Summary
Background
Maternal and neonatal mortality rates remain high in many low-income and middle-income countries. Different approaches for the improvement of birth outcomes have been used in community-based interventions, with heterogeneous effects on survival. We assessed the effects of women’s groups practising participatory learning and action, compared with usual care, on birth outcomes in low-resource settings.
Methods
We did a systematic review and meta-analysis of randomised controlled trials undertaken in Bangladesh, India, Malawi, and Nepal in which the effects of women’s groups practising participatory learning and action were assessed to identify population-level predictors of effect on maternal mortality, neonatal mortality, and stillbirths. We also reviewed the cost-effectiveness of the women’s group intervention and estimated its potential effect at scale in Countdown countries.
Findings
Seven trials (119 428 births) met the inclusion criteria. Meta-analyses of all trials showed that exposure to women’s groups was associated with a 37% reduction in maternal mortality (odds ratio 0·63, 95% CI 0·32–0·94), a 23% reduction in neonatal mortality (0·77, 0·65–0·90), and a 9% non-significant reduction in stillbirths (0·91, 0·79–1·03), with high heterogeneity for maternal (I2=58·8%, p=0·024) and neonatal results (I2=64·7%, p=0·009). In the meta-regression analyses, the proportion of pregnant women in groups was linearly associated with reduction in both maternal and neonatal mortality (p=0·026 and p=0·011, respectively). A subgroup analysis of the four studies in which at least 30% of pregnant women participated in groups showed a 55% reduction in maternal mortality (0·45, 0·17–0·73) and a 33% reduction in neonatal mortality (0·67, 0·59–0·74). The intervention was cost effective by WHO standards and could save an estimated 283 000 newborn infants and 41 100 mothers per year if implemented in rural areas of 74 Countdown countries.
Interpretation
With the participation of at least a third of pregnant women and adequate population coverage, women’s groups practising participatory learning and action are a cost-effective strategy to improve maternal and neonatal survival in low-resource settings.
Funding
Wellcome Trust, Ammalife, and National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care for Birmingham and the Black Country programme.
Introduction
Between 1990 and 2010, substantial improvements were noted in maternal and child survival—maternal mortality decreased by 47% and the mortality in children younger than 5 years fell by 37%.1 However, in 2011, an estimated 273 465 mothers died from complications of pregnancy and childbirth and 2·9 million infants did not survive the first month of life, representing 43% of all deaths in children younger than 5 years.2,3 Achievement of the Millennium Development Goals 4 and 5 requires a doubling of the reduction in maternal mortality ratio and a renewed focus on neonatal survival.2 Community-based interventions are crucial for the attainment of these goals.4
In a systematic review and meta-analysis of community-based intervention studies, reductions were noted in the neonatal mortality (12 studies, risk ratio 0·76, 95% CI 0·68–0·84), but the evidence of reductions in maternal mortality was inconclusive (ten studies, 0·77, 0·59–1·02).5 This and other reviews included different approaches to community interventions,6,7 and the policy implications of their findings are uncertain. One approach involved home visits to counsel mothers, provide newborn care, and facilitate referral.8,9 Another involved home-based counselling combined with community activities to improve newborn care.10,11
A third approach involved women’s groups in a four-phase participatory learning and action cycle. Phase 1 was to identify and prioritise problems during pregnancy, delivery, and post partum; phase 2 was to plan and phase 3 implement locally feasible strategies to address the priority problems; phase 4 was to assess their activities.12–14 Women’s groups aimed to increase appropriate care-seeking (including antenatal care and institutional delivery) and appropriate home prevention and care practices for mothers and newborns. The women’s group approach was inspired by a commitment to the participation of people in health care after Alma Ata. It also drew on Paolo Freire’s work, which provided insights applicable to health: many health problems are rooted in powerlessness, and would be addressed by social and political empowerment; health education is more empowering if it involves dialogue and problem solving, rather than message giving; communities can develop critical consciousness to recognise and address the underlying social and political determinants of health.15,16 For example, where gender inequity constrains improvements in maternal survival, empowered groups could give women the understanding, confidence, and support to choose a healthy diet in pregnancy, and seek care or advice outside of their homes.
The effects of the different approaches for the improvement of birth outcomes need to be reviewed and population-level predictors of the effects need to be identified to guide policy and practice. We therefore did a systematic review of randomised controlled trials to assess the effect of women’s groups practising participatory learning and action. Our objectives were to ascertain the effects of these groups, compared with usual care, on maternal mortality, neonatal mortality, and stillbirths in low-resource settings. We did a meta-analysis of the data retrieved in the systematic review, investigated potential population-level predictors of effect, assessed cost-effectiveness, and estimated how many lives could be saved if the approach was scaled up in the Countdown countries.
Methods
Systematic review
AW and CMa searched databases for literature about interventions with participatory women’s groups in low-income and middle-income countries: PubMed, Embase, Cochrane library, CINAHL, African Index Medicus, Web of Science, the Reproductive Health Library, and the Science Citation Index, using the inception date for each database and Oct 13, 2012, as inclusion dates. Search terms were a combination of “community mobilisation”, “community participation”, “participatory action”, “participatory learning and action*”, “women* group*”, and “women” (appendix p 1). No language restrictions were applied. AW and CMa also sought unpublished data from researchers who were known to be active in this specialty.
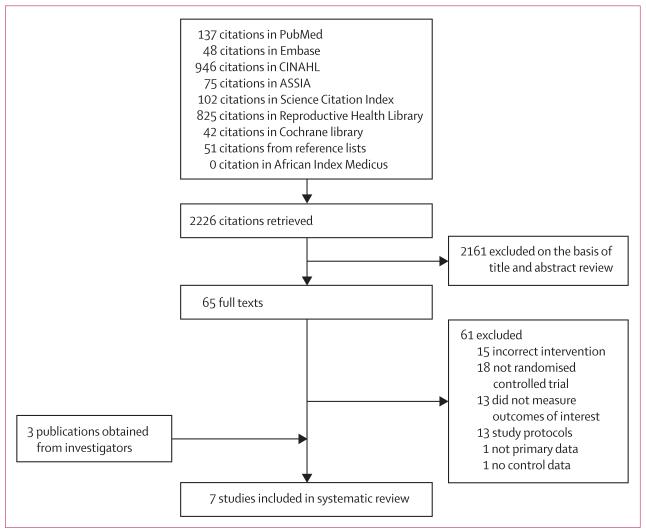
Figure 1 summarises the study selection process. AW and CMa reviewed the results of the electronic searches and acquired electronic reports of published studies, and manuscripts of unpublished studies from the respective investigators. AW and CMa made the final decisions about the inclusion or exclusion of reports or manuscripts separately after inspection, and then independently extracted data for the characteristics, quality, and outcomes of each study. Together, the reviewers checked and verified these data. Investigators for the primary studies were contacted for clarification if there were discrepancies in the extracted data.
