Abstract
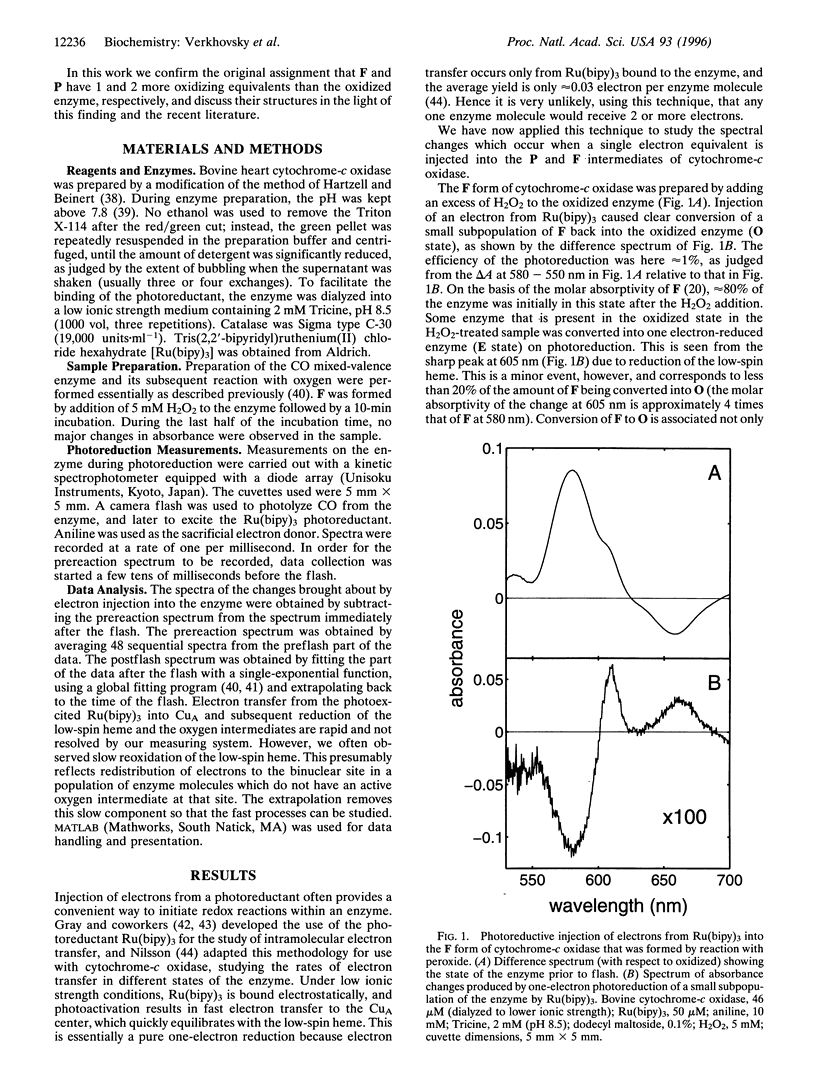
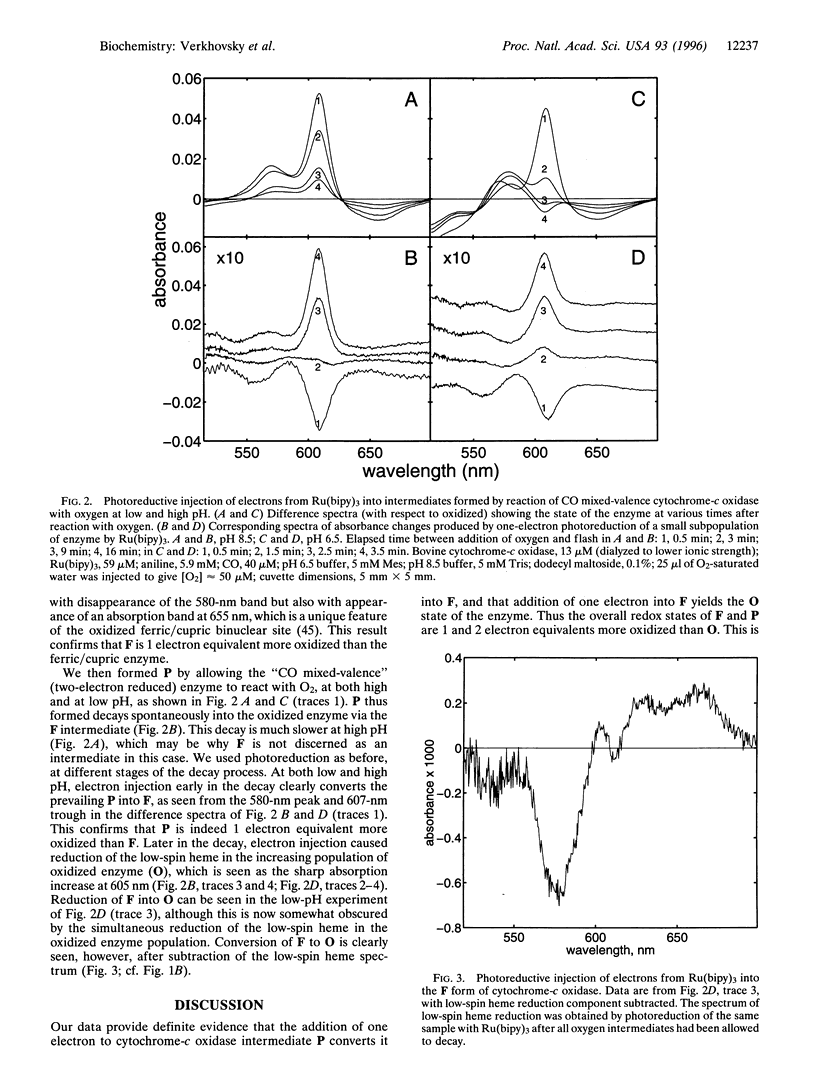
Some intermediates in the reduction of O2 to water by cytochrome-c oxidase have been characterized by optical, Raman, and magnetic circular dichroism spectroscopy. The so-called "peroxy" (P) and "ferryl" (F) forms of the enzyme, which have been considered to be intermediates of the oxygen reaction, can be generated when the oxidized enzyme reacts with H2O2, or when the two-electron reduced ("CO mixed-valence") enzyme reacts with O2. The structures as well as the overall redox states of P and F have recently been controversial. We show here, using tris(2,2'-bipyridyl)ruthenium(II) as a photoinducible reductant, that one-electron reduction of P yields F, and that one-electron reduction of F yields the oxidized enzyme. This confirms that the overall redox states of P and F differ from the oxidized enzyme by two and one electron equivalents, respectively. The structures of the P and F states are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992 Mar 26;356(6367):301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- Baker G. M., Noguchi M., Palmer G. The reaction of cytochrome oxidase with cyanide. Preparation of the rapidly reacting form and its conversion to the slowly reacting form. J Biol Chem. 1987 Jan 15;262(2):595–604. [PubMed] [Google Scholar]
- Blackmore R. S., Greenwood C., Gibson Q. H. Studies of the primary oxygen intermediate in the reaction of fully reduced cytochrome oxidase. J Biol Chem. 1991 Oct 15;266(29):19245–19249. [PubMed] [Google Scholar]
- Carter K., Palmer G. Models of the two heme centers in cytochrome oxidase. The optical properties of cytochrome a and a3. J Biol Chem. 1982 Nov 25;257(22):13507–13514. [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Fabian M., Palmer G. The interaction of cytochrome oxidase with hydrogen peroxide: the relationship of compounds P and F. Biochemistry. 1995 Oct 24;34(42):13802–13810. doi: 10.1021/bi00042a011. [DOI] [PubMed] [Google Scholar]
- Han S. H., Ching Y. C., Rousseau D. L. Cytochrome c oxidase: decay of the primary oxygen intermediate involves direct electron transfer from cytochrome a. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8408–8412. doi: 10.1073/pnas.87.21.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. W., Ching Y. C., Rousseau D. L. Primary intermediate in the reaction of oxygen with fully reduced cytochrome c oxidase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2491–2495. doi: 10.1073/pnas.87.7.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Ching Y. C., Rousseau D. L. Ferryl and hydroxy intermediates in the reaction of oxygen with reduced cytochrome c oxidase. Nature. 1990 Nov 1;348(6296):89–90. doi: 10.1038/348089a0. