Abstract
Purpose
Primary malignant melanoma of the nasal cavity and nasopharynx is rarely seen. Clinically, most patients display initial nonspecific symptoms of unilateral nasal obstruction or epistaxis. The prognosis is generally poor, with a mean survival time of 3.5 years.
Material and methods
In this paper, we have reported the case of malignant melanoma of the nasal cavity and nasopharynx. 79 years old man had presented with the swelling of the nose, nasal blockage and epistaxis during the six months before diagnosis. Functional endoscopic sinus surgery was performed to excised the nasal cavity tumor. Using positron emission tomography/computed tomography examination with 18-fluorodeoxyglucose, the patient was diagnosed with residual nasopharyngeal tumor after surgery.
Results
Following the diagnosis, intracavitary brachytherapy for nasopharynx was administered. Solitary cervical nodal involvement occurred 6 months after the diagnosis when had been completely removed. After that, external beam radiotherapy was performed on the submandibular area on the right side. Thereafter, the patient was given follow-up care in the Department of Radiation Oncology until the time of distant progression of the disease.
Conclusions
We have chosen to discuss this condition, because of its rarity and the possibility of using radiotherapy, even though the malignant melanoma had been regarded as a radioresistant disease, and also to emphasize the importance of a multidisciplinary approach to treatment of such patients.
Keywords: brachytherapy, malignant melanoma, nasal cavity, nasopharynx
Purpose
Melanomas are tumors arising from melanocytes which are neuroectodermally derived cells located in the basal layers of skin, skin adnexas and some of the mucosal membrane. Common sites for melanomas are head, neck and the lower extremities as they are exposed to sunlight – one of the predisposing factor. Less commonly they occur in the oral and genital mucosa, nail beds, conjunctiva, orbit, esophagus, nasal mucosa or nasopharynx, vagina and leptomeninges [1]. Sinonasal malignant melanoma is an extremely rare tumor, with primary mucosal melanoma being more aggressive than its cutaneous counterpart. It accounts for less than 1% of all western melanomas and < 5% of all sinonasal tract neoplasm. Malignant melanoma in the nasal cavity and nasopharynx occurs rarely, from 0.3 to 2% of all malignant melanomas, and 4% of all malignant melanomas of the head and neck [2–4]. In the absence of any characteristic clinical symptoms is often diagnosed based on histopathological findings. The prognosis is always poor, because of high rates of locoregional recurrences or distant metastasis during a several months or years after diagnosis. The basis of treatment consists in the surgical excision. Radiotherapy and chemotherapy have little effect on the prognosis of the disease [5, 6].
In this paper we have described a case of malignant melanoma in the nasal cavity and nasopharynx and the influence of radiotherapy on the control of this disease.
Material and methods
A 79-year-old man, had a feeling of nasal obstruction and had bled from the right nostril for approximately 3 times during the period of 6 months before diagnosis. In the anamnesis, the patient gave information about long-term exposure to sunlight (25 years long residence in Africa), and removal of melanoma on his back in January 2011 – histology of the skin in the area of right shoulder blade (superficial spreading melanoma [SSM], Clark level III, Breslow 0.7 mm, pT1apNxpMx).
