Abstract
Herein we demonstrate that switchable, spontaneous, directional-transport ability to both water and oil fluids can be created on fabric materials through wet-chemistry coating and successive UV irradiation treatment. When the fabric showed directional transport to a liquid, it prevented liquids of higher surface tension from penetration, but allowed liquids of lower surface tension to permeate, from either side. The directional transport ability can be switched from one fluid to another simply by heating the fabric at an elevated temperature and then re-irradiating the fabric with UV light for required period of time. By attaching liquid drops vertically upwards to a horizontally-laid fabric, we further demonstrated that this novel directional fluid transport was an automatic process driven by surface property alone, irrespective of gravity's effect. This novel fabric may be useful for development of “smart” textiles and functional membranes for various applications.
Directional liquid transport driven just by the surface property of solid substrate has received considerable interest because of the intriguing science underpinning the phenomena and the exciting application potential. Beettle's back1,2 and spider silk3 are good examples, which combine two opposite wetting properties, i.e. hydrophobicity and hydrophilicity, on the surface. When moisture (liquid) attaches to the surface, tiny water drops move spontaneously towards hydrophilic areas where they coalesce into easily-collected large volume3. Such a directional-wetting property has inspired the development of innovative water harvesting devices4. More controllable directional-wetting was reported on synthetic surfaces having a wettability gradient5,6,7.
In comparison with directional wetting on open surfaces, directional liquid transport through thin porous medium is more complex but interesting. The liquid attachment, spreading and transport in porous medium are highly determined by not only surface property but also porous structure, because of the capillary effect involved. Spontaneous, directional liquid transport allows a thin porous membrane to remove liquid proactively from undesirable area, hence preventing liquid accumulation, and meanwhile eliminates back flow and associated fluid contamination during liquid transport. These unique features are very useful for development of novel membranes and separation technology, desalination, fuel cell, and biomedical materials, as well as smart textiles.
Despite the wide application potential, effective techniques to imbue thin porous materials with directional fluid transport ability have been seldom reported. Wang et al.8 in our group reported directional water transport effect on fabrics through the formation of a wettability gradient from superhydrophobicity to hydrophilicity across the fabric thickness. When water was dropped on the superhydrophobic side, it penetrated through the fabric rapidly and spread on hydrophilic area. However, no water can be transferred in opposite way through the fabric unless an extra pressure was applied to assist in the penetration. A similar result was also reported by Kong et al.9 who showed that directional water transport can be formed on superhydrophobic fabric through light irradiation. Apart from the formation of wettability gradient across the thickness of fabric, abruptly changed wettability between hydrophobicity and hydrophilicity in thin nanofibrous membranes also showed directional water transport effect10. In this case, electrospinning was used to prepare hydrophilic and hydrophobic nanofibres and deposit into a dual-layer fibrous membrane. All the works reported to date, however, have been confined to transport of liquid water8,10,11. Thin porous media with directional transport ability to oil fluids have not demonstrated in the research literature. Oil fluids have much wider choice in comparison to water, and directional transport of oil fluids through thin porous media hence offers larger space for industry applications. Since oil fluids vary in surface tension depending on the type, a thin membrane that have directional transport ability to one oil fluid may have a completely different response to others. Understanding of directional oil transport would lead to development of smart membranes having selective oil transport ability and novel applications.
In principle, directional liquid motion within a capillary channel can be achieved through either pore structure or surface property. Liquid within a porous membrane having gradient pore size change across the thickness transfers preferably in the direction from large to smaller pores. However, pore size gradient does not warrant fluid transport directionality because liquid can still move from smaller to large pores when sufficient liquid is fed from the smaller pores. Although directional water transport through thin porous membrane driven by the surface properties was reported, the transport directionality was mainly demonstrated by dropping liquid water on the upper surface of a horizontally-laid fabric. Consequently, gravity effect was suggested to play a key role to induce spontaneous directional water transport11. Directional liquid transport through a thin porous media driven just by surface properties irrespective of gravity's effect has not been demonstrated in research literature.
