Abstract
Purpose
To determine predictors of distant metastases (DM) in prostate cancer patients treated with high dose rate brachytherapy boost (HDR-B) and external beam radiation therapy (EBRT).
Material and methods
From 1991 to 2002, 768 men with localized prostate cancer were treated with HDR-B and EBRT. The mean EBRT dose was 37.5 Gy (range: 30.6-45 Gy), and the HDR-B was 22 or 24 Gy delivered in 4 fractions. Univariate and multivariate analyses using a Cox proportional hazards model including age at diagnosis, T stage, Gleason score (GS), pretreatment PSA, biologically equivalent dose (BED), and use of androgen deprivation therapy (ADT) was used to determine predictors of developing distant metastases.
Results
The median follow-up time for the entire patient population was 4.2 years (range: 1-11.2 years). Distant metastases were identified in 22/768 (3%) of patients at a median of 4.1 years. PSA failure according to the Phoenix definition developed in 3%, 5%, and 14% of men with low, intermediate, and high risk disease with a median time to failure of 3.8 years. Prostate cancer specific mortality was observed in 2% of cases. T stage, GS, and use of ADT were significantly associated with developing DM on univariate analysis. GS, and use of ADT were the only factors significantly associated with developing DM on multivariate analysis (p < 0.01). Patients who received ADT had significantly higher risk features suggesting patient selection bias for higher DM in this group of patients rather than a negative interaction between HDR-B and EBRT.
Conclusions
In men treated with HDR-B and EBRT, GS is a significant factor on multivariate analysis for developing distant metastasis.
Keywords: brachytherapy, distant metastases, high-dose-rate, prostate cancer
Purpose
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer-related deaths amongst men in the United States [1]. Brachytherapy is an effective treatment modality that can be utilized either alone or in combination with external beam radiation therapy (EBRT) for patients with localized prostate cancer of all risk groups [2–5]. The combination of EBRT with brachytherapy can improve prostate cancer outcomes by allowing meaningful dose escalation while minimizing dose to organs at risk [6]. Predictors of PSA progression free survival following prostate brachytherapy have been extensively evaluated, whereas fewer studies have characterized predictors of distant metastases [2, 7]. The bulk of the data on predictors of distant metastasis are from external beam radiation therapy and low dose rate brachytherapy [2, 7–9]. Data with respect to predictors of distant metastasis outcomes following high-dose-rate brachytherapy are more limited [10]. Higher Gleason score has been a significant predictor of distant metastasis in most series, whereas total dose has not always been [11–13]. Determining predictors of metastatic disease following combination therapy is important, because it identifies men who could benefit from additional treatment. We reviewed the clinical outcomes on our single institution experience treating localized prostate cancer with high-dose-rate brachytherapy boost (HDR-B), and EBRT to identify predictors of distant metastases.
Material and methods
An Institutional Review Board approved retrospective study that was performed on men with clinically localized biopsy-proven prostate cancer who were treated between 1991 and 2002 at a single center with HDR-B, and EBRT with at least one year of follow-up. Pretreatment evaluation included history and physical examination, digital rectal examination (DRE), transrectal ultrasonography (TRUS), prostate biopsies with Gleason score, and PSA determination. Staging procedures, such as bone scan and CT of the abdomen and pelvis were performed for intermediate and higher risk patients. T stage was based exclusively on the DRE.
The HDR brachytherapy method has been previously described [10]. HDR-B and EBRT were administered with a 2 week interval in between (most commonly HDR first followed by EBRT). Two implants were performed 1 week apart with each implant used to deliver 2 HDR fractions (i.e. total of 4 fractions). The procedure consisted of transperineal interstitial implantation of 17-18 flexiguides directed by TRUS, cystoscopy, and fluoroscopy. Simulation radiography, during the period of study, was based on plain film simulation, and dosimetry was computed using Nucletron, versions 10 through 11.4 (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden). The 100% isodose was normalized to a 5-6 mm margin anterior and lateral to the prostatic capsule to include microscopic disease extension, but the margin was limited to approximately 2-3 mm posteriorly to prevent rectal injury. Bladder balloon and rectal dose limits were ≤ 80%. The urethral dose limits were 120%, except for cases of prior transuretheral resection of the prostate, where the maximum urethral dose constraint was reduced to 105%. Fluoroscopic verification of the brachytherapy catheter position was performed prior to the second treatment delivery. Dosimetric quality was determined by evaluation of dose volume histograms, analysis of clinical target volume coverage (D90 – dose to 90% prostate, V100 – prostate volume receiving 100% prescription dose, and V150 – prostate volume receiving 150% of the prescription dose) and normal tissue dose constraints. Pelvic EBRT was delivered using 3D conformal treatment planning, and was delivered in 1.8-2.0 Gy per fraction initially to 36 Gy and later to 39.6 Gy.