Figure 1. Flow chart of the study selection process.
The four criteria for inclusion of the studies in the systematic review were that they were randomised controlled trials; the intervention contained the stages of a participatory learning and action cycle; most of the participants were women of reproductive age (15–49 years); and the study outcomes included maternal mortality, neonatal mortality, and stillbirths. AW and CMa independently assessed the studies for quality using the CONSORT statement extension for cluster-randomised controlled trials,17 and risk of bias using the Cochrane Collaboration’s tool.18 The review protocol was not registered in any database.
Meta-analysis
NS and AP extracted study-specific odds or risk ratios for each outcome, using the main estimates reported in each study. These ratios accounted for clustering, stratification, and, where appropriate, adjustments for other covariates. We did not undertake data analysis of individual participants because of differences in methods to adjust for clustering and in the range of variables that were adjusted for in each study. When a required outcome was not reported in a study, we used methods identical to those reported in the original study to calculate an effect size from the trial datasets. We did a meta-analysis of the study-level data with the metan command in Stata (version 12.1) using random-effects models because we assumed that the effects seen in each trial were taken from an underlying distribution.
We planned a-priori meta-analyses to ascertain the effect of women’s groups on maternal mortality, neonatal mortality, and stillbirths with all identified trials, followed by subgroup analyses to identify population-level predictors of effect. We postulated that these might include the population coverage of women’s groups, proportion of pregnant women participating, and background mortality and institutional delivery rates as measured in the control areas during the trials. In previous studies, the hypothesis was that having one women’s group per 450–750 population and between 30% and 50% of pregnant women attending groups would be key determinants of effect.14,19 We used meta-regression analysis20 to assess whether each of the predictors was associated with intervention effects. When there was evidence of statistical heterogeneity (I2>50%, p<0·05), we separated the trials into groups according to the results of the meta-regression analyses. We assessed potential publication bias and small-study effects using funnel plots and Egger tests.21
Cost-effectiveness analysis
To compare the cost-effectiveness of the interventions, we used incremental cost-effectiveness ratios for trials in which significant effects on neonatal mortality rate were reported. We independently assessed the quality of the studies using guidelines adapted from Drummond and Jefferson.22 In each trial, the economic costs of setting up and running the women’s group intervention were gathered from the provider’s perspective, using project accounts as the main data source. Costs linked to health-service strengthening, monitoring, and evaluation were excluded. Capital costs were annualised over the expected lifetime of the item and women’s groups were allocated a share of any costs incurred jointly with other activities or programmes. We converted the reported US$ back into the local currency using the average exchange rate for that year, and used the local consumer price index to account for inflation in the interim period and calculate the value of the cost in 2011. Local currencies were then converted to international dollars using purchasing power parity conversion factors for 2011, creating ratios comparable across trials at a common timepoint. Cost-effectiveness is expressed as the incremental cost per neonatal death averted and life-year saved. Consistent with WHO-recommended methods, we classified each intervention as highly cost effective if it averted a year of life lost for less than the national gross domestic product (GDP) per person, cost effective if one-to-three times GDP per person, and not cost effective if greater than three times the GDP per person.23
Effect in Countdown countries
We estimated the effect of implementation of the intervention in rural areas of all Countdown countries.1 Mortality rates for deliveries with and without skilled birth attendance (SBA) are very different, and many Countdown countries have higher rates of deliveries with SBA than do the study areas in the trials, so we could not ignore the difference between deliveries with and without SBA. Although the inter vention could reduce the mortality by increasing SBA deliveries or improving their outcomes, its largest effect seems to be on deliveries without SBA. Thus, in estimating the effect, we applied an overall risk ratio derived from the meta-analysis for rural trials in which a third or more of pregnant women participated in groups only to deaths in rural deliveries without SBA. We believe this method provides a conservative estimate of the effect that captures most of the intervention benefit.
We generated two estimates of effect: one in which we assumed that the intervention would have the same effect at scale as that from the meta-analysis of rural trials in which 30% or more of the pregnant women participated in groups, and another in which we assumed a 30% loss of effectiveness for implementation at scale. This estimate was intended to provide a conservative lower bound for effect (appendix pp 2–4 provides a detailed description of assumptions and methods).
Role of the funding source
The funders had no role in the design of the study, data gathering, analysis, interpretation, or writing up of the report. The corresponding author had access to all the data and had final responsibility for the decision to submit for publication.
Results
We found and analysed seven cluster, randomised controlled trials with a total of 119 428 births.12–14,24–27 Table 1 summarises the characteristics of these trials. The studies were done between 1999 and 2011 in four countries: Bangladesh, India, Malawi, and Nepal.
Table 1. Characteristics of cluster-randomised controlled trials included in the systematic review and meta-analysis.
