ABSTRACT
BACKGROUND
In 2009, the U.S. Preventive Service Task Force changed its recommendation regarding screening mammography in average-risk women aged 40–49 years.
OBJECTIVE
To evaluate the effects of the 2009 recommendation on reported mammogram use in a population-based survey.
DESIGN
Secondary data analysis of data collected in the 2006, 2008, and 2010 Behavioral Risk Factor Surveillance System surveys.
PARTICIPANTS
Women ages 40–74 years in the 50 states and Washington, DC who were not pregnant at time of survey and reported data on mammogram use during the 2006, 2008, or 2010 survey.
MAIN MEASURES
Mammogram use was compared between women ages 40–49 and women ages 50–74 before and after the recommendation. We performed a difference-in-difference estimation adjusted for access to care, education, race, and health status, and stratified analyses by whether women reported having a routine checkup in the prior year.
KEY RESULTS
Reported prevalence of mammogram use in the past year among women ages 40–49 and 50–74 was 53.2 % and 65.2 %, respectively in 2008, and 51.7 % and 62.4 % in 2010. In 2010, mammography use did not significantly decline from 2006–2008 in women ages 40–49 relative to women ages 50–74.
CONCLUSION
There was no reduction in mammography use among younger women in 2010 compared to older women and previous years. Patients and providers may have been hesitant to comply with the 2009 recommendation.
KEY WORDS: mammography, cancer screening, women’s health, preventive health
In 2009, the U.S. Preventive Services Taskforce (USPSTF) recommended that while women ages 50–74 years should undergo biennial screening mammography, breast cancer screening among women between the ages of 40 and 49 should be “an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.”1,2 Evidence behind this decision included a meta-analysis that revealed that while mammography does reduce mortality in this age group, there are more false positives than in older women and the number needed to treat is higher due to lower incidence.3 Therefore, screening mammography in this younger age group could lead to costly and anxiety-provoking workups, which are associated with their own health risks, as well as to potential over-diagnosis.4
The new guidelines led to resistance among the media, patients, providers, and medical organizations.5–9 Surveys of providers demonstrated their lack of understanding of the new USPSTF recommendation and skepticism about applying it.10
Rates of mammography screening vary by age group, with lower screening rates among younger women.11–13 Few physicians recommend cessation of screening mammograms to women in older age groups, despite lack of evidence of effectiveness.14,15 In a population-based sample in 2006, rates of mammography use in the preceding 2 years were 69.2 % among women ages 40–49, 80.5 % among women ages 50–59, and 69.3 % among women ages 80 and older.12
To our knowledge, there has been only one study using nationally representative, population-based data from the National Health Interview Survey to investigate whether mammogram use among women ages 40–49 declined after the 2009 USPSTF recommendation.16 We used data from the Behavioral Risk Factor Surveillance Study (BRFSS) to examine the difference in mammography rates before and after 2009 between women ages 40–49, for whom screening mammography is not universally recommended, and women ages 50–74, for whom screening mammography continues to be recommended. We hypothesized that relative to older women, women ages 40–49 would report reduced use of mammography in 2010 as compared to past years.
METHODS
Overall Design
We conducted a population-based secondary data analysis in 2012, comparing mammography among cross sectional samples from survey data collected by the BRFSS in 2006, 2008, and 2010. BRFSS is a nationally representative annual telephone survey of health behaviors in adults age 18 years and older. State health departments administer the survey and the Centers for Disease Control and Prevention provides national coordination. Each state contacts an independent probability sample through random digit dialing, after which results are pooled. Median response rates in 2006, 2008, and 2010 were 51.4 %, 53.3 %, and 54.6 %, respectively.17 Dozens of studies have evaluated the reliability and validity of items included in the BRFSS. One review found the BRFSS item measuring timing of mammography utilization to be moderately reliable and valid (κ range 0.50–0.79), while items measuring sociodemographic characteristics were found to be highly reliable and valid.18 Another recent study found both measures of mammography utilization (κ =0.62) and Pap test utilization (κ = 0.54) to be valid relative to data from chart review.19 Data subsequent to 2010 was not used, as BRFSS survey methodology and weighting changed in 2011, and subsequent survey data is not directly comparable to previous years.20 As secondary data analysis using a publicly available data set with de-identified participants does not meet the federal definition of human subjects research, institutional review board (IRB) approval was not needed.