When ADT was utilized, it consisted of a luteinizing hormone releasing agonist usually with an antiandrogen. It was typically initiated 3 months before EBRT and consisted of a short course (≤ 6 months). Indications for ADT were quite variable over the course of the study. In the early years, it was widely administered by referring physicians regardless of risk group. Over time, the use of ADT was less commonly prescribed for favorable disease. In some cases, it was used, without regard to risk group, to reduce the prostate volume prior to brachytherapy.
Follow up consisted of office visits with digital rectal examination, PSA testing and search of patient medical records for evidence of distant metastasis. PSA measurements were done for 3-month intervals for the first 2 years following completion of treatment, every 6 months for years 2-5, and then yearly thereafter. Distant metastasis was identified primarily by radiological means.
A Cox proportional hazards model was employed to examine the risk of developing DM by several patient disease and treatment parameters, including age at diagnosis, T stage, Gleason score, use of ADT, and BED (an α/β ratio of 1.2 Gy was assumed) [11]. BEDs were also converted to an equivalent 2 Gy dose (EQD2) for ease of interpretation, and are presented in the discussion section [14, 15]. A two-sided p value < 0.05 was considered significant for all tests.
Results
We identified 768 men with localized prostate cancer who were treated at our institution with HDR-B and EBRT (Table 1A). According to NCCN risk stratification criteria, the distribution of patients was 26% low, 50% intermediate, and 24% high risk. ADT was administered to 380 patients (49%) for a median duration of 4.5 months (maximum 6 months). The mean pre-treatment PSA ± standard deviation was 10.2 ± 7 ng/ml. The mean EBRT dose was 37.5 Gy (range: 30.6-45 Gy) and HDR-B dose 22 Gy for 15%, and 24 Gy for 85% of patients. The median BED for HDR-B plus EBRT was 234 Gy (range: 212-249 Gy).
Table 1A.
Patient characteristics
| N = 768 (%) | SD | |
|---|---|---|
| Mean age at diagnosis (years) | 66.3 | 7.9 |
| Mean Gleason score: | 6.5 | 1.1 |
| 6 | 406 (53) | |
| 7 | 264 (34) | |
| ≥ 8 | 98 (13) | |
| Mean pre-treatment PSA (ng/ml) | 10.2 | 7 |
| < 10 | 505 (66) | |
| 10-20 | 195 (25) | |
| ≥ 20 | 68 (9) | |
| T-Stage | ||
| T1 | 287 (37.4) | |
| T2 | 421 (54.8) | |
| T3 | 60 (7.8) | |
| NCCN risk group | ||
| Low | 197 (25.7) | |
| Intermediate | 383 (49.9) | |
| High | 188 (24.5) | |
| Androgen deprivation | ||
| No | 388 (51) | |
| Yes | 380 (49) | |
| Low | 63 (32) | |
| Intermediate | 190 (50) | |
| High | 127 (68) | |
| Mean duration of hormones (m) | 4.5 | 1.3 |
