [Google Scholar]
- 11.Keum KC, Suh YG, Koom WS, et al. The role of postoperative external-beam radiotherapy in the management of patients with papillary thyroid cancer invading the trachea. Int J Radiat Oncol Biol Phys. 2006;65:474–480. doi: 10.1016/j.ijrobp.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Hu A, Clark J, Payne RJ, Eski S, Walfish PG, Freeman JL. Extrathyroidal extension in well-differentiated thyroid cancer: macroscopic vs microscopic as a predictor of outcome. Arch Otolaryngol Head Neck Surg. 2007;133:644–649. doi: 10.1001/archotol.133.7.644. [DOI] [PubMed] [Google Scholar]
- 13.Kim TH, Yang DS, Jung KY, Kim CY, Choi MS. Value of external irradiation for locally advanced papillary thyroid cancer. Int J Radiat Oncol Biol Phys. 2003;55:1006–1012. doi: 10.1016/s0360-3016(02)04203-7. [DOI] [PubMed] [Google Scholar]
- 14.Farahati J, Reiners C, Stuschke M, et al. Differentiated thyroid cancer: impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration (stage pT4) Cancer. 1996;77:172–180. doi: 10.1002/(SICI)1097-0142(19960101)77:1<172::AID-CNCR28>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Lin JD, Tsang NM, Huang MJ, Weng HF. Results of external beam radiotherapy in patients with well differentiated thyroid carcinoma. Jpn J Clin Oncol. 1997;27:244–247. doi: 10.1093/jjco/27.4.244. [DOI] [PubMed] [Google Scholar]
- 16.Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. doi: 10.1210/jcem.75.3.1517360. [DOI] [PubMed] [Google Scholar]
- 17.Sun XS, Sun SR, Guevara N, et al. Indications of external beam radiation therapy in non-anaplastic thyroid cancer and impact of innovative radiation techniques. Crit Rev Oncol Hematol. 2013;86:52–68. doi: 10.1016/j.critrevonc.2012.09.007. [DOI] [PubMed] [Google Scholar]