Study Population
We restricted our sample to female respondents ages 40–74 years in the 50 states and Washington, DC, who were not pregnant at the time of survey administration and who reported data on mammogram use during the 2006, 2008, or 2010 survey.
Outcome and Predictor Variable Definitions
The primary outcome was whether or not a woman reported having a mammogram in the prior year, measured as a dichotomous variable. Although the USPSTF recommends biennial screening mammography, given the short time interval between the 2009 recommendation and 2010 survey data collection, mammography in prior year was chosen as the outcome, since the analysis focused on relative differences between age groups. The main independent variables were: 1) age group, defined as 40–49 years versus 50–74 years; and 2) time period, defined as before (2006–2008) and after (2010) the USPSTF 2009 mammography recommendation.
Additional covariates included access to health care, highest level of education obtained, race and ethnicity, and health status. To measure access to health care, a composite variable was constructed to denote poor, fair, or good access to care based on health insurance status and having a regular primary care physician, both of which have been positively correlated with access to preventive health services.21,22 Poor access to care was defined as having neither a primary physician nor health insurance. Fair access to care was defined as having either a primary physician or health insurance, and good access to care was defined as reporting both health insurance and a primary physician. As another measure of access to health care, a dichotomous variable indicating whether or not a woman reported having to forego health care in the prior year due to cost was included. Health status was dichotomized as “very good” or “excellent” health versus “good,” “fair,” or “poor” health. We considered including state fixed effects (indicator variables for each state) in the analyses to control for time-invariant state-level characteristics; however, the inclusion of these state variables did not change our results and so were not included in the final analyses.
Because women are typically referred for screening mammography during a primary care visit, analyses were stratified by whether women reported having a checkup within the previous year. Checkup was defined as a “visit to a doctor for a…general physical exam, not an exam for a specific injury, illness, or condition”.17
Analysis
We employed a difference-in-differences approach using logistic regression to estimate the effect of the USPSTF recommendation on mammogram use over time, comparing women of the different age groups. This approach compares the change in odds of mammography in 2006/2008 versus 2010 between women ages 40–49 years (the treatment group) and women ages 50–74 years (the comparison group). The estimated effect of the 2009 mammography recommendation is therefore the difference in the pre-recommendation and post-recommendation time periods, comparing differences between the two age groups. The final model included variables indicating age 40–49 versus 50–74, the year 2010 versus 2006/2008, and an interaction term of the two age and time indicator variables. The interaction term represents a ratio of two odds ratios, or the difference-in-difference in mammography utilization between 2006–2008 and 2010, in women ages 40–49 relative to women ages 50–74.23,24 A strength of this analytic approach is that it can minimize bias due to unmeasured confounders, and control for secular trends that might impact mammography rates.25
The model was adjusted for access to care, education, race and ethnicity, and health status. Standard errors were adjusted to account for non-independence of observations within states.26 The results of the logistic regression models were used to calculate predicted probabilities of mammogram use by age group within each year, adjusting for access to care, education, race and ethnicity, and health status.
Sensitivity Analyses
To test the robustness of the results, we performed two sensitivity analyses. First, it is possible that the trends in utilization observed over time might not be unique to mammography, but rather associated with more widespread trends affecting women’s cancer screening. To address this concern, a parallel analysis was conducted using the Pap test as the outcome of interest. Because national recommendations for cervical cancer screening did not change between 2006 and 2010, screening rates would not be expected to have changed during these years. As in the primary analysis described above, an interaction term for age group and time pre- and post- 2009 USPSTF mammography recommendation was created to provide an estimate of the time-treatment effect. The same covariates of access to care, education, race and ethnicity, and health status were used in the model. We expected that if a relative change in mammogram use was due to a broader trend in utilization rather than the USPSTF recommendation, then Pap test use would follow the same pattern.
Second, a key assumption underlying the difference-in-differences approach is that in the absence of the USPSTF mammography recommendation, women ages 40–49 would have had the same trends in mammography utilization as women ages 50–74. Given the major economic recession that occurred during our study time period, we were concerned that Medicare coverage might have protected women ages 65 and older from decreased health care utilization due to loss of employer-sponsored insurance or decreased income. To address this concern, we duplicated our primary analysis, comparing mammography use among women ages 40–49 to women ages 50–64 before and after the USPSTF recommendation. The results were nearly identical to the analyses comparing women ages 40–49 to women ages 50–74; therefore, the primary analysis with women ages 50–74 is presented as the comparison group.