| Study population and setting | Intervention | Control | Outcomes | |
|---|---|---|---|---|
| Manandhar et al,12 2004 (Nepal) |
24 clusters; population of about 7000 per cluster Closed cohort of married women of reproductive age (15–49 years) living in Makwanpur district, rural Nepal; pregnancies registered during Nov 1, 2001, to Oct 31, 2003, were followed up |
12 clusters (2972 births) Each cluster had a local literate female facilitator who was given a brief training in perinatal health issues and a facilitation manual; facilitators supported women’s groups through ten monthly meetings using a participatory learning and action cycle and a picture card game that addressed prevention and treatment for typical problems in mothers and infants; one supervisor supported three facilitators Health service strengthening and training of traditional birth attendants were as in the control group |
12 clusters (3303 births) Health service strengthening activities and training of traditional birth attendants: primary health centres given resuscitation equipment, phototherapy units, and warm cots; essential newborn-care training for local health staff and traditional birth attendants; and newborn-care kits given to community-based workers |
Primary: neonatal mortality rate Secondary: stillbirth rate, maternal mortality ratio, uptake of maternity services, care practices at home, neonatal morbidity, and health-care seeking |
| Tripathy et al,13 2010 (India) |
36 clusters; mean population 6338 per cluster (SD 2101) Open cohort of women aged 15–49 years, living in rural areas of three districts of Jharkhand and Orissa, eastern India, who gave birth between July 31, 2005, and July 30, 2008 |
18 clusters (9770 births) A local woman facilitated 20 monthly meetings with women’s groups after 7 days of training; each facilitator convened 13 groups per month; groups followed a four-phase participatory learning and action cycle and were open to all members of the community though primarily targeting pregnant women and new mothers Facilitators and group members used stories, participatory games, and picture cards to facilitate discussions about prevention and care-seeking Health service strengthening was as in the control group |
18 clusters (9260 births) Health service strengthening activities: health committees formed so community members could express opinions about local health services; committees met every 2 months to discuss maternal and newborn health entitlement issues; and workshops using appreciative inquiry provided to frontline government health staff |
Primary: neonatal mortality rate and maternal depression scores Secondary: stillbirths, maternal mortality ratio, and perinatal mortality, uptake of maternity services, care practices at home, and health-care seeking |
| Azad et al,14 2010 (Bangladesh) |
18 clusters; mean population 27 953 per cluster (SD 5953) Open cohort of women aged 15–49 years living in three rural districts of Bangladesh, who gave birth between Feb 1, 2005, and Dec 31, 2007 |
Nine clusters (15 695 births) A local woman facilitated groups using a participatory learning and action cycle after receiving five training sessions that covered communication, maternal and neonatal health issues; she visited every tenth household in the intervention clusters and invited married women of reproductive age to join the groups; mothers-in-law, adolescent girls, and other women joined at a later date Health service strengthening and training of traditional birth attendants were as in the control group |
Nine clusters (15 257 births) Health service strengthening activities and training of traditional birth attendants: improvements to referral systems and links between communities and health services; and provision of basic and refresher training in essential maternal and newborn care |
Primary: neonatal mortality rate Secondary: maternal mortality ratio, stillbirths, perinatal mortality rate, uptake of maternity services, care practices at home, neonatal morbidity, and health-care seeking |
| More et al,24 2012 (India) |
48 clusters; mean population 5865 per cluster (SD 1077) Women were recruited between Oct 1, 2006, and Sept 30, 2009, in urban Mumbai slums; women from transient communities and areas for which resettlement was being negotiated were excluded |
24 clusters (9155 births) A facilitator (local woman with secondary education and leadership skills) set up ten groups in a cluster of 1000 households; groups met fortnightly, and the facilitator met weekly with other facilitators and her supervisor; women’s groups followed a cycle of 36 meetings and were open to all women. Participatory methods with seven phases, based on the principles of appreciative inquiry, were used in the meetings |
24 clusters (9042 births); no details were provided about control clusters |
Primary: stillbirths, neonatal mortality rate and extended perinatal mortality rate, perinatal care, and maternal morbidity Secondary: maternal mortality ratio, antenatal care, institutional delivery, breastfeeding, and care- seeking for newborn illness |
| Lewycka et al,25 2013 (Malawi)* |
48 clusters; mean population 3958 per cluster (SD 404) A cohort of women aged 10–49 years in Mchinji district, rural Malawi, who delivered a child between Feb 1, 2006, and Jan 31, 2009 |
24 clusters and 9374 births in factorial analysis, 12 clusters and 3129 in stratified analysis for women’s groups Women’s groups were supported by a female facilitator through a participatory learning and action cycle of 20 meetings Facilitators were local, literate women aged 20–49 years; they were trained for 11 days, with refresher training every 4 months, and supported by one supervisor per six facilitators Meetings followed a four-phase participatory learning and action cycle; group membership was restricted to women, but expanded to men in later stages Health service strengthening was as in the control group |
24 clusters and 9749 births in overall analysis; 12 clusters and 3329 births in stratified analysis for women’s groups Health service strengthening activities: health workers received training in essential newborn care and safe motherhood; neonatal resuscitation equipment donated to all facilities; a project for prevention of mother-to-child transmission of HIV introduced in 2005 was scaled up to all facilities by 2008 |
Primary: neonatal, perinatal, and infant mortality rates, and maternal mortality ratio Secondary: maternal and infant morbidity, use of skilled maternity services, immunisation, malaria prophylaxis, use of prevention of mother-to-child transmission services, and breastfeeding |
| Colbourn et al,26 2013 (Malawi)† |
32 clusters; mean population of 3934 per cluster (SD 1332) An open cohort of pregnant women was recruited from three rural districts of Malawi between Oct 1, 2008, and Dec 31, 2010; women were excluded if they were living in urban areas, or areas with facilities providing comprehensive emergency obstetric care or non-functioning facilities |
15 clusters (10329 births); 81 volunteer facilitators supported by nine MaiKhanda study staff, each formed a women’s group that followed a participatory learning and action cycle to improve maternal and neonatal health |
17 clusters (10 247 births): no details reported |
Primary: maternal mortality ratio, and perinatal, and neonatal mortality rates Secondary: institutional delivery, percentage of maternal deaths subjected to audit, case fatality rates, practice of signal obstetric- care functions |
| Fottrell et al,27 2013 (Bangladesh) |
Clusters were the same as in Azad et al14 An open cohort of women residing in three rural districts of Bangladesh, who were permanent residents of the union in which their delivery was identified from January, 2009, to June, 2011; temporary residents were excluded |
Nine cluster (9106 births) In addition to the 162 women’s groups already set up previously (Azad et al14), 648 new groups were formed by newly recruited facilitators to increase population coverage; from January, 2009, the new groups followed a participatory learning and action cycle with monthly meetings about maternal and newborn health Health service strengthening was as in the control group |
Nine clusters (8834 births) Health service strengthening: provision of basic medical equipment to local facilities; training of traditional birth attendants in essential newborn care; and refresher training in essential newborn care for physicians |
Primary: neonatal mortality rate Secondary: stillbirth, perinatal mortality rate, pregnancy- related mortality, institutional delivery, home-care practices, and health-care seeking |
2×2 factorial, cluster-randomised controlled trial of volunteer peer counselling support for breastfeeding and infant care.
2×2 factorial, cluster-randomised controlled trial of quality improvement of health facilities.
In all trials, variants of a participatory learning and action cycle were tested. Women’s group facilitators, all local women who were not health workers, coordinated between nine and 13 group meetings per month after receiving 7–11 days of basic training in maternal and newborn health and participatory facilitation techniques. In six of seven studies, women’s groups had monthly meetings; in the urban trial,24 groups met fortnightly. In all trials, both intervention and control clusters had context-specific health services strengthening (table 1).