Herein, we describe a fabric-based thin porous membrane which has novel, spontaneous directional transport ability to both water and oil fluids. The liquid transport also has a selective feature. When it shows transport directionality to a fluid, it allows fluids with lower surface tension penetration from both sides, but prevents the fluids of higher surface tension from permeation from either side. The transport directionality is switchable from one fluid to another simply by a heating treatment of the membrane followed by UV-irradiation for required period of time. By attaching liquid drops vertically upwards to a horizontally-laid fabric, we further prove that the directional transport is controlled by surface properties, irrespective of gravity's effect. This novel fabric may be useful for development of “smart” energy-efficient, functional membranes for various applications.
Results
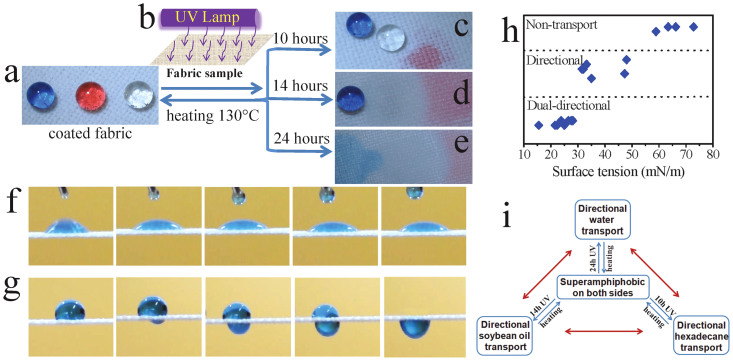
A commercial polyester fabric (thickness 512 μm) was employed as a model. The directional fluid transport effect was prepared by pre-treatment of the fabric with a two-step wet-chemistry coating technique developed in our previous study12, and subsequent UV irradiation. Without UV irradiation, the coated fabric had a superamphiphobic surface on both sides, with a contact angle (CA) of 172°, 165°, 160° to water, soybean oil and hexadecane, respectively. Figure 1a shows water, and oil drops (10 μL) on the coated fabric, which all look like a spherical ball and stay stably on the fabric for a long time.
Figure 1.
(a) Photos of blue coloured water droplets, red coloured hexadecane droplets and clear soybean oil droplets on the coated polyester fabric (10 μL for each drop; The small amount of dye used, reactive blue in water and oil red in hexadecane, had no influence on the contact angles), (b) illustration of asymmetric UV irradiation of the fabric, (c), (d) & (e) water, soybean oil and hexadecane droplets on the coated fabric after (c) 10 hours, (d) 14 hours and (e) 24 hours of UV irradiation, (f) & (g) still frames taken from videos showing dropping water (45 μl) on the horizontally-laid polyester fabric (after 24-hour UV irradiated), dropping on: (f) UV exposed surface (time interval, 0.26 s) and (g) unexposed side (time interval, 0.24 s), (h) selective transport to different liquid fluids (fabric, 14-hour UV-irradiated), (i) Switching feature of directional fluid transport on single piece of fabric.
Figure 1b illustrates the UV irradiation process. A UV lamp (wavelength mainly at 254 nm) was employed to treat the superamphiphobic fabric just on one fabric side. Upon irradiating with strong UV light, the irradiated fabric side showed a decrease in fluids repellence. After 10 hours of UV irradiation, the UV exposed surface reduces to 0° to hexadecane, but still amphiphobic for water and soybean oil (Figure 1c). After 14 hours of UV irradiation, the CA of the UV exposed surface is 0° for soybean oil and hexadecane, but not for water (Figure 1d). After 24 hours of irradiation, the UV irradiated fabric surface can be wetted by water, soybean oil and hexadecane, with a CA of zero degree (Figure 1e).
Figure 1f shows a series of still frames taken from a video during dropping water on the 24-hour UV irradiated fabric surface. Although water spread on the fabric surface, it did not penetrate through the fabric. When water was dropped onto the unexposed side, which did not receive the UV light directly, it moved through and spread on the opposite side immediately (Figure 1g). These clearly indicate that the coated fabric after 24-hour UV irradiation shows directional water transport effect. However, oil fluids such as soybean oil and hexadecane can spread throughout the fabric thickness from either fabric side.