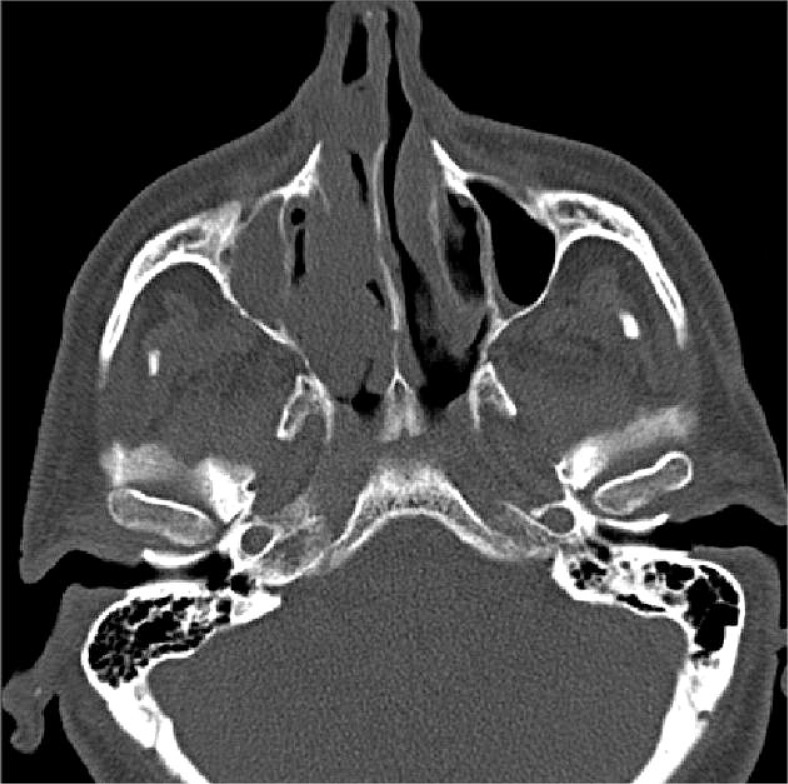
Initial ENT (ear-nose-throat) examination had found “polyp” in the right nasal cavity in late January 2012. After that, native computed tomography (CT) scan of the paranasal sinuses (PNS) had been done on 31st January 2012, which was described as a thickening of mucoperiosteum of both maxillary sinuses – diffuse on the right side, ethmoidal and frontal sinuses significantly to the right side, also polypoid thickening of mucoperiostium of both nasal vents – much more on the right side where there was a total occlusion of ostiomeatal unit (OMU), left side was free. Sphenoidal cavities were airy and loose (Fig. 1).
Fig. 1.
CT image of the tumor in the right nasal cavity
In February 2012, the patient was admitted to the 2nd ENT Clinic of Medical School, Comenius University in Bratislava for the purpose of functional endoscopic sinus surgery (FESS) in general anesthesia (GA). ENT examination was: nose – on the right side and 1 cm from the entrance of the nose, around 4/5 of the space of the nasal cavity was filled with tumor with bumpy surface and several traces of bleeding. Tumor size was 1.7 x 1.4 