Quality assessment and risk of bias appraisals for the seven trials included in the systematic review are described in appendix pp 5–8. The studies were of good quality and had low risk of bias, according to the standards of the CONSORT statement17 and Cochrane Collaboration’s tool18 for assessing risk of bias in randomised trials, for all items except masking of participants, personnel, and outcome assessment. These shortcomings were due to the nature of the intervention and study designs. In all trials, analyses were by intention to treat—ie, data from all women who had recently delivered in a study cluster, whether they participated in a group or not, were included. According to the CONSORT statement, all trials had appropriate randomisation, accounted for the effect of clustering, and had no loss of clusters at follow-up. The panel shows the outcome definitions used, which were the same in all studies included in the systematic review and meta-analysis.
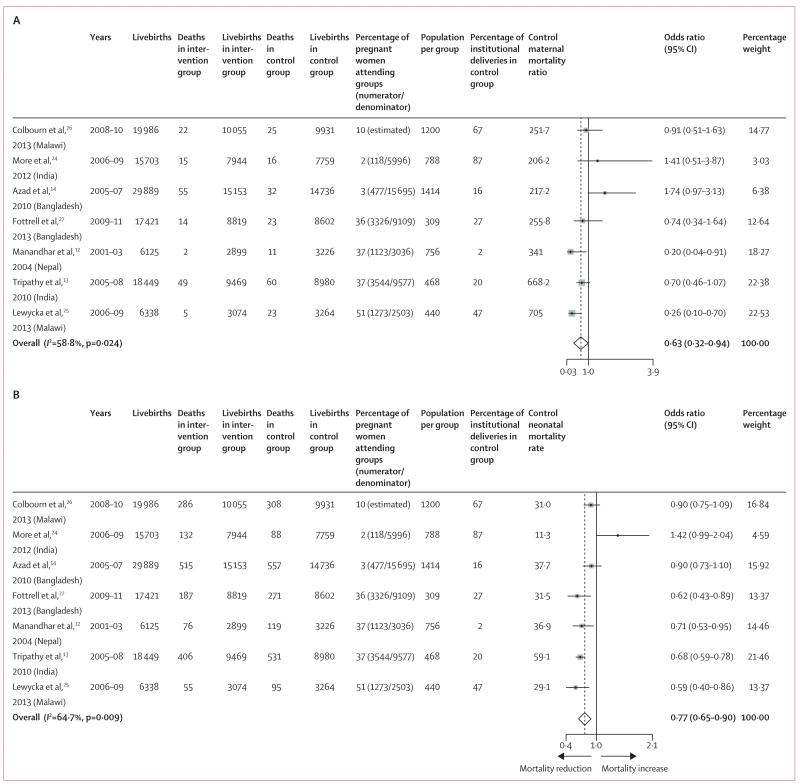
Figure 2 shows the forest plots for meta-analyses of the effects of women’s groups on maternal and neonatal mortality in the seven trials. Exposure to women’s groups was associated with a 37% reduction in maternal mortality (figure 2A) and a 23% reduction in neonatal mortality (figure 2B), but with high statistical heterogeneity (figure 2A, B). This heterogeneity warranted further exploration through meta-regression and subgroup analyses. There was no evidence of reduction in stillbirths (odds ratio 0·91, 95% CI 0·79–1·03, I2=37·7%, p=0·141; appendix p 11). Appendix pp 12–14 show the effects on perinatal mortality, and early and late neonatal mortality rates. Funnel plots for all outcomes were broadly symmetric (appendix pp 14–15). Results of Egger tests suggested no evidence of publication or small-study bias for neonatal mortality (p=0·040), but there was some evidence of maternal mortality (p=0·059).
Figure 2. Meta-analysis of the effect of women’s groups practising participatory learning and action on maternal mortality (A) and neonatal mortality (B).
Weights are from random-effects analysis.
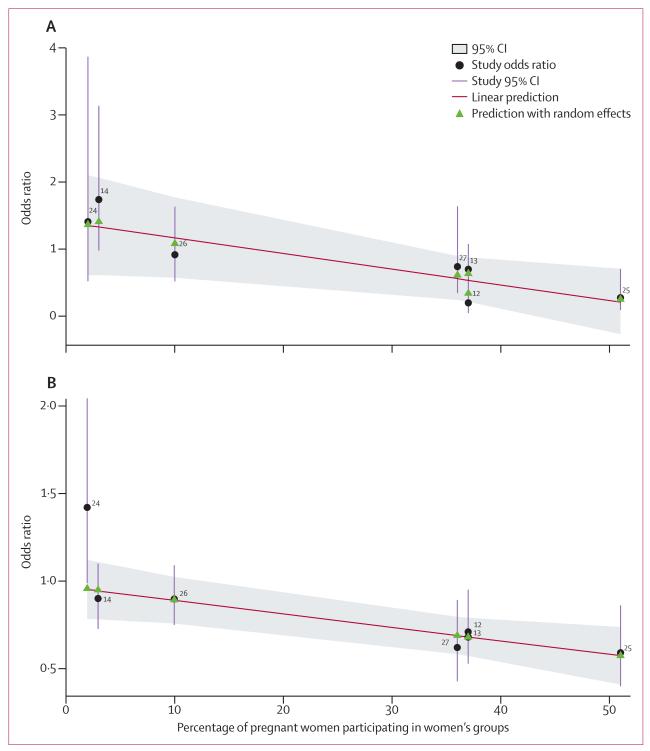
In all but one study,26 the coverage of pregnant women in groups was calculated as the proportion of women who had delivered between 28 days and 8 weeks before the interview and reported ever attending a women’s group, irrespective of the number of meetings attended. Results of meta-regression analyses indicated that the proportion of pregnant women participating in groups was linearly associated with reduction of both maternal and neonatal mortality (odds ratio −0·023, 95% CI −0·043 to −0·004, p=0·026; −0·008, –0·013 to −0·003, p=0·011, respectively; figure 3). We found no evidence of associations between intervention effects and background mortality or institutional delivery rates (appendix p 17), but did find evidence of an association between the size of the population covered by a women’s group and neonatal mortality (0·0003, <0·0001 to 0·0005, p=0·042).
Figure 3. Meta-regression analysis of the effect of women’s groups by percentage of pregnant women participating in groups for (A) maternal mortality and (B) neonatal mortality.
Green triangles show the predicted effect with random-effects meta-regression to allow for between study variation.