Changing the UV irradiation time shifts the fluid transport directionality. When the coated fabric was UV irradiated for 14 hours, the fabric showed transport directionality to soybean oil (see Supplementary Figure S1). In this case, soybean oil can transfer directionally from the unexposed to the UV exposed surface rapidly when it was dropped on the unexposed side. When soybean oil was dropped on the UV exposed surface, it can only spread on the UV exposed surface without penetrating through the fabric. However, hexadecane can spread through the fabric from both sides, but water was strongly repelled and cannot penetrate from both sides. A list of fluids having different surface tensions (see Supplementary Table S1) was employed to examine the transport directionality of the fabric after 14 hours of UV irradiation. As shown in Figure 1h, directional transport takes place when the liquid has a surface tension in the range of 29 mN/m to 50 mN/m. Liquids with a surface tension below 29 mN/m could penetrate from both sides (i.e. dual directional transport), while the liquids with surface tension above 50 mN/m were not able to transfer through the fabric from either side.
Ten hours of UV irradiation led to transport directionality to hexadecane (see Supplementary Figure S2). In this case, the fabric had strong repellency to soybean oil and water, preventing them from penetration from either fabric side.
Apart from the selectivity to liquid type, the directional fluid transport can also be switched from one fluid to another. To prove this, the directional liquid transport fabric was heated at 130°C for 10 minutes. After heat treatment, the directional fluid-transport ability was eliminated completely from the fabric, and the fabric turned to be superamphiphobic on both sides. When the heat treated fabric was subjected to a UV irradiation for certain period of time, the fabric showed directional fluid transport again. However, the transport directionality can be set to either the same or different fluid depending on the UV irradiation time chosen. For example, heating a fabric which had directional water transport property (24-hour UV-irradiated fabric) and then re-irradiating the fabric with UV light under the same irradiation condition for 10 hours switched the directional fluid transport ability from water to hexadecane. This switchable feature was reversible and can be repeated for several times (Figure 1i). To our knowledge, this is the first to demonstrate that selective, directional fluid transport ability and the reversibly switchable feature to change the fluid transport directionality between different fluids. Such novel selectivity and switchability allow having on-demand directional fluid transport ability from a single piece of membrane.
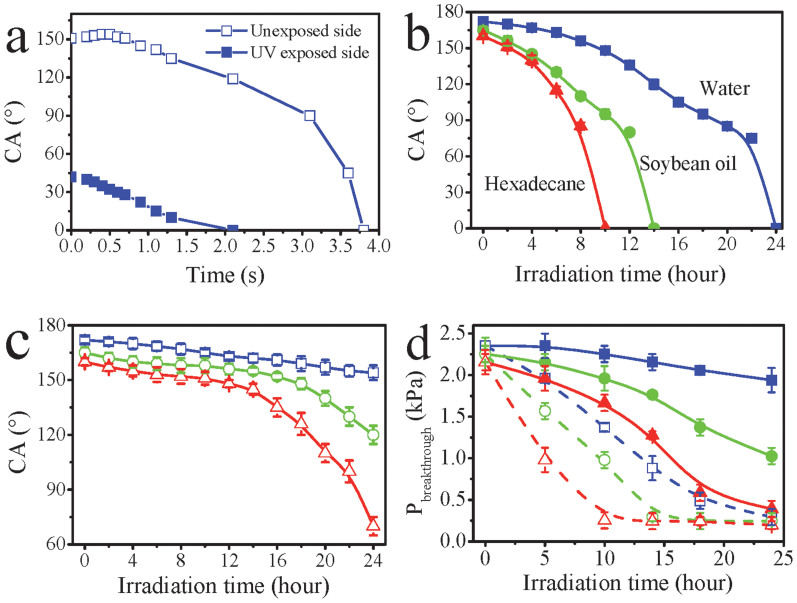
To understand the novel liquid transport property, the CA of the UV irradiated fabric was measured. When water (5 μL) was dropped on the unexposed side of the UV-irradiated fabric, its CA changed with time (Figure 2a). Starting from the droplet landing on the fabric surface, the CA increased initially from 152° to 154° within 0.2 second, then decreased monotonously to 90° in 0.6 second, and finally reduced to zero (in less 0.3 second). The first increase in the CA was explained by the relaxation vibration of the water drop on the superhydrophobic surface. The later reduction in the CA was attributed to the permeation of the droplet into the fabric matrix. The droplet from contacting the fabric surface to complete penetration took around 1.1 seconds. In contrast, the CA of water drop (the same volume) on the UV irradiated surface changed rapidly from 42° to zero (in 0.9 seconds) (Figure 2a).