cm. Nasopharynx was free of tumor. The endoscopic extirpation of nasal cavity tumor had been performed under GA on 28th February 2012. Histological result was mucosal malignant melanoma of nasal cavity (epithelioid type).
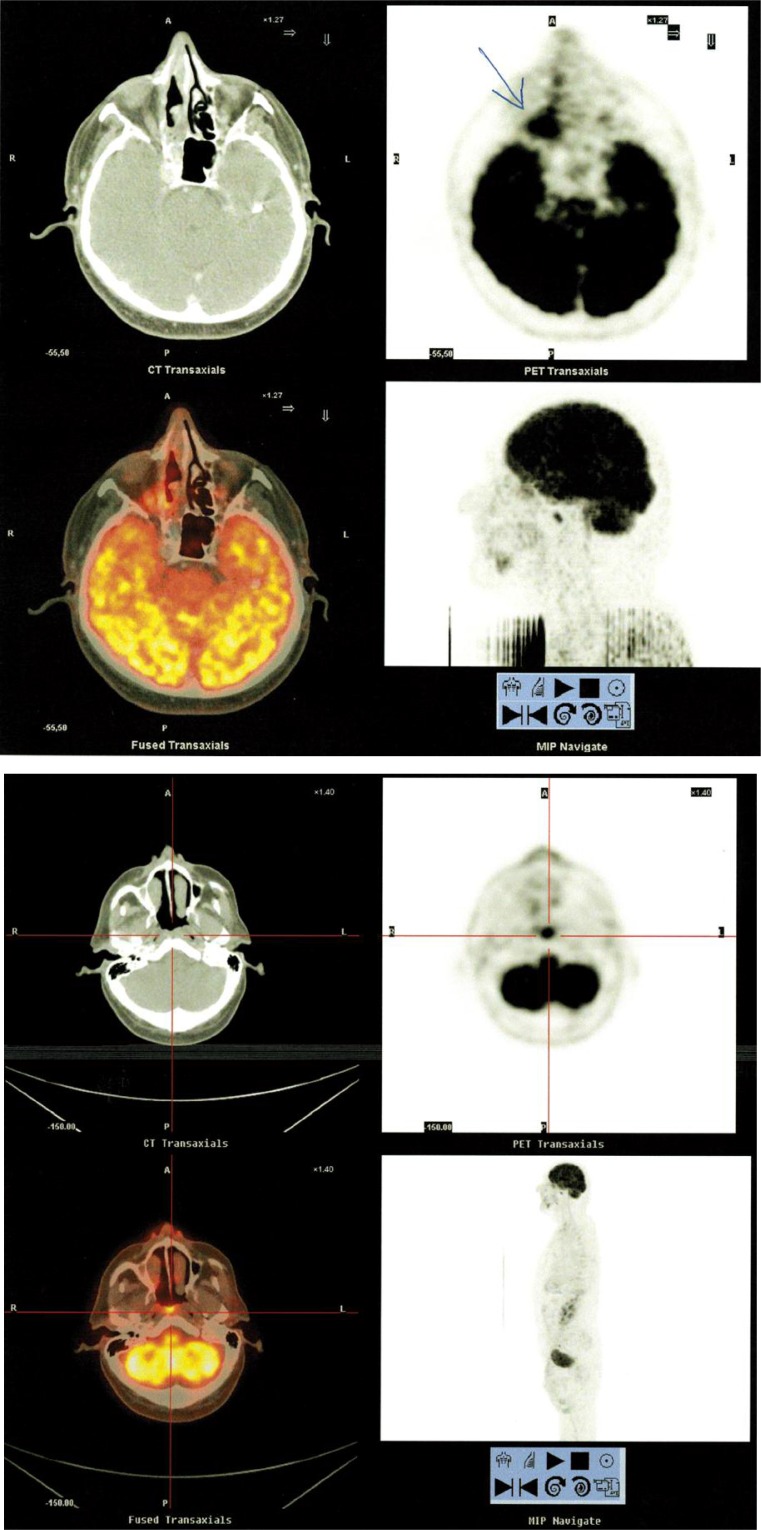
On 4th March 2012, the Positron Emission Tomography/Computed Tomography (PET/CT) examination with 18-fluorodeoxyglucose (18FDG) had been performed, and in the right nasal cavity it showed mucosal thickening and increased metabolic activity on the dorsal wall of nasopharynx with 15 mm in size (LL – lateral lateral), SUVmax (standardized uptake value) 8.24-11.05. Frontal and right maxillary sinuses were filled with content, but with no metabolic activity. There were no further distinct pathological changes in PET/CT images in relation to the underlying disease, no signs of lymph nodes or metastatic involvement of other organs (Fig. 2).
Fig. 2.
PET/CT images of the tumor on the dorsal wall of nasopharynx
On 24th April 2012, postoperative ENT examination was: nose – thicker red mucosa on the right side, nasal passage loose without discharge. There was no residual tumor in the right nasal cavity.