All analyses were performed using Stata 11 (StataCorp, College Station, TX). Results are reported using the sampling weights provided by BRFSS to account for the complex sampling design and robust variance estimated to account for potential in-state clustering effects.27
RESULTS
A total of 484,296 women aged 40–74 years reported data on mammogram use in the 2006, 2008, and 2010 surveys. Table 1 shows characteristics of the study population. Thirty-six percent of women were ages 40–49 years and 64 % were ages 50–74 years. Most women (90.4 %) reported ever having a mammogram, and 59.7 % reported having had a mammogram in the previous year. 83.3 % of women ages 40–49 and 94.4 % of women ages 50–74 reported ever having a mammogram (p < 0.01), and 52.2 % of women ages 40–49 reported mammogram use in the prior year relative to 63.9 % of women ages 50–74 (p < 0.01). Rates of mammogram use in the past year among women ages 40–49 and 50–74 were 51.8 % and 64.3 % in 2006, respectively, 53.2 % and 65.2 % in 2008, and 51.7 % and 62.4 % in 2010. Seventy-five percent of women reported a check-up in the prior year. Most women (83 %) had good access to care, 13 % had fair access to care, and 5 % had poor access to care. Fourteen percent of women reported having foregone needed health care within the prior year due to cost.
Table 1.
Descriptive Statistics of Non-Pregnant U.S. Women Ages 40–74 Years: Behavioral Risk Factor Surveillance System
| 2006 N = 134,795* % | 2008 N = 165,273* % | 2010 N = 184,228* % | All years N = 484,296* % | |||||
|---|---|---|---|---|---|---|---|---|
| Age | 40–49 | 50–74 | 40–49 | 50–74 | 40–49 | 50–74 | 40–49 | 50–74 |
| 37.2 | 62.8 | 35.8 | 64.2 | 36.0 | 64.0 | 36.3 | 63.7 | |
| Mammogram utilization | ||||||||
| Ever had a mammogram | 83.8 | 94.8 | 83.1 | 94.1 | 83.0 | 94.4 | 83.3 | 94.4 |
| Mammogram in prior year | 51.8 | 64.3 | 53.2 | 65.2 | 51.7 | 62.4 | 52.2 | 63.9 |
| Care utilization | ||||||||
| Checkup in prior year | 68.2 | 77.2 | 70.8 | 78.9 | 69.6 | 78.3 | 69.6 | 78.2 |
| Pap test in prior year | 62.3 | 53.2 | 62.3 | 52.2 | 60.1 | 47.8 | 61.6 | 50.9 |
| Access to care† | ||||||||
| Low access | 6.6 | 3.3 | 6.3 | 3.5 | 6.7 | 3.6 | 6.5 | 3.4 |
| Medium access | 15.5 | 11.5 | 15.0 | 11.3 | 15.7 | 10.9 | 15.4 | 11.2 |
| High access | 77.9 | 85.3 | 78.8 | 85.3 | 77.6 | 85.6 | 78.1 | 85.4 |