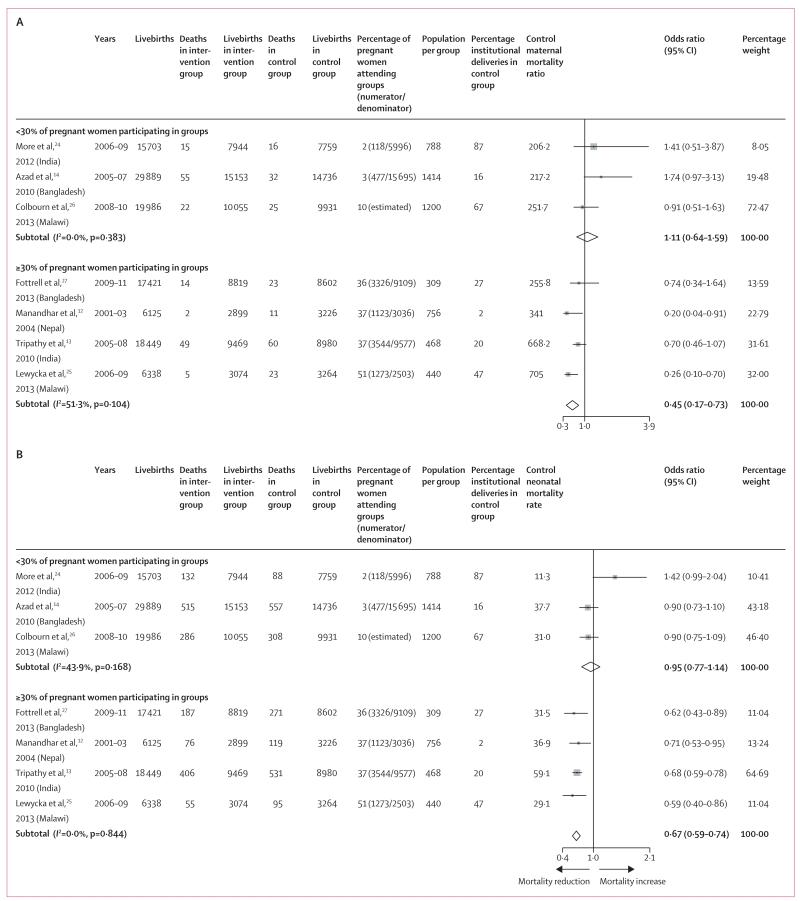
Since the proportion of pregnant women participating in groups was a key predictor of mortality reduction, for our subgroup analyses we separated the trials into categories of high (≥30% of pregnant women participating in women’s groups) and low coverage (<30% participating). Figure 4 shows that in high-coverage studies (48 333 livebirths), exposure to women’s groups was associated with a 55% reduction in maternal mortality (figure 4A) and a 33% reduction in neonatal mortality (figure 4B). No effects were noted in the low coverage studies for any of the birth outcomes.
Figure 4. Subgroup analysis of the effect of women’s groups on maternal mortality (A) and neonatal mortality (B), by percentage of pregnant women participating in groups.
Weights are from random-effects analysis.
Table 2 shows the behavioural mechanisms, based on reported data, through which the interventions might have affected birth outcomes. In three12,13,27 of four14 south Asian trials in which the behavioural mechanisms were reported, women’s groups showed strong (including significant and non-significant) effects on clean delivery practices for home deliveries (especially handwashing and use of clean delivery kits), and noticeable effects on breastfeeding (table 2). Use of women’s groups resulted in significant increases in the uptake of any antenatal care in two studies,12,25 and institutional deliveries in one study (table 2).12 The largest behavioural effects on mortality that were seen in the south Asian studies are likely to have been determined by changes in clean delivery practices for home deliveries and improved immediate postnatal care at home.
Table 2. Behavioural mechanisms for the effect of the interventions on pregnancy and birth outcomes.
| Manandhar et al,12 2004 (Nepal) |
Tripathy et al,13 2010 (India) |
Azad et al,14 2010 (Bangladesh) |
More et al,24 2012 (India) |
Lewycka et al,25 2013 (Malawi) |
Colbourn et al,26 2013 (Malawi) |
Fottrell et al,27 2013 (Bangladesh) |
|
|---|---|---|---|---|---|---|---|
| Increased uptake of any antenatal care | * | † | † | † | * | Not reported | †,‡ |
| Increased care-seeking for a problem in pregnancy | * | † | Not reported | †,§ | Not reported | Not reported | Not reported |
| Increased institutional deliveries | * | † | † | † | † | † | † |
| Increased handwashing by attendant before home deliveries | * | * | † | Not reported | † | Not reported | * |
| Increased use of clean delivery kits for home births | * | * | † | Not reported | Not reported | Not reported | * |
| Increased appropriate cord care for home births (nothing or use of antiseptic) | * | † | † | Not reported | Not reported | Not reported | * |
| Increased appropriate thermal care for the newborn infant | † | † | † | Not reported | † | Not reported | 1 of 3¶ |
| Increased care-seeking for the newborn in case of a health problem | * | † | † | †,∥ | Not reported | Not reported | †,** |
| Early initiation of breastfeeding (within 1 h of birth) | † | † | Not reported | † | † | Not reported | * |
| Exclusive breastfeeding for the first 6 weeks of life | Not reported | * | † | †,†† | *,‡‡ | Not reported | * |
| Increased care-seeking for the mother in case of a post-partum problem | Not reported | † | Not reported | *,§§ | Not reported | Not reported | †,¶¶ |
Not reported indicates that the secondary outcome was not measured or not reported by the investigators.
Significant difference (p<0·05) between intervention and control groups in published data; we used analyses adjusted for clustering and variables that differed at baseline.
Secondary outcome was measured but not significant.
Four or more antenatal-care visits.
Sentinel antepartum symptom (leaking of waters, vaginal bleeding, baby not moving, convulsion, or loss of consciousness).
Infant not bathed in the first 24 h of life.
Sought clinical care for specified newborn illness within 24 h.
In Fottrell and colleagues,27 this is infant check-up within 6 weeks, so not necessarily in the event of a problem.
Exclusively breastfed for at least 28 days.
First 6 months.
Postpartum check.
In Fottrell and colleagues,27 this is postpartum check-up within 6 weeks so not necessarily in the event of a problem.
