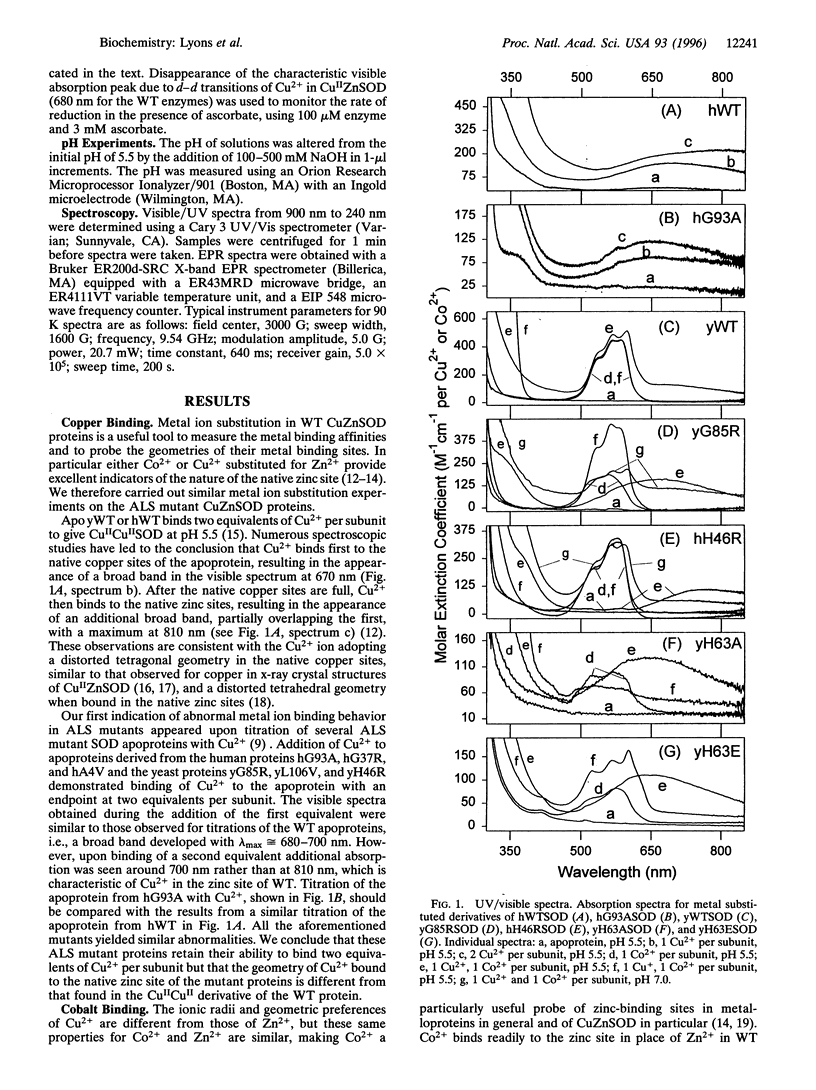
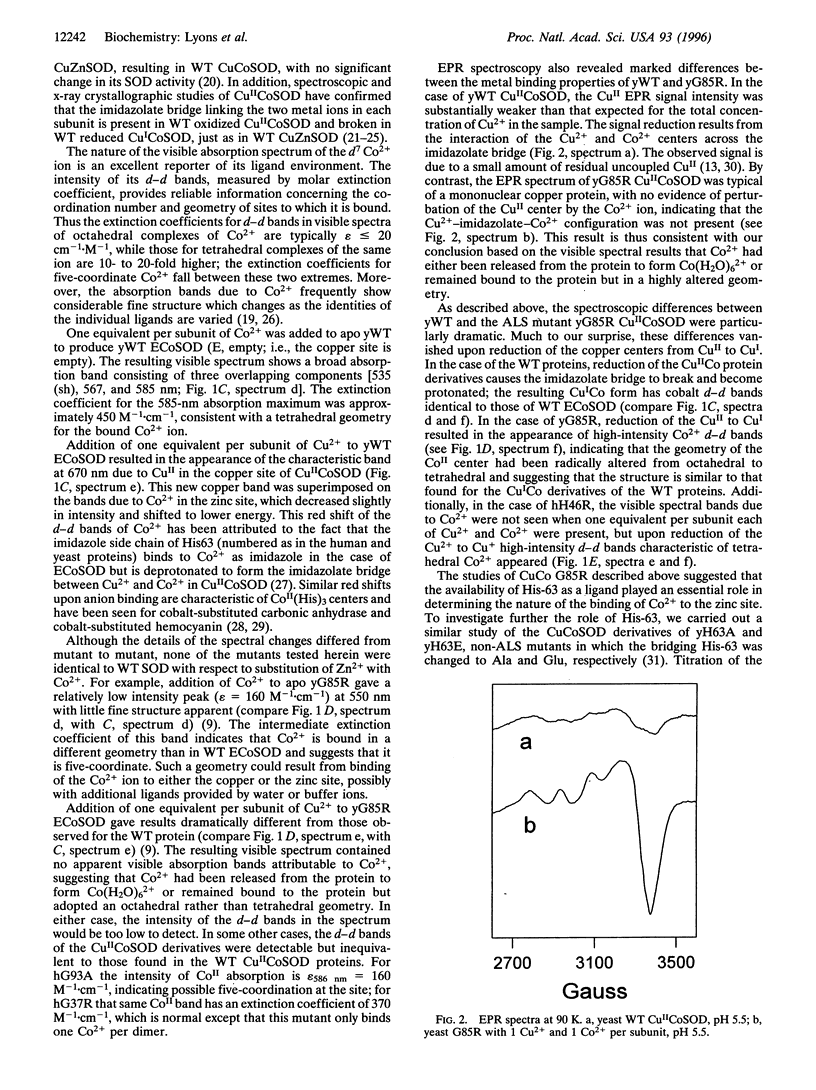
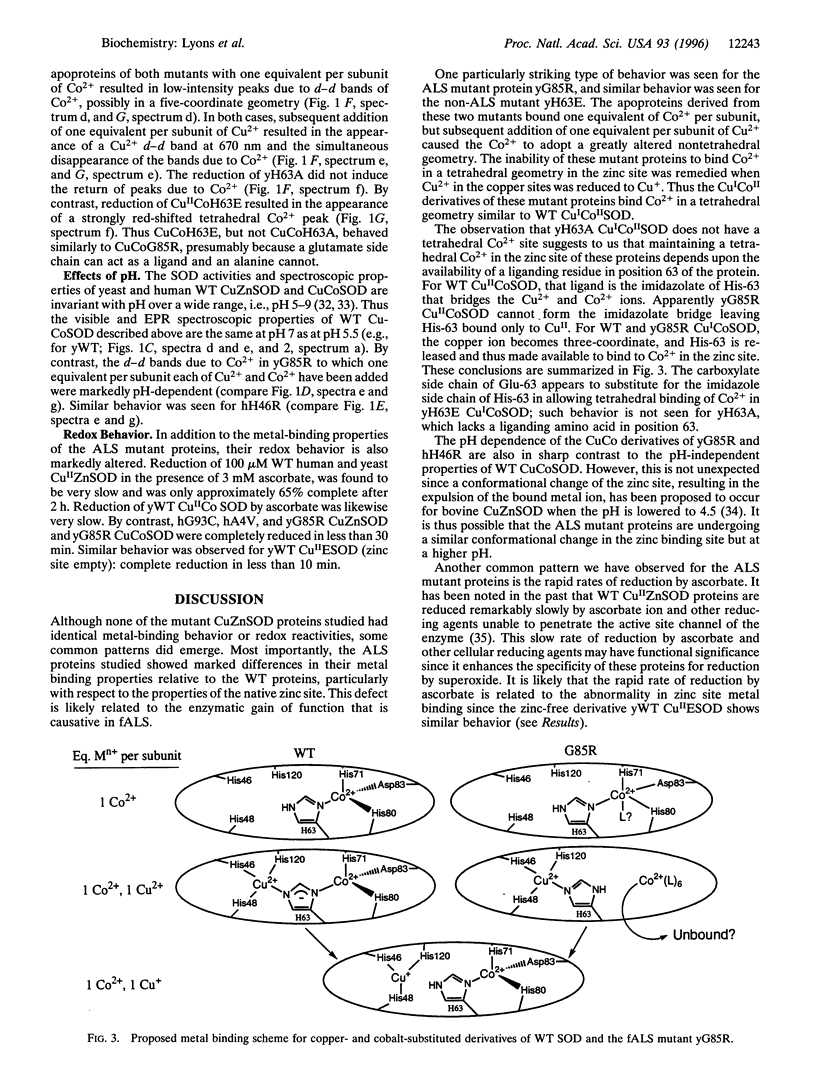
Abstract
A series of mutant human and yeast copper-zinc superoxide dismutases has been prepared, with mutations corresponding to those found in familial amyotrophic lateral sclerosis (ALS; also known as Lou Gehrig's disease). These proteins have been characterized with respect to their metal-binding characteristics and their redox reactivities. Replacement of Zn2+ ion in the zinc sites of several of these proteins with either Cu2+ or Co2+ gave metal-substituted derivatives with spectroscopic properties different from those of the analogous derivative of the wild-type proteins, indicating that the geometries of binding of these metal ions to the zinc site were affected by the mutations. Several of the ALS-associated mutant copper-zinc superoxide dismutases were also found to be reduced by ascorbate at significantly greater rate than the wild-type proteins. We conclude that similar alterations in the properties of the zinc binding site can be caused by mutations scattered throughout the protein structure. This finding may help to explain what is perhaps the most perplexing question in copper-zinc superoxide dismutase-associated familial ALS-i.e., how such a diverse set of mutations can result in the same gain of function that causes the disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertini I., Luchinat C., Piccioli M., Oliver M. V., Viezzoli M. S. 1H NMR investigation of reduced copper-cobalt superoxide dismutase. Eur Biophys J. 1991;20(5):269–279. doi: 10.1007/BF00450562. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Jr Superoxide dismutase in familial amyotrophic lateral sclerosis: models for gain of function. Curr Opin Neurobiol. 