| Foregone care due to cost in prior year | 16.7 | 11.5 | 17.6 | 12.6 | 19.5 | 13.3 | 17.9 | 12.5 |
| Education | ||||||||
| Less than high school | 9.3 | 11.0 | 8.3 | 9.8 | 8.5 | 9.0 | 8.7 | 9.9 |
| High school | 26.4 | 31.9 | 25.3 | 31.4 | 23.4 | 29.4 | 25.0 | 30.9 |
| Some college | 27.9 | 26.8 | 28.3 | 27.0 | 26.1 | 27.9 | 27.4 | 27.2 |
| College degree or higher | 36.4 | 30.3 | 38.1 | 31.7 | 42.1 | 33.7 | 38.9 | 32.0 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 69.7 | 76.4 | 69.1 | 74.8 | 67.7 | 75.3 | 68.8 | 75.5 |
| Non-Hispanic Black | 11.0 | 9.0 | 10.6 | 10.1 | 11.2 | 10.2 | 10.9 | 9.8 |
| Non-Hispanic other race | 4.4 | 3.5 | 4.8 | 3.9 | 4.9 | 3.4 | 4.7 | 3.6 |
| Hispanic | 12.9 | 8.7 | 13.5 | 8.9 | 13.8 | 8.4 | 13.4 | 8.7 |
| Multiple races reported‡ | 1.3 | 1.4 | 1.3 | 1.4 | 1.4 | 1.4 | 1.3 | 1.4 |
| Health status | ||||||||
| Fair or poor | 14.4 | 22.2 | 15.2 | 21.8 | 15.1 | 21.7 | 14.9 | 21.9 |
| Excellent, very good, or good | 85.6 | 77.8 | 84.8 | 78.2 | 84.9 | 78.3 | 85.1 | 78.1 |
*Annual and total sample sizes shown are not weighted. All other data shown are weighted
†Access to care is defined as follows: Low: No health insurance and no regular source of care. Medium: Either health insurance or regular source of care. High: Health insurance and a regular source of care
‡This variable represents non-Hispanic women who reported multiple races
Comparison of Mammography Utilization Between Younger and Older Women
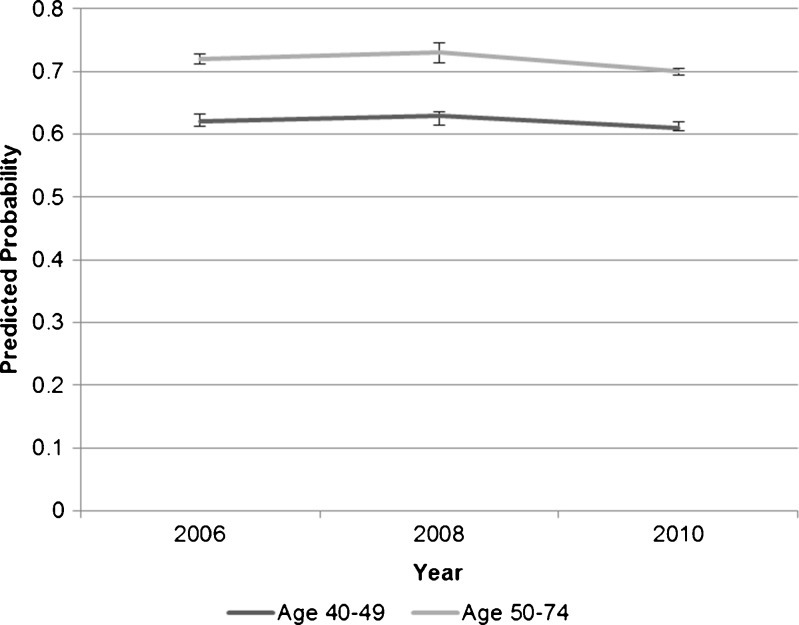
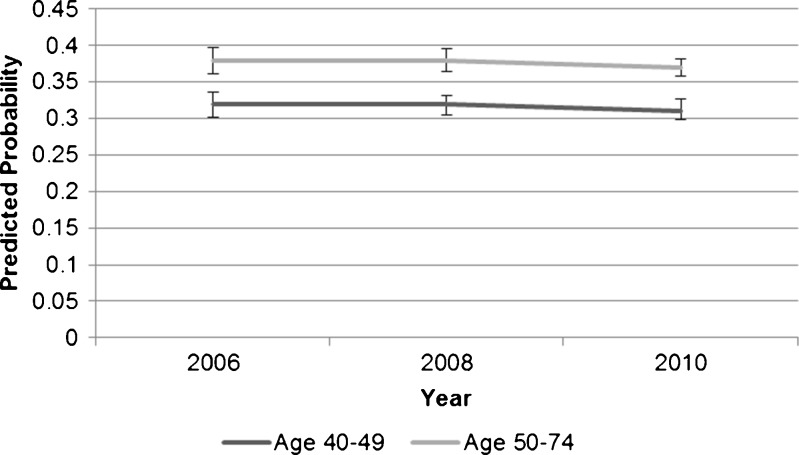
During each year of survey administration, women ages 40–49 reported lower rates of mammogram receipt than older women. In both younger and older age groups, women who reported having a checkup in the previous year were more likely to report receiving mammograms than those without a checkup. Among those who reported checkups, the predicted probabilities of mammography were 62 %, 63 %, and 61 % for younger women in the years 2006, 2008, and 2010, respectively, and 72 %, 73 %, and 70 % for older women in the same years (Fig. 1). Among older women without a checkup in the past year, the predicted probabilities of mammography were 38 %, 38 %, and 37 % in 2006, 2008, and 2010, respectively. Younger women who did not report checkups had lower predicted probabilities in these same years (32 %, 32 %, and 31 %, respectively) (Fig. 2).