| BED (EBRT + HDR) | ||
| ≤ 234 | 492 | 64.1 |
| > 234 | 276 | 35.9 |
| Mean EBRT dose (Gy) | 37.5 | 2 |
| Range (Gy) | 30.6-45 | |
| HDR dose (Gy) | ||
| 22 | 113 (14.7) | |
| 24 | 655 (85.3) |
SD – standard deviation, BED – biologically equivalent dose, EBRT – external beam radiation therapy, HDR – high dose rate
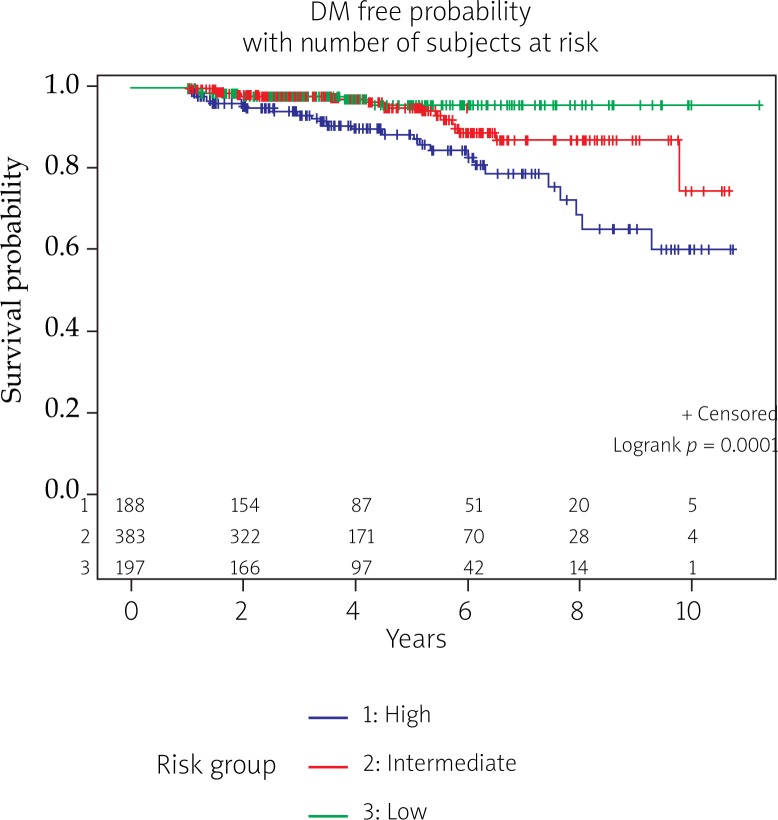
The median follow-up time for the entire patient population was 4.2 years (range: 1.0-11.2 years). The median follow-up for patients with low, intermediate, and high risk disease was 3.9, 3.6, and 3.8 years. Three percent (22/768) of patients developed distant metastases with a median time to a diagnosis of metastatic disease of 4.1 years (range: 1.0-7.2 years). All but one patient who developed metastatic disease had high (n = 17) or intermediate (n = 4) risk disease. All distant metastasis, except for one, occurred in patients who received BED ≤ 234 Gy (Table 1B). Actuarial curves for freedom from distant metastasis for low, intermediate, and high risk prostate cancer patients are shown in Figure 1.
Table 1B.
Patient characteristics grouped by distant metastasis
| Patient characteristics | All (N = 768) | DM | ||||
|---|---|---|---|---|---|---|
| No (n = 746) | Yes (n = 22) | |||||
| N | % | N | % | N | % | |
| Age at diagnosis | ||||||
| ≤ 67 | 395 | 51.4 | 384 | 51.5 | 11 | 50 |
| > 67 | 373 | 48.6 | 362 | 48.5 | 11 | 50 |
| Pre-treatment PSA | ||||||
| < 10 | 505 | 65.8 | 491 | 65.8 | 14 | 63.6 |
| 10-20 | 195 | 25.4 | 190 | 25.5 | 5 | 22.7 |
| ≥ 20 | 68 | 8.9 | 65 | 8.7 | 3 | 13.6 |
| Gleason score | ||||||
| ≤ 6 | 406 | 52.9 | 405 | 54.3 | 1 | 4.5 |
| 7 | 264 | 34.4 | 255 | 34.2 | 9 | 40.9 |
| ≥ 8 | 98 | 12.8 | 86 | 11.5 | 12 | 54.5 |
| T-Stage | ||||||
| T1 | 287 | 37.4 | 284 | 38.1 | 3 | 13.6 |
| T2 | 421 | 54.8 | 409 | 54.8 | 12 | 54.5 |
| T3 | 60 | 7.8 | 53 | 7.1 | 7 | 31.8 |
| Androgen deprivation | ||||||
| No | 388 | 50.5 | 382 | 51.2 | 6 | 27.3 |
| Yes | 380 | 49.5 | 364 | 48.8 | 16 | 72.7 |
| Risk group | ||||||
| Low | 197 | 25.7 | 196 | 26.3 | 1 | 4.5 |
| Intermediate | 383 | 49.9 | 379 | 50.8 | 4 | 18.2 |
| High | 188 | 24.5 | 171 | 22.9 | 17 | 77.3 |
| Distant metastases | . | |||||
| No | 746 | 97.1 | 746 | 100 | . | |
| Yes | 22 | 2.9 | . | . | 22 | 100 |
| Androgen deprivation | ||||||
| No | 388 | 50.5 | 382 | 51.2 | 6 | 27.3 |
| Yes | 380 | 49.5 | 364 | 48.8 | 16 | 72.7 |
| BED | ||||||
| ≤ 234 | 492 | 64.1 | 471 | 63.1 | 21 | 95.5 |
| > 234 | 276 | 35.9 | 275 | 36.9 | 1 | 4.5 |
| HDR | ||||||
| 22 | 113 | 14.7 | 105 | 14.1 | 8 | 36.4 |
| 24 | 655 | 85.3 | 641 | 85.9 | 14 | 63.6 |
DM – distant metastases, BED – biologically equivalent dose
Fig. 1.