Figure 2.
(a) change of CA with time during dropping water on 24-hour UV-irradiated fabric, (b) & (c) CA change with the UV irradiation time on (b) UV exposed side (UV exposed surface) and (c) unexposed side, (d) pressure required for breakthrough the coated fabrics (from UV exposed side for  water,
water,  soybean oil, and
soybean oil, and  hexadecane; from unexposed side for
hexadecane; from unexposed side for  water,
water, 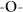 soybean oil, and
soybean oil, and 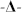 hexadecane). (The breakthrough pressure of the un-coated pristine polyester fabric was 0.29 kPa, 0.26 kPa and 0.24 kPa for water, soybean oil and hexadecane, respectively, with no difference shown on either side).
hexadecane). (The breakthrough pressure of the un-coated pristine polyester fabric was 0.29 kPa, 0.26 kPa and 0.24 kPa for water, soybean oil and hexadecane, respectively, with no difference shown on either side).
The effect of UV irradiation time on the CA was also examined. As shown in Figure 2b, after 10 hours of UV irradiation, the CA for hexadecane on the UV exposed fabric surface became 0°, while the surface was still amphiphobic to water and soybean oil. Longer UV irradiation time, i.e. 14 hours, resulted in the UV exposed surface being completely wettable to soybean oil and hexadecane with a zero CA. However, the CA to water was still 120°. After 24 hours of UV irradiation, the UV exposed surface was completely wettable to all the three liquids.
Figure 2c shows the CA change with the irradiation time on the unexposed fabric surface. After 10 hours of UV irradiation, the CA was still as high as 165°, 158° and 150° for water, soybean oil and hexadecane, while after 14 hours of irradiation, the CA was still high, respectively being 164°, 155° and 145°. 24 hours of UV irradiation led to decrease of the CA respectively to 154°, 120° and 70°. It was evident that the wettability of the UV-irradiated surface considerably increased with increasing the irradiation time.
The surface morphology of the UV-irradiated fabric was observed under SEM. As expected, the original rough surface feature was still maintained after the UV irradiation (Supplementary Figure S3). Obviously, the directional-fluid transport property should not come from the surface morphology changes.
The influence of UV irradiation on the chemical components of the coated fabric was characterized by FTIR (Supplementary Figure S4 and Table S2). After UV irradiation, polar groups such as -COOH or -OH were formed on the coating surface, while C-F bonds was removed. The change on the UV exposed surface was more noticeable than the unexposed surface.
To further understand the directional liquid transport property, the initial pressure required for a fluid to break through the fabric was measured. As shown in Figure 2d, the breakthrough pressure on the UV exposed side (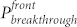 ) is always higher than that on the unexposed side (
) is always higher than that on the unexposed side (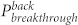 ), regardless of UV irradiation time. The pressure value on both sides decayed with the UV irradiation time. However, the rate of the decay on the UV exposed side was lower when compared to the unexposed side. This led to the difference between the UV exposed and the unexposed breakthrough pressure (
), regardless of UV irradiation time. The pressure value on both sides decayed with the UV irradiation time. However, the rate of the decay on the UV exposed side was lower when compared to the unexposed side. This led to the difference between the UV exposed and the unexposed breakthrough pressure (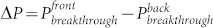 ) changing with irradiation time. It was interesting to find that the directional transport effect always took place at the point where the ΔP reached the maximum (see Supplementary Figure S5). When the
) changing with irradiation time. It was interesting to find that the directional transport effect always took place at the point where the ΔP reached the maximum (see Supplementary Figure S5). When the 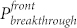 was lower than 1.27 kPa (e.g. hexadecane on 14-hour irradiated fabric), the liquid penetrated through the fabric. However, when the
was lower than 1.27 kPa (e.g. hexadecane on 14-hour irradiated fabric), the liquid penetrated through the fabric. However, when the 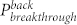 was above 0.89 kPa (e.g. water on 14-hour irradiated fabric, unexposed side), it prevented the liquid from penetration.
was above 0.89 kPa (e.g. water on 14-hour irradiated fabric, unexposed side), it prevented the liquid from penetration.