Figure 1.
Average predicted probabilities of mammography by age group among those who had a checkup in the past year.
Figure 2.
Average predicted probabilities of mammography by age group among those who did not have a checkup in the past year.
Table 2 shows the results of the multivariable regression analysis comparing mammogram utilization within prior year pre- and post- USPSTF mammography recommendation, stratified by checkup status. Women ages 40–49 who had a checkup in the past year as well as those without checkups in the past year did not have a lower odds of receiving mammograms in 2010 relative to women ages 50–74 in 2010 and all women in prior years (OR = 1.11, 95 % CI 1.04, 1.18 and OR = 1.04, 95 % CI 0.93, 1.16, respectively), as shown in the year 2010 and age interaction. Several covariates were associated with higher utilization of mammograms, including higher access to care, higher educational attainment, higher health status, and not having to forego care due to cost.
Table 2.
Odds of Mammogram Utilization Within Prior Year Pre- and Post- USPSTF Mammography Recommendation
| Checkup within prior year | No checkup within prior year | |
|---|---|---|
| OR (95 % CI) | OR (95 % CI) | |
| Age | ||
| Ages 40–49 | 0.61 (0.59, 0.64) | 0.75 (0.70, 0.80) |
| Year 2010 | 0.87 (0.84, 0.90) | 0.94 (0.88, 1.00) |
| Ages 40–49*2010 interaction | 1.11 (1.04, 1.18) | 1.04 (0.93, 1.16) |
| Access to care | ||
| Low access to care (Ref) | 1.0 | 1.0 |
| Medium access to care | 1.32 (1.15, 1.53) | 1.50 (1.29, 1.73) |
| High access to care | 2.03 (1.77, 2.33) | 3.90 (3.41, 4.45) |
| Forgone care due to cost | 0.68 (0.65, 0.72) | 0.80 (0.74, 0.86) |
| Education | ||
| Less than high school diploma (Ref) | 1.0 | 1.0 |
| High school diploma | 1.18 (1.10, 1.25) | 0.94 (0.83, 1.07) |
| Some college | 1.23 (1.15, 1.31) | 1.05 (0.93, 1.20) |
| College degree or higher | 1.46 (1.36, 1.55) | 1.26 (1.12, 1.43) |
| Race/ethnicity | ||
| Non-Hispanic White (Ref) | 1.0 | 1.0 |
| Non-Hispanic Black | 1.05 (1.00, 1.10) | 1.06 (0.94, 1.19) |
| Non-Hispanic Other race | 0.79 (0.72, 0.86) | 0.84 (0.72, 1.00) |
| Hispanic | 0.73 (0.65, 0.82) | 0.89 (0.72, 1.10) |
| Multiple races reported | 1.17 (1.09, 1.26) | 1.58 (1.40, 1.78) |
| Health status | ||
| Health status fair–poor (Ref) | 1.0 | 1.0 |
| Health status good–excellent | 1.45 (1.39, 1.50) | 0.88 (0.81, 0.95) |
USPSTF is U.S. Preventive Services Task Force. All results shown used the weights provided in the BRFSS (Behavioral Risk Factor Surveillance System), to account for the complex survey sampling design and non-independence of observations within states. Age variable is relative to women ages 50–74 years. Year variable is relative to pooled data from years 2006 and 2008
Parallel Analysis of Pap Test Utilization
In the parallel analysis using Pap test as the outcome of interest, women ages 40–49 were more likely to receive Pap tests than older women (OR = 1.53, 95 % CI 1.48–1.59). Similar to results with mammogram use, the year 2010 was associated with lower rates of Pap tests (OR = 0.81, 95 % CI 0.79–0.83) compared with the previous two survey dates. Women ages 40–49 had an increased odds of receiving a Pap smear in 2010 than women ages 50–74 and all women in prior years (OR of interaction term = 1.12, 95 % CI 1.06–1.18). The Appendix shows our regression results for Pap test as the outcome.