Kaplan Meier actuarial survival curve for PSA progression free probability stratified by risk groups
PSA failure (Phoenix definition) developed in 7% of men with a median time to failure of 3.8 years (range: 1.0-9.8 years). By NCCN risk group PSA failure developed in 3%, 5%, and 14% of men with low, intermediate, and high risk disease (Table 2). Actuarial curves for PSA progression free survival for low, intermediate, and high risk patients are shown in Figure 2. Local tumor control (i.e. failure to manifest clinical disease progression in the prostate) was observed in 99% of cases. Prostate cancer specific mortality was observed in 2% of cases.
Table 2.
Patient characteristics grouped by PSA failure
| Patient characteristics | All (N = 768) | DM | ||||
|---|---|---|---|---|---|---|
| No (n = 746) | Yes (n = 22) | |||||
| N | % | N | % | N | % | |
| Age at diagnosis | ||||||
| ≤ 67 | 395 | 51.4 | 362 | 50.6 | 33 | 62.3 |
| > 67 | 373 | 48.6 | 353 | 49.4 | 20 | 37.7 |
| Pre-treatment PSA | ||||||
| < 10 | 505 | 65.8 | 475 | 66.4 | 30 | 56.6 |
| 10-20 | 195 | 25.4 | 178 | 24.9 | 17 | 32.1 |
| ≥ 20 | 68 | 8.9 | 62 | 8.7 | 6 | 11.3 |
| Gleason score | ||||||
| ≤ 6 | 406 | 52.9 | 393 | 55 | 13 | 24.5 |
| 7 | 264 | 34.4 | 241 | 33.7 | 23 | 43.4 |
| ≥ 8 | 98 | 12.8 | 81 | 11.3 | 17 | 32.1 |
| T-Stage | ||||||
| T1 | 287 | 37.4 | 275 | 38.5 | 12 | 22.6 |
| T2 | 421 | 54.8 | 392 | 54.8 | 29 | 54.7 |
| T3 | 60 | 7.8 | 48 | 6.7 | 12 | 22.6 |
| Androgen deprivation | ||||||
| No | 389 | 50.7 | 364 | 50.9 | 25 | 47.2 |
| Yes | 379 | 49.3 | 351 | 49.1 | 28 | 52.8 |
| NCCN risk group | ||||||
| Low | 197 | 25.7 | 191 | 26.7 | 6 | 11.3 |
| Intermediate | 383 | 49.9 | 363 | 50.8 | 20 | 37.7 |
| High | 188 | 24.5 | 161 | 22.5 | 27 | 50.9 |
| Distant metastasis | ||||||
| No | 746 | 97.1 | 707 | 98.9 | 39 | 73.6 |
| Yes | 22 | 2.9 | 8 | 1.1 | 14 | 26.4 |
| Androgen deprivation | ||||||
| No | 389 | 50.7 | 364 | 50.9 | 25 | 47.2 |
| Yes | 379 | 49.3 | 351 | 49.1 | 28 | 52.8 |
| BED | ||||||
| ≤ 234 | 492 | 64.1 | 450 | 62.9 | 42 | 79.2 |
| > 234 | 276 | 35.9 | 265 | 37.1 | 11 | 20.8 |
| HDR | ||||||
| 22 | 113 | 14.7 | 103 | 14.4 | 10 | 18.9 |
| 24 | 655 | 85.3 | 612 | 85.6 | 43 | 81.1 |
BED – biologically equivalent dose
Fig. 2.
Kaplan Meier actuarial survival curve for distant metastasis free probability stratified by risk groups
On univariate analysis, T stage, GS, and use of ADT were significantly associated with developing DM (Table 3). Multivariate analysis showed increasing GS was associated with an increased risk of developing distant metastasis (p < 0.05) (Table 3). Multivariate analysis also showed an increased risk of developing distant metastasis with the use of ADT. This lead us to compare the makeup of patients treated with ADT to those without ADT to determine if patient selection bias could explain this finding. We found that patients selected for ADT had significantly higher GS, T stage, and pre-treatment PSA (all p < 0.0001) compared to those who did not receive ADT (Table 4).