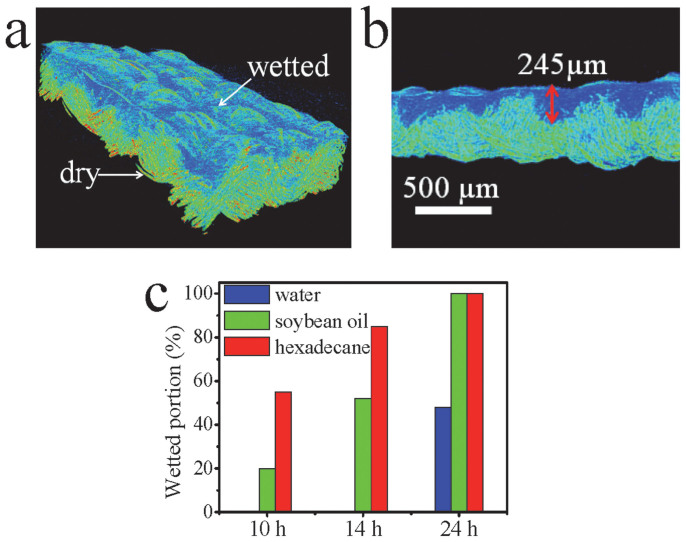
X-ray microtomograph (micro-CT) was used to observe the wetted fabric. Figure 3a shows a typical 3D micro-CT image of the 24-hour UV-irradiated fabric after being wetted with water. A large image contrast was observed between the wetted (in blue) and the non-wetted (in green) fabric areas. The 2D cross-sectional view obtained from the 3D image clearly shows the wetting profile (Figure 3b). The depth of wetting was around 245 μm from the UV exposed surface (the total fabric thickness was 512 μm). Based on the 2D images, the wetting portion of the fabric was estimated. As shown in Figure 3c, the wetting portion increases with increasing the UV irradiation time for all liquids tested. The directional transport always occurred at the condition that the transport portion to the liquid was around 50%. In that case, the CA on the unexposed side was always greater than 150° (i.e. superphobic), 0° on the UV exposed surface (see a table summarizing the directional transport in the Supplementary Table S3). When a thicker fabric was used as substrate, longer UV irradiation time was required for achieving the corresponding directional liquid transport effect. The thickness of the liquid repellent layer plays an important role in determining the directional liquid transport ability.
Figure 3.
(a) 3D micro-CT image of the 24-hour UV-irradiated fabric after wetted with water, (b) 2D micro-CT cross-sectional image showing partially-wetted fabric (24-hour UV-irradiated), (c) wetting portion of different liquids on UV-irradiated fabrics.
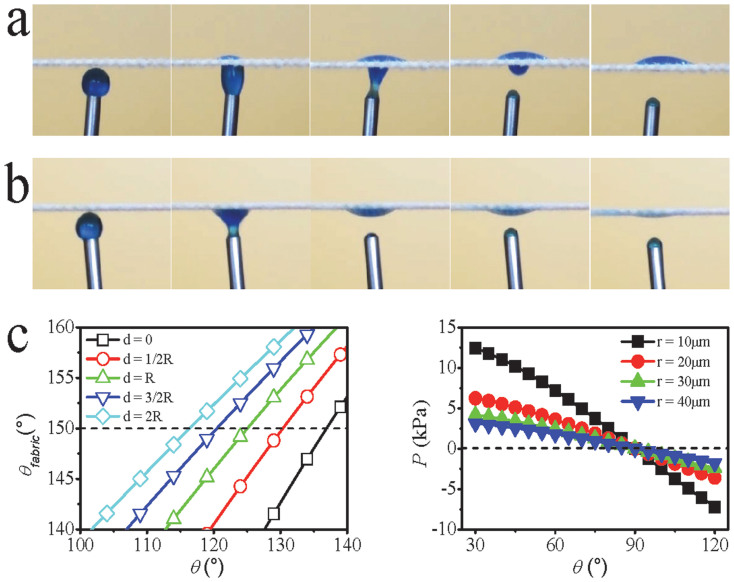
Having demonstrated the novel fluid transport properties, we next examined the effect of fabric spatial arrangement and liquid feeding style on the directional fluid transport. Figures 4a & b show an example in which liquid water is attached upwards to the lower surface of a horizontally-laid fabric (24-hour UV irradiated). When the lower surface was hydrophobic (i.e. the unexposed side of the UV irradiated fabric), water was attracted up and then moved to spread on the upper hydrophilic surface (i.e. UV exposed side) (Figure 4a). Attached water upwards to contact the downwards hydrophilic surface leads to spreading just on the lower surface, and there is no water transport and spread on the upper hydrophobic side (Figure 4b). (Photos from high-speed videos are also provided in Supplementary Figure S6). For comparison, hexadecane was also fed in a similar way to the fabric, which showed a normal wetting performance (see Supplementary Figure S7). These results clearly indicate that this directional transport is not triggered by the gravity. Directional fluid transport performance can be also obtained on a vertically-placed fabric (Supplementary Figure S8).