DISCUSSION
Contrary to our hypothesis, we found no significant reduction in mammogram use in 2010 among younger women relative to older women. Among women who reported having a checkup in the prior year, women ages 40–49 actually had a small but significantly increased odds of mammogram use in the time period after the USPSTF recommendation relative to women ages 50–74. Among women who did not report a checkup in the prior year, there were no significant differences by age group, comparing mammogram use before and after the USPSTF recommendation. While younger women were overall less likely than older women to receive mammograms in each of the three time points, there was no relative decrease seen in 2010 that could be attributed to the recommendations, even after adjusting for covariates that might impact mammogram utilization, including access to care, education, race and ethnicity, and health status. The parallel analysis with Pap test use as the outcome of interest yielded results that were nearly identical to those with mammogram use as the outcome, further suggesting that the USPSTF recommendation had no effect on mammogram use among women ages 40–49 as of 2010.
The finding that older women have higher rates of mammography use and lower rates of Pap smear use than younger women has been documented previously.11–13 This age difference was present irrespective of check-up status. Differences in perceived cancer risk on the part of providers, variable levels of patient concern, competing healthcare priorities, differing rates of Medicare insurance, or higher levels of accumulated health factors that drive cancer screening behaviors among older women, such as hysterectomy, prior history of cancer, or prior finding on mammogram, may account for these age differences in screening rates.28
In the current study, rates of mammogram use were lower for women who reported no checkup in the previous year, compared to those who had a checkup. In our sample, women who obtained checkups also reported better health care access and lower general health status, either of which might lead to higher mammogram use. Among women who do not get checkups, it is conceivable that those who do obtain mammograms may do so for different reasons, such as to address health concerns or suspicious findings on breast self-exams or for high-risk surveillance due to medical or family history.
While certain preventive health services, including colorectal cancer screening and influenza vaccination, remain underused, there is growing evidence that other services may be overused.15,29–31 Following publication of USPSTF recommendations regarding prostate-specific antigen (PSA) testing, there was only a small decrease of 3 % in screening 2 years later.32 Additionally, despite the 2003 USPSTF recommendation that women receive combined Pap smear and human papillomavirus testing no more than once every 3 years and that average risk women cease Pap testing at age 65, annual testing remains common among women over age 65.33,34
This study had several limitations that are worth noting. First, the data reported in the study are derived from self-report via national survey sample rather than from prospective follow-up or chart review. The use of self-reported survey data may introduce recall bias as well as misclassification bias, which may limit our ability to attribute any changes in mammography use solely to the USPSTF recommendation. Previous research has shown that people, particularly older women, may increasingly misclassify time since screening test for tests performed several years earlier.35,36 For this reason, the report of mammogram use within the prior year was selected as the outcome. Checkup in prior year is also self-reported, and women may inaccurately classify sick or urgent visits as checkups.
Second, while several relevant covariates were included in the model, there may be residual confounding by factors, such as income levels and access to care, which may vary over time, particularly in the context of rising unemployment levels. Another limitation was that income level was not included in the model. However, given that a sizable portion of study subjects are of retirement age, we believe that education was a better indicator of socioeconomic status. While access to care was included in the model, the fact that over 80 % of women reported high access to care by the metric used indicates a limitation in ability to stratify by this variable.
Third, women with a history of breast cancer or suspicious lesions, whose mammograms were performed for reasons other than screening, could not be excluded from the sample based on lack of survey data. This may have led to an overestimation of the rate of screening mammogram use. However, we would not expect this overestimate to change over time. By analyzing the difference in the changes in mammography use over time between the two age groups, this limitation is minimized.
Finally, because the USPSTF issued its recommendations regarding mammograms in women in their forties just a year prior to the 2010 survey, the impact on actual practice may not yet be evident. This might bias results towards the null in the short-term. One determining factor may be whether health plans continue to cover the test for women in their forties.
While cancer screening saves lives, practice should be informed by an understanding of the recommendations. Overuse of cancer screening tests, including mammography and Pap smear, in populations where evidence is lacking may contribute to the increasing cost of medical care and convey additional risks to individuals.15,37 This study points to a need for longer-term follow-up on the population impact of the USPSTF recommendations on mammography rates, as well as breast cancer-associated morbidity and mortality. As research continues to reveal overuse of cancer screening in the U.S., we will need further research on how providers interpret and use recommendations on cancer screening, how patient–provider communication around mammography decisions might be improved, and where individualized recommendations for higher risk groups may be indicated.
Acknowledgements
Funding
None.