Table 3.
Univariate and multivariate analysis for predictors of PSA failure (Phoenix definition) and distant metastasis
| Parameter | Univariate analysis p value | Multivariate analysis p value | ||
|---|---|---|---|---|
| BF | DM | BF | DM | |
| Pre-treatment PSA: | ||||
| 10-20 vs. < 10 | 0.23 | 0.87 | 0.14 | 0.99 |
| ≥ 20 vs. < 10 | 0.62 | 0.65 | 0.95 | 0.92 |
| T-stage: | ||||
| T2 vs. T1 | 0.47 | 0.16 | 0.47 | 0.61 |
| T3 vs. T1 | 0.03 | 0.0014 | 0.03 | 0.17 |
| Age at diagnosis: | ||||
| > 67 vs. ≤ 67 | 0.009 | 0.96 | 0.009 | 0.34 |
| Gleason score: | ||||
| 7 vs. ≤ 6 | 0.0005 | 0.007 | 0.002 | 0.02 |
| 8 vs. ≤ 6 | < 0.0001 | 0.0001 | < 0.0001 | 0.0003 |
| BED: | ||||
| ≥ 234 vs. < 234 | 0.38 | 0.20 | 0.38 | 0.13 |
| Androgen deprivation | 0.44 | 0.003 | 0.44 | 0.04 |
BF – biochemical failure, DM – distant metastasis
Table 4.
Patient characteristics based on androgen deprivation therapy (ADT)
| Patient characteristics | All (N = 768) | ADT | P-value | ||||
|---|---|---|---|---|---|---|---|
| Without ADT | With ADT | ||||||
| N | % | N | % | N | % | ||
| Age at diagnosis | |||||||
| ≤ 67 | 395 | 51.4 | 208 | 53.5 | 187 | 49.3 | 0.25 |
| > 67 | 373 | 48.6 | 181 | 46.5 | 192 | 50.7 | |
| Pre-Treatment PSA | |||||||
| < 10 | 505 | 65.8 | 280 | 72 | 225 | 59.4 | < 0.001 |
| 10-20 | 195 | 25.4 | 83 | 21.3 | 112 | 29.6 | |
| ≥ 20 | 68 | 8.9 | 26 | 6.7 | 42 | 11.1 | |
| Gleason score | |||||||
| ≤ 6 | 406 | 52.9 | 245 | 63 | 161 | 42.5 | < 0.0001 |
| 7 | 264 | 34.4 | 115 | 29.6 | 149 | 39.3 | |
| ≥ 8 | 98 | 12.8 | 29 | 7.5 | 69 | 18.2 | |
| T-Stage | |||||||
| T1 | 287 | 37.4 | 164 | 42.2 | 123 | 32.5 | < 0.001 |
| T2 | 421 | 54.8 | 207 | 53.2 | 214 | 56.5 | |
| T3 | 60 | 7.8 | 18 | 4.6 | 42 | 11.1 | |
BF – biochemical failure, DM – distant metastasis
Discussion
Most clinical reports following definitive treatment of prostate cancer use PSA control as a primary endpoint, but it is clear from longitudinal studies that biochemical failure does not always lead to other evidence of disease progression. Such an observation was made in a series of 1997 men with clinically localized prostate cancer treated with radical prostatectomy, of whom 15% (n = 315) developed PSA progression. After a median follow-up of eight years only, 30% of men of those with PSA failure went on to developed metastasis [16]. This discordance between PSA failure and the development of distant metastases underscores the need to identify factors that predict for a higher likelihood of developing clinically meaningful events such as the occurrence of distant metastasis which can impact quality of life and survival.
One of the largest series looking at predictors of distant metastasis was performed on 8669 men with clinically localized or locally advanced non-metastatic prostate cancer, treated with either EBRT alone or surgery. They found that a post-treatment PSA doubling time of less than 3 months predicted a 20-fold increase in the risk of dying from prostate cancer [8]. Combining Gleason score with PSA doubling time further improves predicting prostate cancer-specific mortality [17]. Other studies in patients treated definitively with EBRT have identified T stage, Gleason score, pretreatment PSA, ADT use, and higher radiation dose as other significant predictors of distant metastasis free survival [9]. While some information is known regarding predictors of distant metastasis following EBRT, less is known following brachytherapy. In two of the largest low dose rate (LDR) brachytherapy series Forsythe et al. showed Gleason score and PSA doubling time as significant predictors [2], whereas Taira et al. found that metastasis free survival was most closely related to Gleason score and year of treatment [7].