Figure 4. Water being fed upwards to attach the lower surface of a horizontally-laid fabric (24-hour UV-irradiated), (a) lower surface hydrophobic (time interval 0.26 s), (b) lower surface hydrophilic (time interval 0.18 s), (c) estimated θfabric ~ θ and Pcp ~ θ relationships.
To find out the mechanism behind the directional transport, time required for the directional transport under different temperatures (5 ~ 70°C) was recorded (Supplementary Figure S9). For all the three liquids studied, the transport became faster at a higher temperature. This was presumably because of effect of temperature on the surface tension, saturated vapour pressure and contact angle. With increasing the temperature, the liquid surface tension decreased, the saturated vapour pressure increased, while the CA did not change much in the temperature range tested (Supplementary Figure S9). In addition, liquid viscosity is expected to have an influence on the directional liquid transport. However, the effect is much less noticeable in comparison to the surface tension (see Supplementary Table S1).
Discussion
Before UV irradiation, the fabric sample used is superamphiphobic to liquids of surface tension above 27.5 mN/m12. Such liquid repellency derives from the amplification effect of the hierarchical rough-structure of fabric texture and rough surface of coated fibres on contact angle, in addition to the low wettability of fibre material. The effect of roughness on the apparent contact angle (θfabric) of fabric can be described as: cosθfabric = rf
f cosθ + f − 1 (where θ is the liquid contact angle of the coating material on fibre surface, rf is the roughness of solid that is in contact with liquid, and f is the fraction of the projected solid surface area that is in contact with the liquid). The relationship between θfabric and θ for a plain weave fabric made of multifilament yarns has been estimated as: 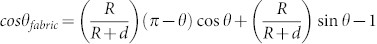 13 (where R is fibre radius, d is half of inter-fibre distance on the yarn locating on the fabric surface). The θfabric ~ θ relationship at a few different d/R ratios is shown in Figure 4c. Clearly, θ
is much lower than θfabric, and the fabric shows superhydrophobicity when the fibres have water contact angle over 117°.
13 (where R is fibre radius, d is half of inter-fibre distance on the yarn locating on the fabric surface). The θfabric ~ θ relationship at a few different d/R ratios is shown in Figure 4c. Clearly, θ
is much lower than θfabric, and the fabric shows superhydrophobicity when the fibres have water contact angle over 117°.
When the fabric is irradiated by strong UV light, the degradation of fibre surface leads to formation of polar groups (e.g. –COOH) on the irradiated surface. The increased surface energy because of the polar groups reduces contact angle despite the topological feature remains unchanged. Since UV light can penetrate the fabric but its intensity is significantly weakened when it reaches the unexposed side of the fabric, the reduction of contact angle from the UV exposed to the unexposed fabric surface should have a gradient change. At certain condition, the UV exposed surface becomes highly wettable while the unexposed surface is still super repellent to a liquid, which forms a Janus surfaced fabric. Longer UV irradiation time increases the degradation yield, resulting in lower contact angle value.