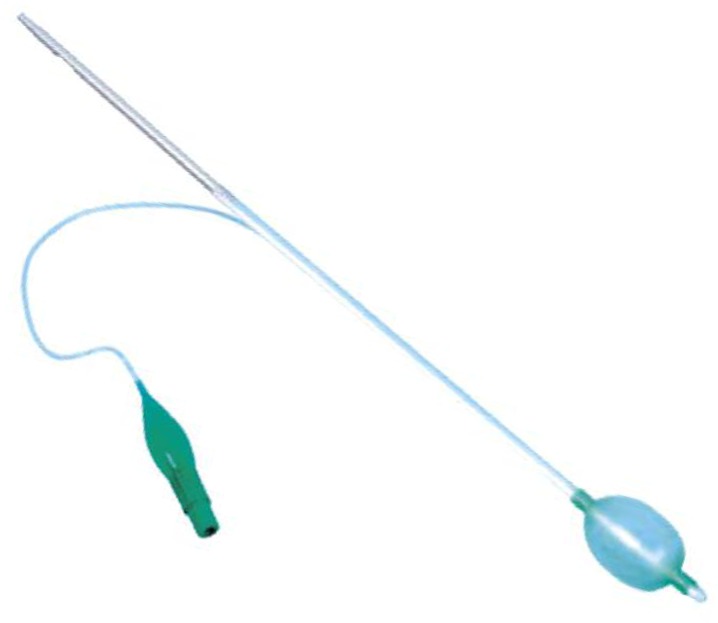
In May 2012, on the recommendation of the Joint clinic for malignant melanoma, the patient had been presented to Department of Radiation Oncology of National Oncology Institute in regards to brachytherapy of the nasopharyngeal dorsal wall. We used a high dose rate (HDR) brachytherapy using MicroselectronHDR® (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden), with source 192Ir HDR (air-kerma strength was 21698 cGy x cm 2/h during the first fraction of brachytherapy), and for nasopharynx using an inflatable balloon applicator. It is a simple, convenient and secure method that does not require general anesthesia. The balloon applicator ensures fixation with a good connection between the applicator and the vault of nasopharynx, whilst it reduces the surface radiation dose. The wire with outer diameter 4mm had been inserted into the nasopharynx with the deflated balloon which was inflated in the cavity of nasopharynx to the maximal diameter of 30 mm (Fig. 3). Local anesthesia was achieved with lidocaine spray and adrenaline gauze in the nasal cavity.
Fig. 3.
Nasopharyngeal balloon applicator
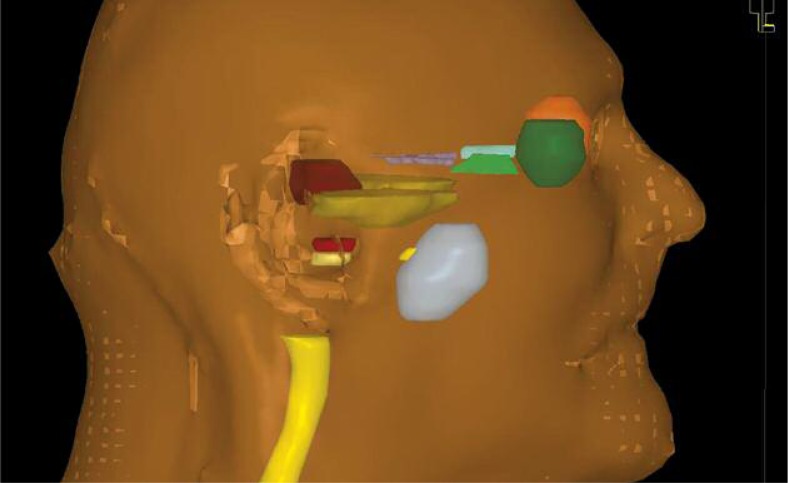
Subsequently, X- ray images (anterior-posterior and lateral projections) were taken with inserted wire into the closed end of the balloon, the patient was in the supine position. We made a cone beam CT (100 kV, 4 mA, reconstruction diameter 27 cm, pixel spacing 0.527 mm, slices thickness 2 mm) on the simulator, followed by the registration with previous PET/CT images on which has been drawn balloon, clinical target volume (CTV) (CTV was equal planning target volume – PTV) that was equal to balloon plus 3 mm margins, and surrounding organs (brainstem, chiasma, spinal cord, temporal lobes, the visual apparatus, cochlea on both side) (Figs. 4 and 5). Brachytherapy was planned on the treatment planning system Oncentra v4® (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden), number of source positions was 13, with a spacing of 2.5 mm.