HDR is significantly different from LDR with respect to dose rate, and technique suggesting there may be differences in predictors of outcomes between the two modalities [18]. In terms of HDR, the largest published study of predictors of distant metastasis following HDR and EBRT comes from the experience at William Beaumont Hospital (WBH) by Martinez et al., who reported on 472 men with intermediate and high risk disease [11]. They performed a dose escalation investigation starting with HDR doses of 5.5 Gy x 3 and ending with 11.5 Gy x 2 for the HDR component of treatment. The mean dose of EBRT was 46 Gy. They found that when the BED (α/β ratio 1.2) was ≥ 268 Gy (which corresponded to 9.5 Gy x 2) there was less biochemical failure, better local control, and fewer cases of distant metastasis. They also observed that Gleason score predicted biochemical and overall clinical control, but did not independently correlate with freedom from distant metastasis. In another series from Memorial Sloan Kettering Cancer Center (MSKCC), Kotecha et al. reported on the outcomes of 229 patients with clinically localized prostate cancer treated with a HDR brachytherapy boost (5.5 Gy x 3 to 7.5 Gy x 3) followed by EBRT (most patients were treated to 50.4 Gy). Amongst high risk patients a higher BED (> 190 Gy, α/β ratio of 2) resulted in improved biochemical control and distant metastases free survival [12]. In a final large series from Prada et al. that included outcomes of 313 patients with localized prostate cancer with 46 Gy of EBRT, and HDR brachytherapy boost of 11.5 Gy x 2, Gleason score was the only factor significantly associated with developing distant metastasis [13].
Our data is consistent with the published literature demonstrating the importance of GS in predicting the risk of developing distant metastasis, however we did not find that BED was significant on multivariate analysis. Our early standard treatment consisted of 36 Gy EBRT plus 6 Gy x 4 fractions of HDR. In 1999 we increased the EBRT dose from 36 Gy to 39.6 Gy without changing the HDR program. If we convert the doses used in this series (EBRT 39.6 Gy + 6 Gy x 4 HDR), the WBH series over which they saw improved outcomes (EBRT 46 Gy + 9.5 Gy x 2 HDR), the MSKCC series over which they saw improved outcomes in high risk patients (EBRT 50.4 Gy + 7.5 Gy x 3 HDR), and the Prada series (EBRT 46 Gy + 11.5 Gy x 2) to a BED assuming an α/β ratio of 1.2 Gy we get 243 Gy, 292 Gy, 289 Gy, and 366 Gy. Converting these BEDs to EQD2s we get 203, 243, 241, and 305 Gy. While there is variation in the total BED; the BED from the HDR component of treatment for the four series are similar at 144 Gy, 169 Gy, and 163 Gy. It is speculative whether this suggests the HDR component of treatment is more critical to treatment outcomes than the EBRT dose. Differences in dosimetry policies between institutions may also affect the actual biologically significant dose delivered that is not manifest in the HDR prescription alone. For example, the dose was prescribed to the prostate plus a several millimeter margin beyond the prostate (except where it was immediately adjacent to the bladder and rectum). Consequently, the 110% isodose line typically encompassed the prostate. The BED dose to the prostate was then EBRT 39.6 + HDR 6.6 Gy x 4 (BED 99 + 172 = 271). Ultimately one explanation for why we did not find a significant relationship in our cohort of 768 patients between developing distant metastasis and BED could be because the total EBRT plus brachytherapy dose, and range of total dose delivered was lower than the thresholds seen for improved outcomes in the WBH series. Use of ADT was also associated with a lower FFDM. Upon further analysis, patients who received ADT had significantly higher risk features such as Gleason score, T stage, and pre-treatment PSA that we believe explains the higher incidence of distant metastases as opposed to a negative interaction between HDR-B and ADT. In addition, the median duration of hormone therapy was 4.5 months which is much shorter than is now commonly recommended for high risk patients, and as a result some men may have been undertreated.