Prior presentations
Presented as a poster at the AcademyHealth Annual Research Meeting on June 26, 2012.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Appendix
Table 3.
Logistic Regression Results Estimating the Odds of Pap Test Utilization Within Prior Year
| OR (95 % CI) | |
|---|---|
| Age | |
| Ages 40–49 | 1.53 (1.48, 1.59) |
| Year 2010 | 0.81 (0.79, 0.83) |
| Ages 40–49*2010 interaction | 1.12 (1.06, 1.18) |
| Access to care | |
| Low access to care (ref) | 1.0 |
| Medium access to care | 1.82 (1.66, 1.98) |
| High access to care | 3.43 (3.16, 3.72) |
| Forgone care due to cost | 0.71 (0.68, 0.74) |
| Education | |
| Less than high school diploma (ref) | 1.0 |
| High school diploma | 1.05 (0.99, 1.10) |
| Some college | 1.17 (1.11, 1.24) |
| College degree or higher | 1.53 (1.45, 1.61) |
| Race/ethnicity | |
| Non-Hispanic White (ref) | 1.0 |
| Non-Hispanic Black | 1.34 (1.28, 1.39) |
| Non-Hispanic Other race | 0.86 (0.80, 0.92) |
| Hispanic | 0.96 (0.88, 1.06) |
| Multiple races reported | 1.57 (1.48, 1.66) |
| Health status | |
| Health status fair-poor (ref) | 1.0 |
| Health status good-excellent | 1.40 (1.35, 1.44) |
USPSTF is U.S. Preventive Services Task Force. All results shown used the weights provided in the BRFSS (Behavioral Risk Factor Surveillance System) to account for the complex survey sampling design and non-independence of observations within states. Age variable is relative to women ages 50–74 years. Year variable is relative to pooled data from years 2006 and 2008
REFERENCES
- 1.US Preventive Services Task force. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: Systematic evidence review update for the U.S. Preventive Services Task Force. Evidence review update. Rockville: Agency for Healthcare Research and Quality; 2009. [PubMed] [Google Scholar]
- 3.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive services task force. Ann Intern Med. 2009;151:727–37. doi: 10.7326/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalager M, Adami HO, Bretthauer M, Tamimi RM. Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian screening program. Ann Int Med. 2012;156(7):491–9. doi: 10.7326/0003-4819-156-7-201204030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Partridge AH, Winer EP. On mammography—more agreement than disagreement. N Engl J Med. 2009;361:2499–501. doi: 10.1056/NEJMp0911288. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis CD, Fontanarosa PB. U.S. preventive services task force and breast cancer screening. JAMA. 2010;303(2):173–4. doi: 10.1001/jama.2009.1990. [DOI] [PubMed] [Google Scholar]
- 7.Squiers LB, Holden DJ, Dolina SE, et al. The public’s response to the U.S. preventive services task force’s 2009 recommendations on mammography screening. Am J Prev Med. 2010;40(5):497–504. doi: 10.1016/j.amepre.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Davidson AS, Liao X, Magee D. Attitudes of women in their forties toward the 2009 USPSTF mammogram guidelines: a randomized trial on the effects of media exposure. Am J Obstet Gynecol. 2011;205:e1–7. doi: 10.1016/j.ajog.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. American Cancer Society guidelines for the early detection of cancer. www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed April 21, 2013.
- 10.Hinz EK, Kudesia R, Rolston R, et al. Physician knowledge of and adherence to the revised breast cancer screening guidelines by the U.S. preventive services. Task Force. Am J Obstet Gynecol. 2011;205:201. doi: 10.1016/j.ajog.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Miller JW, King JB, Ryerson AB, et al. Mammography use from 2000–6: state-level trends with corresponding breast cancer incidence rates. Am J Roentology. 2009;192:352–60. doi: 10.2214/AJR.08.1757. [DOI] [PubMed] [Google Scholar]
- 12.Ryerson AB, Miller JQ, Eheman CR, et al. Recent trends in U.S. mammography use from 2000–6: a population-based analysis. Prev Med. 2008;47:477–82. doi: 10.1016/j.ypmed.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Cancer Screening- United States, 2010. Morbidity and Mortality Weekly Reports. 2012. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6103a1.htm. Accessed May 1, 2013.