Fig. 4.
Registration of PET/CT and cone beam CT images
Fig. 5.
3D contours of therapeutical volumes and surrounding organs
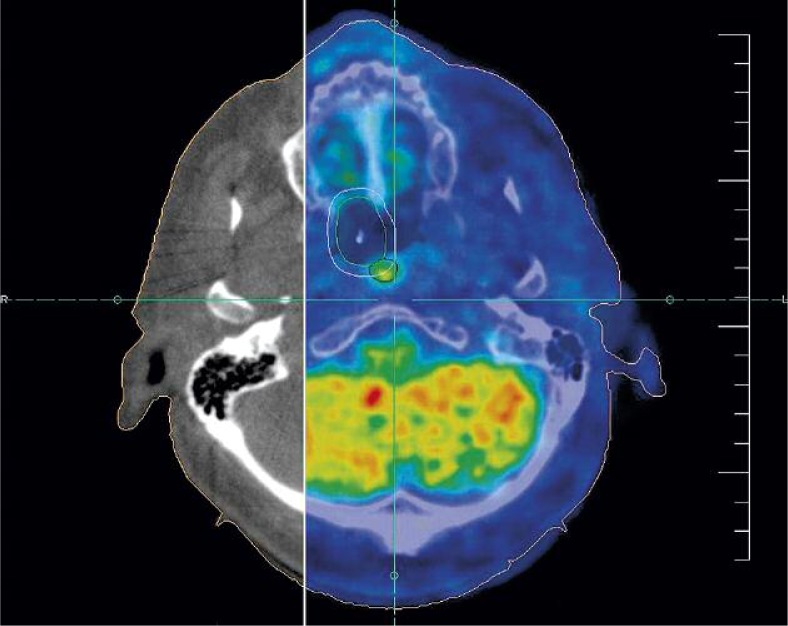
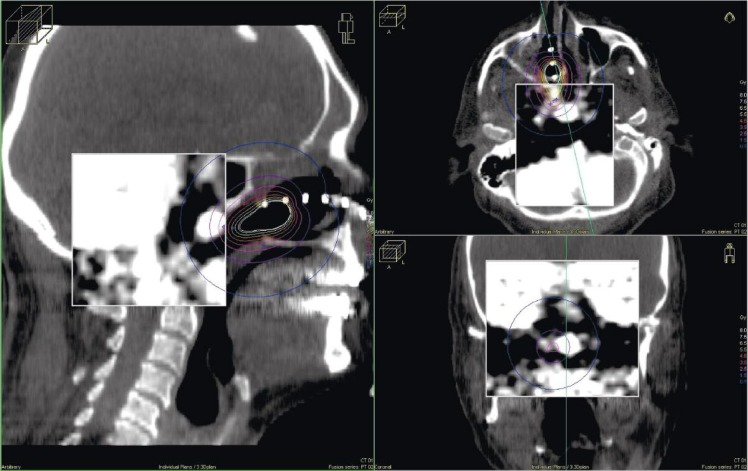
Dose of brachytherapy was 10 x 4 Gy (for α/β0.5Gy EQD2 {dose in 2 Gy fractions biologically equivalent to dose D given in fractions of size d, in units of Gray} was 72 Gy), 2 times per week (Tuesday and Thursday), 100% dose was 3 mm from the surface of the applicator, the applicator had diameter 20 mm, irradiated length was 30 mm, D90 (the median dose delivered to 90%), for gross tumor volume (GTV) was 85% according PET/CT. Surrounding organs received a minimum dose of radiation (Fig. 6 and Table 1). Dose of brachytherapy was high, but malignant melanoma as radioresistant disease requires such high dose. During brachytherapy, dryness and burning sensation in the mucosa of the nasal cavity appeared.