On another hand, bringing liquid to contact with a porous media causes the liquid either to stay on the surface or to flow into the porous matrix, depending on the capillary effect. Young–Laplace capillary pressure for an idealized pore having circular cross-section is expressed as 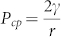 (where θ is the contact angle between liquid drop and capillary wall, r is the pore radius and γ is the surface tension of liquid). When the pore surface is wettable (θ < 90°), liquid is spontaneously drawn into the pore because of the positive capillary pressure. However, a negative capillary pressure would result when the pore surface is non-wettable wall (θ > 90°), repelling the liquid out of the pore. In this case, extra pressure is needed to force the liquid to permeate into the pore. The minimal external pressure to enable liquid to flow into the pore, i.e. breakthrough pressure (Pbreakthrough), can be established as
(where θ is the contact angle between liquid drop and capillary wall, r is the pore radius and γ is the surface tension of liquid). When the pore surface is wettable (θ < 90°), liquid is spontaneously drawn into the pore because of the positive capillary pressure. However, a negative capillary pressure would result when the pore surface is non-wettable wall (θ > 90°), repelling the liquid out of the pore. In this case, extra pressure is needed to force the liquid to permeate into the pore. The minimal external pressure to enable liquid to flow into the pore, i.e. breakthrough pressure (Pbreakthrough), can be established as 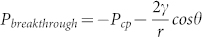 . Pcp at a few different pore radii was estimated based on the Young–Laplace equation (Figure 4c). For polyester fabric used in this study, the pore radius is in the range of 3 ~ 41 μm (average radius 20 μm). When the fabric has a superhydrophobic surface (θfabric = 150°, θ = 117°), the breakthrough pressure is 1.63 kPa, which is on a similar level to our experiment result.
. Pcp at a few different pore radii was estimated based on the Young–Laplace equation (Figure 4c). For polyester fabric used in this study, the pore radius is in the range of 3 ~ 41 μm (average radius 20 μm). When the fabric has a superhydrophobic surface (θfabric = 150°, θ = 117°), the breakthrough pressure is 1.63 kPa, which is on a similar level to our experiment result.
Several wetting regimes have been reported on a hierarchical rough-surface, including Wenzel, Cassie–Baxter, Lotus and petal regimes14,15,16. A superhydrophobic surface can be non-sticking and self-cleaning (Lotus effect) or adhesive to water strongly (petal effect). In the petal regime, water can penetrate either in the micron or/and nanostructure, while the micro/nanostructure can be partially impregnated by air15,16. Superhydrophobic fabric in petal regime allows water penetration slightly into the fabric. However, if the fabric has a wettability gradient from superhydrophobicity to hydrophilicity across the thickness, the penetration of water could be further enhanced by the fibres beneath because of the reduced contact angle. In this way, liquid penetration is accelerated until it completely spreads into the hydrophilic zone. However, when water drop is fed from the hydrophilic size, capillary effect allow the liquid easily spreading into the hydrophilic area. The capillary pressure turns negative when the liquid meets the high contact angle zone, preventing itself from further penetration. Therefore, the novel directional liquid transport property is attributed to photo-induced formation of gradient wettability across the fabric thickness and the petal superphobic surface on one side.
It is known that the contact angle of liquid drop on a solid surface is affected by surface tension. Smaller surface tension leads to smaller contact angle. The same trend happens to the capillary pressure. It is easy to understand that if a porous membrane allows one type of liquid to penetrate, it should allow other liquids of lower surface tension to permeate as well, because of more wettable nature. However, the membrane may stop liquid of higher surface tension to penetrate if the contact angle is large enough. In this way, directional fluid transport should be liquid specific to have a surface-tension-controlled selectivity.
The switchable feature comes from the self-healing ability of the superamphiphobic coating. Our previous paper has indicated that the superamphiphobic coating used has a temperature-driven self-healing ability against chemical damages12. Long-hour UV irradiation causes chemical damage to the coating, which can be healed through a heat treatment, allowing the coating to restore its superamphiphobicity. The heat treatment erases the liquid transport ability, which can be regenerated through second time UV treatment.
The novel switchability and selectivity of liquid transport would significantly enhance the functionality of directional liquid-transport membranes, especially for applications in separation of emulsive or immiscible fluids, healthcare, protection of hazardous liquid chemicals, electrochemical or energy devices, and protective/functional clothing.
In summary, a fibrous-based thin porous media with selective, switchable, directional fluid-transport ability to both water and oil fluids has been prepared. Such a novel liquid transport membrane may be useful for development of “smart” energy-efficient, functional membranes for various fluid transport applications.