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Greenwood C. Spectroscopic evidence for the participation of compound A (Fea32+-O2) in the reaction of mixed-valence cytochrome c oxidase with oxygen at room temperature. Biochem J. 1983 Dec 1;215(3):659–667. doi: 10.1042/bj2150659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C., Greenwood C. The reaction of fully reduced cytochrome c oxidase with oxygen studied by flow-flash spectrophotometry at room temperature. Evidence for new pathways of electron transfer. Biochem J. 1984 Mar 15;218(3):913–921. doi: 10.1042/bj2180913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C. The reaction of the electrostatic cytochrome c-cytochrome oxidase complex with oxygen. J Biol Chem. 1991 Feb 5;266(4):2219–2226. [PubMed] [Google Scholar]
- Iwata S., Ostermeier C., Ludwig B., Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995 Aug 24;376(6542):660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- Kumar C., Naqui A., Chance B. Peroxide interaction with pulsed cytochrome oxidase. Optical and EPR studies. J Biol Chem. 1984 Oct 10;259(19):11668–11671. [PubMed] [Google Scholar]
- Lauraeus M., Morgan J. E., Wikström M. Peroxy and ferryl intermediates of the quinol-oxidizing cytochrome aa3 from Bacillus subtilis. Biochemistry. 1993 Mar 16;32(10):2664–2670. doi: 10.1021/bi00061a026. [DOI] [PubMed] [Google Scholar]
- Mitchell R., Mitchell P., Rich P. R. The assignment of the 655 nm spectral band of cytochrome oxidase. FEBS Lett. 1991 Mar 25;280(2):321–324. doi: 10.1016/0014-5793(91)80321-s. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Verkhovsky M. I., Puustinen A., Wikström M. Identification of a "peroxy" intermediate in cytochrome bo3 of Escherichia coli. Biochemistry. 1995 Dec 5;34(48):15633–15637. doi: 10.1021/bi00048a005. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Chanady G. A. Interactions of cytochrome aa3 with oxygen and carbon monoxide. The role of the 607 nm complex. Biochim Biophys Acta. 1981 Feb 12;634(2):256–265. doi: 10.1016/0005-2728(81)90144-4. [DOI] [PubMed] [Google Scholar]
- Nilsson T. Photoinduced electron transfer from tris(2,2'-bipyridyl)ruthenium to cytochrome c oxidase. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6497–6501. doi: 10.1073/pnas.89.14.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proshlyakov D. A., Ogura T., Shinzawa-Itoh K., Yoshikawa S., Appelman E. H., Kitagawa T. Selective resonance Raman observation of the "607 nm" form generated in the reaction of oxidized cytochrome c oxidase with hydrogen peroxide. J Biol Chem. 1994 Nov 25;269(47):29385–29388. [PubMed] [Google Scholar]
- Proshlyakov D. A., Ogura T., Shinzawa-Itoh K., Yoshikawa S., Kitagawa T. Microcirculating system for simultaneous determination of Raman and absorption spectra of enzymatic reaction intermediates and its application to the reaction of cytochrome c oxidase with hydrogen peroxide. Biochemistry. 1996 Jan 9;35(1):76–82. doi: 10.1021/bi9511705. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Verkhovsky M. I., Morgan J. E., Belevich N. P., Wikstrom M. Reaction of the Escherichia coli quinol oxidase cytochrome bo3 with dioxygen: the role of a bound ubiquinone molecule. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1545–1548. doi: 10.1073/pnas.93.