Each study had a process evaluation for the interventions, evidence from which enabled us to develop a working hypothesis about the way in which the women’s groups bring about improvements in birth outcomes (appendix p 18): the intervention builds the capacities of communities to organise and mobilise to take individual, group, and community action to address the structural and intermediary determinants of health.29–31
Although the incremental cost per neonatal death averted differed widely between trials (table 3), according to WHO-recommended standards, women’s groups practising participatory learning and action were a highly cost-effective intervention in these trials. Quality assessment for the four trials in table 3 is described in appendix p 19.
Table 3. Cost-effectiveness ratios for the participatory women’s group intervention (in 2011 international dollars).
| Cost of women’s group intervention per neonatal death averted |
Cost of women’s group intervention per neonatal year of life lost averted* |
Gross domestic product per person (2011) |
|
|---|---|---|---|
| Manandhar et al,12 2004 (Nepal)* |
22 961 | 753 | 1252 |
| Tripathy et al,13 2010 (India) | 2770 | 91 | 3629 |
| Lewycka et al,25 2013 (Malawi) |
17 604 | 577 | 893 |
| Fottrell et al,27 2013 (Bangladesh) |
19 810 | 650 | 1777 |
To standardise calculations of the years of life lost averted, we recalculated cost per year of life lost averted for Manandhar12 and Tripathy13 and their colleagues’ studies by dividing the reported cost per death averted by 30·5—ie, years of life lost associated with an infant death assuming standard life expectancy and no age weights. Lewycka25 and Fottrell27 and their colleagues included all start-up costs, whereas Manandhar12 and Tripathy13 and their colleagues included a proportion of the start-up cost because it was annualised over 10 years.
Reported in Borghi and colleagues.32
We applied the meta-analysis results from rural, high-coverage studies to deliveries in rural areas and without SBA in 74 of 75 Countdown countries. We estimate that the intervention could prevent the deaths of up to 58 800 mothers and 404 000 newborn infants per year if the effect was the same as in the high-coverage trials, and 41 100 mothers and 283 000 newborn infants per year with a 30% loss of efficacy through scale-up. These numbers correspond to upper and lower estimates of 13% and 9% for neonatal deaths and 21% and 15% for maternal deaths for delivery types and rural and urban regions. Appendix pp 20–21 shows the seven countries where the most maternal and newborn deaths would be saved, and those in which the most lives could be saved as a proportion of total deaths for each country. A scale-up of women’s groups with adequate coverage in rural areas of two countries (India and Bangladesh) where they have already been tested and implementation guides exist33 could prevent the deaths of about 130 000 newborn infants and 11 400 mothers, taking into account a 30% loss in effect through scale-up. Appendix pp 22–23 shows the estimated effect for each of the 74 Countdown countries.
Discussion
Women’s groups practising participatory learning and action led to substantial reductions in neonatal and maternal mortalities in rural, low-resource settings. The proportion of pregnant women participating in groups and the population coverage of groups were key predictors of the effect. We included stillbirths as an outcome because we anticipated that an intervention that increased care-seeking and self-care for women during pregnancy might have an effect on stillbirths.
Our analysis has four important limitations. First, the systematic review and meta-analysis included only seven trials, thereby restricting our analyses of potential sources of heterogeneity and bias. More studies would have increased the accuracy of assessments of bias and enabled multivariate meta-regression analyses and analyses of non-linear associations. Second, the complex nature of the intervention means that the attribution of mortality reductions to discrete mechanisms is not straightforward. Many of the factors that might have been linked to reductions in maternal deaths—eg, increased awareness of danger signs and increased individual and community responsiveness to them—were not measured in impact evaluations. Contextual and implementation factors are likely to have altered the effect sizes, and need further cross-site analysis. Third, we were unable to undertake meta-regression analysis of individual participants because the trials adjusted for different sets of covariates and used a mix of individual-level and cluster-level analyses to address clustering. Individual patient data analysis would have allowed us to investigate sources of heterogeneity in more depth. Nevertheless, we think that our hypothesis linking pregnant women and population coverage to the effect of the intervention is both operationally plausible and supported by our meta-regression analyses. Last, the comparative cost-effectiveness analysis presented here constitutes only a starting point. Comparison of the determinants of differences in costs, or the effect of scale on cost, was not possible but they are a priority for future work.
The effect on neonatal mortality in the four high-coverage studies was greater than the overall pooled effect for all community trials analysed in a recent Cochrane review (odds ratio 0·76, 95% CI 0·68–0·84).5 This result is not unexpected because the interventions aggregated were very different (training of birth attendants, health education, and home visits) and the studies had high heterogeneity (I2=69%, p=0·0001). The effect on neonatal mortality is inferior to that in the most intensive home-based newborn-care programme,8 but similar to effect sizes in the less intensive home visits trials.9 When extrapolated to rural areas of Countdown countries, the overall effect of the women’s group intervention compares well with others. For example, according to the results of a 2011 study, broad coverage of basic and comprehensive emergency obstetric care could prevent an estimated 591 000 neonatal deaths per year.34 By comparison, we estimate that the women’s group intervention could save 283 000 lives (assuming no effect on deliveries attended by SBA) and might be easier to implement where health services are weak. A reasonable assumption is that where SBA delivery rates in rural regions are high, the participatory learning and action cycle could have an effect on birth outcomes following SBA deliveries too. In this case, the effect for such countries could be higher than the estimate in appendix pp 22–23.
The results of our study raise three important issues. First, is the potential of community-based, participatory interventions to reduce maternal mortality. The only intervention found to affect maternal mortality so far has been training of birth attendants with antenatal and intrapartum home visits (relative risk 0·70, 95% CI 0·51–0·96).5 For women’s groups, we hypothesise that reduction of maternal mortality might be driven by reduced infection through improved uptake of antenatal care and hygiene during delivery, and small changes in the rapidity of response and care-seeking that make the difference for survival. This last hypothesis is supported by data for the process evaluation that showed that groups discussed danger signs, raised community-wide support for maternal health, organised transport for pregnant women, and contributed to emergency funds for transport and health-care costs.35–37 However, the reduction seen in the high-coverage studies is large and included two trials that had populations of less than 200 000.12,25 Therefore, even with adequate coverage of pregnant women, it is plausible that effects at scale would be smaller than those in the subgroup analysis for high-coverage interventions.
Second, the results of the analysis raise the question of whether participatory learning and action have a role in maternal and newborn health in urban contexts. Rates of antenatal care and institutional delivery tend to be higher in cities, delays in care-seeking shorter, and mortality rates lower, making them potentially less amenable to non-clinical interventions. There is an argument for focusing on improved links between communities and facilities, and on the quality of clinical care.24 Collective action could be instrumental in achieving these objectives, but might require moving beyond women’s groups as the main agents of change if urban women are more isolated and reluctant to commit to group action.