Fig. 6.
Dose distribution around nasopharyngeal applicator with representative cross sections
Table 1.
Dose volume histogram (DVH) per fraction
| ROI | Dose [%] | Dose [%] | Volume [%] | Volume [ccm] |
|---|---|---|---|---|
| OR/Right eye | 13.78 | 0.5511 | 0.13 | 0.01 |
| OR/Right lens | 7.30 | 0.2921 | 8.65 | 0.01 |
| OR/Chiasm | 16.15 | 0.6459 | 6.60 | 0.01 |
| OR/Temporal lobes | 31.81 | 1.2724 | 0.04 | 0.01 |
| OR/Right optical nerve | 16.05 | 0.6420 | 2.40 | 0.01 |
| OR/Right cochlea | 14.57 | 0.5828 | 1.73 | 0.01 |
| OR/Left cochlea | 10.99 | 0.4397 | 1.61 | 0.01 |
| OR/Left eye | 12.95 | 0.5181 | 0.12 | 0.01 |
| OR/Left lens | 6.01 | 0.2406 | 6.22 | 0.01 |
| OR/Left optical nerve | 11.56 | 0.4625 | 4.00 | 0.01 |
| OR/Brainstem | 18.18 | 0.7273 | 0.13 | 0.01 |
| OR/Spinal cord | 15.28 | 0.6111 | 0.10 | 0.01 |
| GTV (PET/CT) | 84.81 | 3.3923 | 90.00 | 0.09 |
| Baloon | 157.61 | 6.3045 | 90.00 | 5.52 |
| PTV | 101.96 | 4.0785 | 90.00 | 11.73 |
Results
ENT examination after brachytherapy on 19th June 2012 was: nose – on the right side in the posterior 2/3 of mucosa was covered with whitish coating (these changes were also seen on the back of nasopharynx). Neck – a palpable solid submandibular gland on the right side and small lymph node beside it. Clinical finding was confirmed by ultrasound, which was done on 28th June 2012: on the right side of neck, precisely in the submandibular region (region IIa), a hypoechogenic pathological formation appeared – its size was 23 x 18 x 19 mm and it was vascularised. Needle biopsy had been performed on 16th July 2012, and cytological finding confirmed the presence of melanoma.
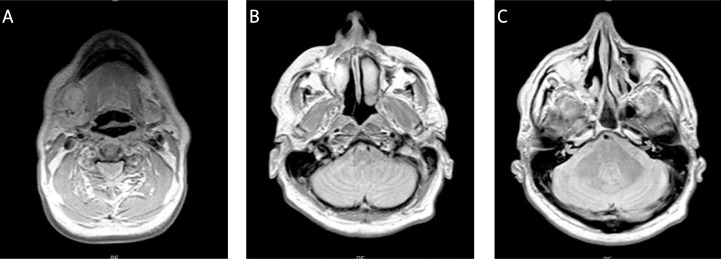
Magnetic resonance imaging (MRI) of brain and paranasal cavities had been done on 30th July 2012 and described: missing middle concha on the right side, hypoplastic right maxillary sinus, diffuse thickening was observed in the mukoperiost of nasal cavity more to the right, also of the right maxillary cavity, ethmoidal cavities, and in the right frontal cavity which was filled with content. Changes were of non-specific nature vs. glial scars in white matter bilaterally supratentorial. In the right submandibular area there was an oval formation – pathological lymph node (Fig. 7).
Fig. 7.