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995 Aug 25;269(5227):1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996 May 24;272(5265):1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- Varotsis C., Zhang Y., Appelman E. H., Babcock G. T. Resolution of the reaction sequence during the reduction of O2 by cytochrome oxidase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):237–241. doi: 10.1073/pnas.90.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky M. I., Morgan J. E., Puustein A., Wikström M. Kinetic trapping of oxygen in cell respiration. Nature. 1996 Mar 21;380(6571):268–270. doi: 10.1038/380268a0. [DOI] [PubMed] [Google Scholar]
- Verkhovsky M. I., Morgan J. E., Wikström M. Oxygen binding and activation: early steps in the reaction of oxygen with cytochrome c oxidase. Biochemistry. 1994 Mar 15;33(10):3079–3086. doi: 10.1021/bi00176a042. [DOI] [PubMed] [Google Scholar]
- Vygodina T. V., Konstantinov A. A. H2O2-induced conversion of cytochrome c oxidase peroxy complex to oxoferryl state. Ann N Y Acad Sci. 1988;550:124–138. doi: 10.1111/j.1749-6632.1988.tb35329.x. [DOI] [PubMed] [Google Scholar]
- Watmough N. J., Cheesman M. R., Greenwood C., Thomson A. J. Cytochrome bo from Escherichia coli: reaction of the oxidized enzyme with hydrogen peroxide. Biochem J. 1994 Jun 1;300(Pt 2):469–475. doi: 10.1042/bj3000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L. C., Baker G. M. Reaction of hydrogen peroxide with the rapid form of resting cytochrome oxidase. Biochemistry. 1991 Jun 11;30(23):5727–5733. doi: 10.1021/bi00237a014. [DOI] [PubMed] [Google Scholar]
- Wikström M. Energy-dependent reversal of the cytochrome oxidase reaction. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4051–4054. doi: 10.1073/pnas.78.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M., Morgan J. E. The dioxygen cycle. Spectral, kinetic, and thermodynamic characteristics of ferryl and peroxy intermediates observed by reversal of the cytochrome oxidase reaction. J Biol Chem. 1992 May 25;267(15):10266–10273. [PubMed] [Google Scholar]
- Winkler J. R., Malmström B. G., Gray H. B. Rapid electron injection into multisite metalloproteins: intramolecular electron transfer in cytochrome oxidase. Biophys Chem. 1995 May;54(3):199–209. doi: 10.1016/0301-4622(94)00156-e. [DOI] [PubMed] [Google Scholar]
- Witt S. N., Blair D. F., Chan S. I. Chemical and spectroscopic evidence for the formation of a ferryl Fea3 intermediate during turnover of cytochrome c oxidase. J Biol Chem. 1986 Jun 25;261(18):8104–8107. [PubMed] [Google Scholar]
- Witt S. N., Chan S. I. Evidence for a ferryl Fea3 in oxygenated cytochrome c oxidase. J Biol Chem. 1987 Feb 5;262(4):1446–1448. [PubMed] [Google Scholar]
- Wrigglesworth J. M. Formation and reduction of a 'peroxy' intermediate of cytochrome c oxidase by hydrogen peroxide. Biochem J. 1984 Feb 1;217(3):715–719. doi: 10.1042/bj2170715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Ioannidis N., Nicholls P. Spectrophotometric characterization of intermediate redox states of cytochrome oxidase. Ann N Y Acad Sci. 1988;550:150–160. doi: 10.1111/j.1749-6632.1988.tb35331.x. [DOI] [PubMed] [Google Scholar]