1995 Dec;5(6):841–846. doi: 10.1016/0959-4388(95)80114-6. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Militello V., Stroppolo M. E., Polticelli F., Desideri A. Low-temperature optical spectroscopy of cobalt in Cu,Co superoxide dismutase: a structural dynamics study of the solvent-unaccessible metal site. Biochemistry. 1995 Dec 19;34(50):16313–16319. doi: 10.1021/bi00050a011. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Djinovic K., Coda A., Antolini L., Pelosi G., Desideri A., Falconi M., Rotilio G., Bolognesi M. Crystal structure solution and refinement of the semisynthetic cobalt-substituted bovine erythrocyte superoxide dismutase at 2.0 A resolution. J Mol Biol. 1992 Jul 5;226(1):227–238. doi: 10.1016/0022-2836(92)90135-7. [DOI] [PubMed] [Google Scholar]
- Djinovic K., Gatti G., Coda A., Antolini L., Pelosi G., Desideri A., Falconi M., Marmocchi F., Rotilio G., Bolognesi M. Crystal structure of yeast Cu,Zn superoxide dismutase. Crystallographic refinement at 2.5 A resolution. J Mol Biol. 1992 Jun 5;225(3):791–809. doi: 10.1016/0022-2836(92)90401-5. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Briggs R. G. Studies on the reconstitution of bovine erythrocyte superoxide dismutase. V. Preparation and properties of derivatives in which both zinc and copper sites contain copper. Biochim Biophys Acta. 1975 Aug 19;400(2):439–450. doi: 10.1016/0005-2795(75)90200-7. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Cutting F. B., Zhai P., Doble A., Taylor C. P., Andrus P. K., Hall E. D. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol. 1996 Feb;39(2):147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippard S. J., Burger A. R., Ugurbil K., Pantoliano M. W., Valentine J. S. Nuclear magnetic resonance and chemical modification studies of bovine erythrocyte superoxide dismutase: evidence for zinc-promoted organization of the active site structure. Biochemistry. 1977 Mar 22;16(6):1136–1141. doi: 10.1021/bi00625a017. [DOI] [PubMed] [Google Scholar]