MRI of brain and paranasal cavities
The patient had come to the 2nd ENT clinic of Medicine Faculty in Bratislava again. Rinoscopical examination was: pink mucosa of sinuses, free passes and ventilation. Nasopharynx: 2 mounds of pink mucous membranes, slightly soaked. The extirpation of lymph node in the right submandibular region had been done on 1st August 2012. Histology was: metastasis of malignant melanoma which was almost completely necrotizing single vital cells. In all probability, the lymph node metastasis on the neck had originated from the mucosal malignant melanoma, not from cutaneous melanoma on the back, because of the anatomical proximity of the submandibular area to the nasal cavity. However, histologically we did not receive originate of this metastasis. On 28th August 2012, ETN examination was: nose – septum in the middle, right nasal cavity was changed (lateral wall without middle concha), roof of nasal cavity scarred with smooth mucosa and without tumor. Nasopharynx – free, vaulted, without tumor. Neck – without palpable lymph nodes, healed scar. Thereafter, the patient had been approached to Joint Clinic for malignant melanoma, where it has been decided to conduct external beam radiotherapy on the submandibular area on the right side, which was done with a dose of 40 Gy, 2.5 Gy per day (for α/β0.5Gy EQD2 was 48 Gy), 3 fields technique with MLCi (80 leafs, 40 x 40), X-rays energy of 6 MV, Elekta Synergy® (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden). Precise planning system v2.2 was used.
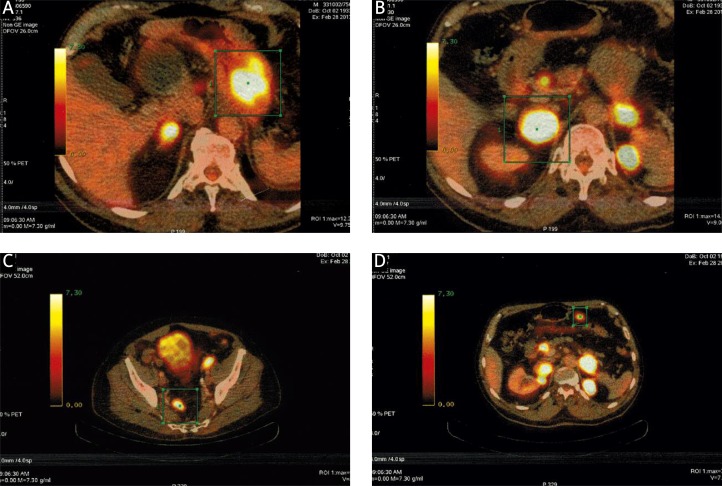
After radiotherapy was finish, the patient was followed up by the ENT specialist and at the Department of Radiation Oncology. The locoregional finding was without signs of recurrence. However, PET/CT scan was made on 28th February 2013, which showed distant progression of disease (gastric fundus, intra and retroperitoneal lymph nodes, adrenal glands and parailiac lymph nodes) (Fig. 8).
Fig. 8.
Distant progression of the disease on PET/CT
Discussion and conclusions
Malignant melanomas are usually found in the skin. But in extraordinary situations they can also arise from mucosal epithelium. In the nose, they are mostly found on the septum and lateral wall, less in the middle and lower concha, and represent 80% of all sinonasal mucosal melanomas (SNMMs). Another 20% of lesions are located in areas of the paranasal sinuses, most commonly in the maxillary cavity, followed by the ethmoid, frontal and sphenoid sinuses [7, 8]. Most patients have non-specific symptoms before the diagnosis. The most common symptoms include obstruction and epistaxis. Pain, diplopia and proptosis are very rare. Due to the non-specificity of symptoms, the majority of lesions are in locally advanced state at the time of diagnosis, since the patient visits their doctor about 6 to 12 months after their appearance [6]. The biggest failure in the treatment of SNMMs is a high incidence of local recurrence. The high local recurrence rates may be a manifestation of the multifocality or clinically unapparent diffuse submucosal lymphatic spread of melanoma cells [9]. Distant metastasis has been observed in 51.5% of patients with a mucosal melanoma of the head and neck [7].
Surgery is the treatment of choice for SNMMs, especially complete resection of the tumor with sufficient free margins. Radiation therapy still holds a controversial role in treating of SNMMs [10, 11]. Melanoma cells are traditionally considered as radioresistant, because they have a high capacity for repair of sublethal damages, in particular when we used a conventional fractionation. Therefore, hypofractionation (higher single dose per fraction) can be considered as a reasonable treatment regimen for this disease [12, 13].