This analysis has shortcomings that include its retrospective design and a short median follow-up. Despite these issues, our overall clinical results are consistent with other published studies reporting outcomes of HDR brachytherapy in combination with external beam radiation therapy [13, 19–23]. This report on a very large group of patients strengthens the evidence regarding the importance of GS in predicting outcomes even after delivering high doses of local radiation.
Conclusions
In men treated with HDR-B and EBRT, GS is a significant factor on multivariate analysis for developing distant metastasis.
References
- 1.Centers for Disease Control and Prevention. Cancer Prevention and Control (2012) Retrieved from http://www.cdc.gov/cancer/dcpc/data/men.htm.
- 2.Forsythe K, Burri R, Stone N, et al. Predictors of metastatic disease after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2012;83:645–652. doi: 10.1016/j.ijrobp.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Stone NN, Potters L, Davis BJ, et al. Multicenter analysis of effect of high biologic effective dose on biochemical failure and survival outcomes in patients with Gleason score 7-10 prostate cancer treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2009;73:341–346. doi: 10.1016/j.ijrobp.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Potters L, Morgenstern C, Calugaru E, et al. 12-Year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2005;173:1562–1566. doi: 10.1097/01.ju.0000154633.73092.8e. [DOI] [PubMed] [Google Scholar]
- 5.Blasko JC, Grimm PD, Sylsvester JE, et al. The role of external beam radiotherapy with I-125/Pd-103 brachytherapy for prostate carcinoma. Radiother Oncol. 2000;57:273–278. doi: 10.1016/s0167-8140(00)00288-7. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch I, Zelefsky MJ, Zhang Z, et al. Comparison of PSA relapse free survival in patients treated with ultra-high-dose IMRT versus combination HDR brachytherapy and IMRT. Brachytherapy. 2010;9:313–318. doi: 10.1016/j.brachy.2010.02.196. [DOI] [PubMed] [Google Scholar]
- 7.Taira AV, Merrick GS, Galbreath RW, et al. Distant metastases following permanent interstitial brachytherapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:225–232. doi: 10.1016/j.ijrobp.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico AV, Moul JW, Carroll PR, et al. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demanes DJ, Rodriguez RR, Altieri GA. High dose rate prostate brachytherapy: the California Endocurietherapy (CET) method. Radiother Oncol. 2000;57:289–296. doi: 10.1016/s0167-8140(00)00291-7. [DOI] [PubMed] [Google Scholar]
- 11.Martinez AA, Gonzalez J, Ye H, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:363–370. doi: 10.1016/j.ijrobp.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Kotecha R, Yamada Y, Pei X, et al. Clinical outcomes of high-dose-rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy. 2013;12:44–49. doi: 10.1016/j.brachy.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Prada PJ, González H, Fernández J, et al. Biochemical outcome after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy: 12 years of experience. BJU Int. 2012;109:1787–1793. doi: 10.1111/j.1464-410X.2011.10632.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoskin PJ, Colombo A, Henry A, et al. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localized prostate cancer: An update. Radiother Oncol. 2013;107:325–332. doi: 10.1016/j.radonc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 17.Zhou P, Chen MH, McLeod D, et al. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–6998. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 18.Skowronek J. Low-dose-rate or high-dose-rate brachytherapy in treatment of prostate cancer – between options. J Contemp Brachytherapy. 2013;5:33–41. doi: 10.5114/jcb.2013.34342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deger S, Boehmer D, Roigas J, et al. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. Eur Urol. 2005;47:441–448. doi: 10.1016/j.eururo.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Demanes DJ, Rodriguez RR, Schour L, et al. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int J Radiat Oncol Biol Phys. 2005;61:1306–1316. doi: 10.1016/j.ijrobp.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Galalae RM, Martinez A, Mate T, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1048–1055. doi: 10.1016/j.ijrobp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Viani GA, Pellizzon AC, Guimarães FS, et al. High dose rate and external beam radiotherapy in locally advanced prostate cancer. Am J Clin Oncol. 2009;32:187–190. doi: 10.1097/COC.0b013e3181841f78. [DOI] [PubMed] [Google Scholar]
- 23.Agoston P, Major T, Fröhlich G, et al. Moderate dose escalation with single-fraction high-dose rate brachytherapy boost for clinically localized intermediate- and high-risk prostate cancer: 5-year outcome of the first 100 consecutively treated patients. Brachytherapy. 2011;10:376–384. doi: 10.1016/j.brachy.2011.01.003. [DOI] [PubMed] [Google Scholar]