- Maret W., Vallee B. L. Cobalt as probe and label of proteins. Methods Enzymol. 1993;226:52–71. doi: 10.1016/0076-6879(93)26005-t. [DOI] [PubMed] [Google Scholar]
- Merli A., Rossi G., Djinovic-Carugo K., Bolognesi M., Desideri A., Rotilio G. Evidence for breaking of the active site dimetal cluster in Cu,Co superoxide dismutase upon copper reduction: a polarized absorption spectra study. Biochem Biophys Res Commun. 1995 May 25;210(3):1040–1044. doi: 10.1006/bbrc.1995.1761. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Fee J. A. On the magnetic properties of cobalt substituted bovine superoxide dismutase derivatives. Biochem Biophys Res Commun. 1975 Sep 16;66(2):799–808. doi: 10.1016/0006-291x(75)90580-x. [DOI] [PubMed] [Google Scholar]
- Nishida C. R., Gralla E. B., Valentine J. S. Characterization of three yeast copper-zinc superoxide dismutase mutants analogous to those coded for in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9906–9910. doi: 10.1073/pnas.91.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill P., Fielden E. M., Cocco D., Rotilio G., Calabrese L. Evidence for catalytic dismutation of superoxide by cobalt(II) derivatives of bovine superoxide dismutase in aqueous solution as studied by pulse radiolysis. Biochem J. 1982 Jul 1;205(1):181–187. doi: 10.1042/bj2050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara N. L., Parge H. E., Hart P. J., Weiss M. S., Goto J. J., Crane B. R., Tsang J., Slater K., Roe J. A., Valentine J. S. Unusual trigonal-planar copper configuration revealed in the atomic structure of yeast copper-zinc superoxide dismutase. Biochemistry. 1996 Feb 20;35(7):2316–2321. doi: 10.1021/bi951930b. [DOI] [PubMed] [Google Scholar]