- 14.Meissner HI, Klabunde CN, Han PK, et al. Breast cancer screening beliefs, recommendations, and practices. Cancer. 2011;117:3101–11. doi: 10.1002/cncr.25873. [DOI] [PubMed] [Google Scholar]
- 15.Bellizzi KM, Breslau ES, Burness A, et al. Prevalence of cancer screening in older and racially diverse adults. Arch Intern Med. 2011;171(22):2031–37. doi: 10.1001/archinternmed.2011.570. [DOI] [PubMed] [Google Scholar]
- 16.Pace LE, He Y, Keating NL. Trends in mammography screening rates after publication of the 2009 US Preventive Services Task Force recommendations. Cancer. 2013. doi:10.1002/cncr.28105. [DOI] [PubMed]
- 17.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2006, 2008, and 2010.
- 18.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS) Soc Prev Med. 2001;46:S03–S42. doi: 10.1007/BF01318790. [DOI] [PubMed] [Google Scholar]
- 19.Caplan LS, McQueen DV, Qualters JR, Leff M, Garrett C, Calonge N. Validity of women’s self-reports of cancer screening test utilization in a managed care population. Cancer Epidemiol Biomark Prev. 2003;12:1182. [PubMed] [Google Scholar]
- 20.Behavioral Risk Factor Surveillance Survey. 2011 survey data information: Comparability of data. CDC. 2011. http://www.cdc.gov/brfss/annual_data/annual_2011.htm. Accessed April 21, 2013.
- 21.Lambrew JM, Defriese GH, Carey TS, Ricketts TC, Biddle AK. The effects of having a regular doctor on access to primary care. Med Care. 1996;34(2):138–51. doi: 10.1097/00005650-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Shoen C, DesRoches C. Uninsured and unstably insured: the importance of continuous insurance coverage. Health Serv Res. 2000;35(1):187–203. [PMC free article] [PubMed] [Google Scholar]
- 23.Bertrand M, Duflo E, Mullainathan S. How much should we trust difference-in-differences estimates? Q J Econ. 2004;119(1):249–75. doi: 10.1162/003355304772839588. [DOI] [Google Scholar]
- 24.Norton EC, Wang H, Ai C. Computing interaction effects and standard errors in logit and probit models. Stata J. 2004;4(2):154–67. [Google Scholar]
- 25.Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among medicare beneficiaries in the first two years following ACGME resident duty hour reform. JAMA. 2007;298(9):975–83. doi: 10.1001/jama.298.9.975. [DOI] [PubMed] [Google Scholar]
- 26.Karaca-Mandic P, Norton EC, Dowd B. Interaction terms in nonlinear models. Health Serv Res. 2012;47(1):255–274. doi: 10.1111/j.1475-6773.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). BRFSS Weighting Formula. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. http://www.cdc.gov/brfss/annual_data/2010/2010_weighting.htm. Accessed April 21, 2013.
- 28.Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: what influences mammography recommendations. J Am Board Fam Pract. 2001;14:352–61. [PubMed] [Google Scholar]
- 29.Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, United States, 1989–2005. Vaccine. 2008;26(14):1786–93. doi: 10.1016/j.vaccine.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Smith RA, Cokkinides V, Brookes D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American cancer society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60(2):99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 31.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomark Prev. 2006;15(2):389–94. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 32.Zelaidt SB, Hoffman RM, Etzioni R, et al. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst. 2011;103:520–3. doi: 10.1093/jnci/djr007. [DOI] [PubMed] [Google Scholar]
- 33.Feldman S. Making sense of the new cervical cancer screening guidelines. N Engl J Med. 2011;365:2145–47. doi: 10.1056/NEJMp1112532. [DOI] [PubMed] [Google Scholar]
- 34.Solomon D, Breen N, McNeel T. Cervical cancer screening rates in the United States and the potential impact of implementation of screening guidelines. CA Cancer J Clin. 2007;57(2):105–11. doi: 10.3322/canjclin.57.2.105. [DOI] [PubMed] [Google Scholar]
- 35.Rauscher GH, Johnson TP, Cho YI, et al. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomark Prev. 2008;17:748–57. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 36.Craig BM, Quinn GP, Vadaparampil ST. Sensitivity of self-report mammography use in older women. Am J Prev Med. 2009;37(5):441–4. doi: 10.1016/j.amepre.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338:1089–96. doi: 10.1056/NEJM199804163381601. [DOI] [PubMed] [Google Scholar]