Last, we should consider how community strategies that were shown to be effective in small-to-mediumsized trials, including home visits and collective action through women’s groups, could be combined at scale. Using participatory women’s groups as a community engagement strategy for maternal and newborn health alongside other evidence-based strategies, including home visits, could alter both the demand and supply side of health care. An intervention from Pakistan combined meetings with women’s groups and home visits led to a large improvement in newborn survival within existing health system structures.11 Can models now be taken to scale and fully integrated within health systems?
With the participation of at least a third of pregnant women and population coverage of 450–750 per group, women’s groups practising participatory learning action are a cost-effective strategy to improve maternal and neonatal survival in resource-poor settings. Their implementation in rural areas of Countdown countries could save many lives. In these settings, policy makers should consider women’s groups as a core strategy complement efforts made to improve safer motherhood and newborn care through better midwifery obstetric care.
Supplementary Material
Panel: Definitions28.
Miscarriage: cessation of a presumptive pregnancy before delivery of the baby’s head at less than 22 weeks of gestation.
Neonatal death: death of a liveborn infant within 28 completed days of birth.
Early neonatal death: deaths arising within 6 completed days of birth.
Late neonatal death: deaths arising from 7 to 28 completed days of birth.
Stillbirth: the International Classification of Diseases and Related Health Problems, 10th revision, defines fetal death as “death prior to the complete expulsion or extraction from its mother of a product of conception, irrespective of the duration of pregnancy”. In all studies included in the systematic review, stillbirths were classified on the basis of verbal autopsies in which no sign of breathing, heartbeat, or any other evidence of life was reported at birth.
Perinatal death: a stillbirth or early neonatal death.
Maternal death: death of a woman while pregnant or within 42 days of cessation of pregnancy from any cause related to the pregnancy or its management, but not from accidental causes.
Acknowledgments
The study was funded by a Wellcome Trust Strategic Award (number 085417MA/Z/08/Z). AW’s PhD, to which this work is related, is funded by Ammalife (UK registered charity number 1120236), and CMa is part funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care for Birmingham and the Black Country programme. We thank Neha Batura and Hassan Haghparast-Bidgoli for their assistance in preparing the cost-effectiveness analysis.
Footnotes
Conflicts of interest With the exception of ACop, ACoo, AW, and CMa, all authors have been involved in some of the studies included in the review.
See Online for appendix
Contributor Information
Audrey Prost, Institute for Global Health, University College London, London, UK.
Tim Colbourn, Institute for Global Health, University College London, London, UK.
Nadine Seward, Institute for Global Health, University College London, London, UK.
Prof Kishwar Azad, Perinatal Care Project, Diabetic Association of Bangladesh, Dhaka, Bangladesh.
Prof Arri Coomarasamy, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK.
Andrew Copas, Centre for Sexual Health and HIV Research, University College London, Mortimer Market Centre, London, UK.
Tanja A J Houweling, Institute for Global Health, University College London, London, UK; Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Edward Fottrell, Institute for Global Health, University College London, London, UK.
Abdul Kuddus, Perinatal Care Project, Diabetic Association of Bangladesh, Dhaka, Bangladesh.
Sonia Lewycka, Institute for Global Health, University College London, London, UK; MaiMwana Project, Mchinji, Malawi.
Prof Christine MacArthur, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK.
Prof Dharma Manandhar, Mother Infant Research Activities, Kathmandu, Nepal.
Joanna Morrison, Institute for Global Health, University College London, London, UK; Mother Infant Research Activities, Kathmandu, Nepal.
Charles Mwansambo, Government of Malawi, Ministry of Health, Lilongwe, Malawi.
Nirmala Nair, Ekjut, Jharkhand, India.
Bejoy Nambiar, Institute for Global Health, University College London, London, UK; Parent and Child Health Initiative, Amina House, Lilongwe, Malawi.
David Osrin, Institute for Global Health, University College London, London, UK; Society for Nutrition, Education and Health Action, Urban Health Centre, Chota Sion Hospital, Maharashtra, India.
Christina Pagel, Clinical Operational Research Unit, Department of Mathematics, London, University College London, UK.
Tambosi Phiri, MaiMwana Project, Mchinji, Malawi.
Anni-Maria Pulkki-Brännström, Institute for Global Health, University College London, London, UK.
Mikey Rosato, Institute for Global Health, University College London, London, UK; MaiMwana Project, Mchinji, Malawi.
Jolene Skordis-Worrall, Institute for Global Health, University College London, London, UK.
Naomi Saville, Institute for Global Health, University College London, London, UK; Mother Infant Research Activities, Kathmandu, Nepal.
Neena Shah More, Society for Nutrition, Education and Health Action, Urban Health Centre, Chota Sion Hospital, Maharashtra, India.
Bhim Shrestha, Mother Infant Research Activities, Kathmandu, Nepal.
Prasanta Tripathy, Ekjut, Jharkhand, India.
Amie Wilson, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK.
Prof Anthony Costello, Institute for Global Health, University College London, London, UK.
References
- 1.WHO. UNICEF [accessed Feb 8, 2013];Countdown 2015: building a future for women and children, the 2012 report. http://countdown2015mnch.org/documents/2012Report/2012-Complete.pdf.