We decided to apply the local excision of nasal tumor at the request of the patient who refused radical surgical treatment. Based on PET/CT method we decided to radiate the change in the nasopharynx, because sensitivity and specificity of this examination was very high (98%, 94%, respectively) [14]. We have decided for brachytherapy, since the change in nasopharynx was small, and due to the proximity of sensitive surrounding structures. Also, brachytherapy allows the use of high doses of radiation with minimal impact on the surrounding healthy organs. Our patient had developed regional progression of the disease 6 months after diagnosis, but the application of postoperative external beam radiotherapy achieved locoregional control of disease. Nearly one year after diagnosis, distant disease progression appeared on which the radiation therapy could not make an impact. The patient lives and his treatment is continued with chemotherapy.
We have chosen to discuss this condition, because of its rarity and the possibility of using radiotherapy, although the malignant melanoma was considered as radioresistant disease, and also to emphasize the importance of a multidisciplinary approach to treatment of these patients. It seems that postoperative radiotherapy may have a role in local control of malignant melanoma, as it had in treatment of our patient.
Acknowledgements
The authors would like to thank the nurses and other staff who participated in the treatment of our patients.
References
- 1.Grewal DS, Mallya SV, Baser B, et al. Malignant melanoma of nasopharynx extending to the nose with metastasis in the neck (a case report) J Postgrad Med. 1994;40:31–33. [PubMed] [Google Scholar]
- 2.Bhave CG, Ogale SB, Sane SY. Malignant melanoma of the nasal cavity (a case report) J Postgrad Med. 1990;36:173–174. [PubMed] [Google Scholar]
- 3.Lin CY, Yang SW, Lai CH. Primary Malignant Melanoma of the Nasal Cavity (a case report) Chang Gung Med J. 2003;26:857–862. [PubMed] [Google Scholar]
- 4.Medhi P, Biswas M, Das D, et al. Cytodiagnosis of mucosal malignant melanoma of nasal cavity: A case report with review of literature. J Cytol. 2012;29:208–210. doi: 10.4103/0970-9371.101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson LDR, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bhagat S, Varshney S, Singh R, et al. Malignant melanoma of nose and paranasal sinuses: 2 case reports. Online J Health Allied Scs. 2009;8:13. [Google Scholar]
- 7.Yen-Fu Cheng, Chien-Chung Lai, Wing-Yin Li, et al. Primary sinonasal mucosal melanoma with unusual long-term survival (a case report) Tzu Chi Med J. 2005;17:177–180. [Google Scholar]
- 8.Manolidis S, Donald PJ. Malignant melanoma of the head and neck: Review of the literature and report of 14 patients. Cancer. 1997;80:1373–1386. doi: 10.1002/(sici)1097-0142(19971015)80:8<1373::aid-cncr3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 10.Harwood AR, Cummings BL. Radiotherapy for mucosal melanoma. Int J Radiat Oncol Biol Phys. 1982;8:1121–1126. doi: 10.1016/0360-3016(82)90058-x. [DOI] [PubMed] [Google Scholar]
- 11.Trotti A, Peters LJ. Role of radiotherapy in the primary management of mucosal melanoma of the head and neck. Semin Surg Oncol. 1993;9:246–250. [PubMed] [Google Scholar]
- 12.Bentzen SM, Overgaard J, Thames HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989;16:169–182. doi: 10.1016/0167-8140(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Peters LJ, Weber RS, et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys. 1994;30:795–798. doi: 10.1016/0360-3016(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 14.Strobel K, Dummer R, Husarik D, et al. High-Risk Melanoma: Accuracy of FDG PET/CT with Added CT Morphologic Information for Detection of Metastases. Radiology. 2007;244:566–574. doi: 10.1148/radiol.2442061099. [DOI] [PubMed] [Google Scholar]