- Parge H. E., Getzoff E. D., Scandella C. S., Hallewell R. A., Tainer J. A. Crystallographic characterization of recombinant human CuZn superoxide dismutase. J Biol Chem. 1986 Dec 5;261(34):16215–16218. [PubMed] [Google Scholar]
- Rabizadeh S., Gralla E. B., Borchelt D. R., Gwinn R., Valentine J. S., Sisodia S., Wong P., Lee M., Hahn H., Bredesen D. E. Mutations associated with amyotrophic lateral sclerosis convert superoxide dismutase from an antiapoptotic gene to a proapoptotic gene: studies in yeast and neural cells. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):3024–3028. doi: 10.1073/pnas.92.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume A. G., Elliott J. L., Hoffman E. K., Kowall N. W., Ferrante R. J., Siwek D. F., Wilcox H. M., Flood D. G., Beal M. F., Brown R. H., Jr Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996 May;13(1):43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Valentine W. A., Williams P. A., Tafoya W. L. Ilizarov external fixation. Surgical principles, nursing implications. AORN J. 1990 Jun;51(6):1530–1545. doi: 10.1016/s0001-2092(07)66905-1. [DOI] [PubMed] [Google Scholar]
- Wiedau-Pazos M., Goto J. J., Rabizadeh S., Gralla E. B., Roe J. A., Lee M. K., Valentine J. S., Bredesen D. E. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996 Jan 26;271(5248):515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Domigan N. M., Broom J. K. Decreased thermal stability of red blood cell glu100-->gly superoxide dismutase from a family with amyotrophic lateral sclerosis. FEBS Lett. 1995 Jul 24;368(3):449–451. doi: 10.1016/0014-5793(95)00708-h. [DOI] [PubMed] [Google Scholar]