- 2.Lozano R, Wang H, Foreman KJ, et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet. 2011;378:1139–65. doi: 10.1016/S0140-6736(11)61337-8. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF . Levels and trends in child mortality: estimates developed by the UN Inter-agency Group for Child Mortality Estimation. UNICEF; New York: [accessed Feb 8, 2013]. 2012. http://www.childinfo.org/files/Child_Mortality_Report_2012.pdf. [Google Scholar]
- 4.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L, for the Lancet Neonatal Survival Steering Team Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 5.Lassi ZS, Haider BA, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database Syst Rev. 2010;11:CD007754. doi: 10.1002/14651858.CD007754.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Gogia S, Ramji S, Gupta P, et al. Community based newborn care: a systematic review and meta-analysis of evidence. Indian Pediatrics. 2011;48:537–46. doi: 10.1007/s13312-011-0096-8. [DOI] [PubMed] [Google Scholar]
- 7.Costello A, Tripathy P. Community based newborn care. Indian Pediatrics. 2012;49:73. [PubMed] [Google Scholar]
- 8.Bang AT, Reddy HM, Deshmukh MD, Baitule SB, Bang RA. Neonatal and infant mortality in the ten years (1993 to 2003) of the Gadchiroli field trial: effect of home-based neonatal care. J Perinatol. 2005;25:S92–S107. doi: 10.1038/sj.jp.7211277. [DOI] [PubMed] [Google Scholar]
- 9.Baqui AH, Arifeen SE, Darmstadt GL, et al. for the Projahnmo Study Group Effect of community based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster randomised controlled trial. Lancet. 2008;371:1936–44. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Mohanty S, Kumar A, et al. for the Saksham Study Group Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomised controlled trial. Lancet. 2008;372:1151–62. doi: 10.1016/S0140-6736(08)61483-X. [DOI] [PubMed] [Google Scholar]
- 11.Bhutta ZA, Soofi S, Cousens S, et al. Improvement of perinatal and newborn care in rural Pakistan through community-based strategies: a cluster-randomised effectiveness trial. Lancet. 2011;377:403–12. doi: 10.1016/S0140-6736(10)62274-X. [DOI] [PubMed] [Google Scholar]
- 12.Manandhar DS, Osrin D, Shrestha BP, et al. members of the MIRA Makwanpur trial team Effect of a participatory intervention with women’s groups on birth outcomes in Nepal: cluster randomised controlled trial. Lancet. 2004;364:970–79. doi: 10.1016/S0140-6736(04)17021-9. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy P, Nair N, Barnett S, et al. Effect of a participatory intervention with women’s groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet. 2010;375:1182–92. doi: 10.1016/S0140-6736(09)62042-0. [DOI] [PubMed] [Google Scholar]
- 14.Azad K, Barnett S, Banerjee B, et al. Effect of scaling up women’s groups on birth outcomes in three rural districts in Bangladesh: a cluster-randomised controlled trial. Lancet. 2010;375:1193–202. doi: 10.1016/S0140-6736(10)60142-0. [DOI] [PubMed] [Google Scholar]
- 15.Rosato M, Laverack G, Howard Grabman L, et al. Community participation: lessons for maternal, newborn, and child health. Lancet. 2008;372:962–71. doi: 10.1016/S0140-6736(08)61406-3. [DOI] [PubMed] [Google Scholar]
- 16.Wallerstein N. Powerlessness, empowerment, and health: implications for health promotion programs. Am J Health Promot. 1992;6:197–205. doi: 10.4278/0890-1171-6.3.197. [DOI] [PubMed] [Google Scholar]
- 17.Campbell MK, Elbourne DR, Altman DG, for the CONSORT Group CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328:702–08. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houweling TA, Azad K, Younes L, et al. The effect of participatory women’s groups on birth outcomes in Bangladesh: does coverage matter? Study protocol for a randomized controlled trial. Trials. 2011;12:208. doi: 10.1186/1745-6215-12-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harbord RM, Higgins JPT. Meta-regression in Stata. In: Sterne JAC, editor. Meta-analysis in Stata: an updated collection from the Stata Journal. Stata Press; College Station, TX: 2009. pp. 70–96. [Google Scholar]
- 21.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313:275–83. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . The world health report. Reducing risks, promoting healthy life. World Health Organization; Geneva: 2002. p. 108. [Google Scholar]
- 24.More NS, Bapat U, Das S, et al. Community mobilization in Mumbai slums to improve perinatal care and outcomes: a cluster randomised controlled trial. PLoS Med. 2012;9:e1001257. doi: 10.1371/journal.pmed.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewycka S, Mwansambo C, Rosato M, et al. Effect of women’s groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster randomised controlled trial. Lancet. 2013;381:1721–35. doi: 10.1016/S0140-6736(12)61959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colbourn T, Nambiar B, Bondo A, et al. Effects of quality improvement in health facilities and community mobilization through women’s groups on maternal, neonatal and perinatal mortality in three districts of Malawi: MaiKhanda, a cluster randomised controlled effectiveness trial. Int Health. doi: 10.1093/inthealth/iht011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fottrell E, Azad K, Kuddus A, Younes L, Shaha S, Nahar T. The effect of increased coverage of participatory women’s groups on neonatal mortality in Bangladesh: a cluster-randomised trial. JAMA. doi: 10.1001/jamapediatrics.2013.2534. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO . International classification of diseases and related health problems. 10th revision Volume 2. World Health Organisation; Geneva: 1993. [Google Scholar]
- 29.Howard-Grabman L, Snetro G. How to mobilize communities for health and social change. Health Communication Partnership, USAID; Baltimore, MD: 2003. [Google Scholar]
- 30.Rosato M. PhD thesis. University College London; [accessed Feb 21, 2013]. 2012. How does community mobilisation through MaiMwana women’s groups work? Addressing the social determinants of mother and child health in rural Malawi. http://discovery.ucl.ac.uk/1365989/ [Google Scholar]
- 31.Laverack G. Addressing the contradiction between discourse and practice in health promotion. Deakin University; 1999. PhD thesis. [Google Scholar]
- 32.Borghi J, Thapa B, Osrin D. Economic assessment of a women’s group intervention to improve birth outcomes in rural Nepal. Lancet. 2005;366:1882–84. doi: 10.1016/S0140-6736(05)67758-6. [DOI] [PubMed] [Google Scholar]
- 33.UCL [accessed Feb 21, 2013];Women and children first. Community mobilization through women’s groups to improve the health of mothers and babies. 2012 http://www.ucl.ac.uk/igh/news-attachments/good-practice-guide.
- 34.Lee ACC, Cousens S, Darmstadt GL, et al. Care during labor and birth for the prevention of intrapartum-related neonatal deaths: a systematic review and Delphi estimation of mortality effect. BMC Public Health. 2011;11:S10. doi: 10.1186/1471-2458-11-S3-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison J, Thapa R, Hartley S, et al. How women’s groups improve maternal and newborn health in Makwanpur, Nepal: a qualitative study. Int Health. 2010;2:25–35. doi: 10.1016/j.inhe.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosato M, Johnson B, Otanez M, MaiMwana Project [accessed Feb 8, 2013];Umodzi (together): a film about MaiMwana women’s groups. 2010 https://vimeo.com/12427420.
- 37.Rath S, Nair N, Tripathy PK, et al. Explaining the impact of a women’s group led community mobilization intervention on maternal and newborn health outcomes: the Ekjut trial process evaluation. BMC Int Health Hum Rights. 2010;10:25. doi: 10.1186/1472-698X-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.