Methods
Materials
1H,1H,2H,2H-perfluorodecyltriethoxysilane (C16H19F17O3Si), poly(vinylidene fluoride-co-hexafluoropropylene), dimethylformamide, tetraethylorthosilicate (98%), ammonium hydroxide (28% in water), oil red and oil blue obtained from Aldrich were used as received. Commercial polyester fabric (plain weave, 168 g/m2, thickness = 512 μm) was purchased from local supermarket, and they were rinsed with acetone and distilled water and cut to 5 cm × 5 cm (each sample) before use.
Preparation of directional fluid transport fabric
Coating solutions were prepared according to our previously report12. The fabric substrate was immersed in the silica particulate solution for 1 minute. After drying at room temperature for 10 minutes, the coated fabric was then immersed in PVDF-HFP/FAS solution for 1 minute, and finally dried at 130°C for 1 hour. The coated fabric was irradiated under a UV lamp just from one side to create directional fluid transport effect.
Characterizations
Contact angles were measured on a contact angle goniometer (KSV CAM 101) using liquid droplets of 5 μL in volume. All the CA values reported represent the mean of 5 measurements. Fourier transform infrared (FTIR) spectra were recorded on a Bruker VERTEX 70 instrument in ATR mode at a resolution of 4 cm−1 accumulating 32 scans. 3D micro-CT images were taken under an X-ray microtomograph (XRadia Inc. USA) using the software (TXM 3DViewer). Breakthrough pressure was measured using customer-built equipment comprising a fluid-feeding system with a flow rate controller, a pressure gauge and a fabric holder. During the measurement, the fluid was loaded on one side of the fabric at a flow rate of 25 ml/min and the minimum pressure under which the fluid starts to pass through the fabric was recorded as the breakthrough pressure.
Author Contributions
H.Z. and H.W. performed the experiment and characterisations. H.Z. and H.W. are equal contribution first authors for this paper. H.N. analysed data. T.L. designed experiments, analysed data and wrote the manuscript.
Supplementary Material
Supplementary Information
References
- Zhai L. et al. Patterned Superhydrophobic Surfaces: Toward a Synthetic Mimic of the Namib Desert Beetle. Nano Letters 6, 1213–1217 (2006). [DOI] [PubMed] [Google Scholar]
- Parker A. R. & Lawrence C. R. Water capture by a desert beetle. Nature 414, 33–34 (2001). [DOI] [PubMed] [Google Scholar]
- Zheng Y. et al. Directional water collection on wetted spider silk. Nature 463, 640–643 (2010). [DOI] [PubMed] [Google Scholar]
- Bai H. et al. Artifi cal Spider Silk: Direction Controlled Driving of Tiny Water Drops on Bioinspired Artificial Spider Silks. Advanced Materials 22, 5435–5435 (2010). [DOI] [PubMed] [Google Scholar]
- Daniel S., Chaudhury M. K. & Chen J. C. Fast Drop Movements Resulting from the Phase Change on a Gradient Surface. Science 291, 633–636 (2001). [DOI] [PubMed] [Google Scholar]
- Fang G., Li W., Wang X. & Qiao G. Droplet Motion on Designed Microtextured Superhydrophobic Surfaces with Tunable Wettability. Langmuir 24, 11651–11660 (2008). [DOI] [PubMed] [Google Scholar]
- Lai Y. H., Yang J. T. & Shieh D. B. A microchip fabricated with a vapor-diffusion self-assembled-monolayer method to transport droplets across superhydrophobic to hydrophilic surfaces. Lab on a Chip 10, 499–504 (2010). [DOI] [PubMed] [Google Scholar]
- Wang H., Ding J., Dai L., Wang X. & Lin T. Directional water-transfer through fabrics induced by asymmetric wettability. Journal of Materials Chemistry 20, 7938–7940 (2010). [Google Scholar]
- Kong Y., Liu Y. & Xin J. H. Fabrics with self-adaptive wettability controlled by "light-and-dark". J. Mater. Chem. 21, 17978–17987 (2011). [Google Scholar]
- Wu J. et al. Unidirectional water-penetration composite fibrous film via electrospinning. Soft Matter 8, 5996–5999 (2012). [Google Scholar]
- Tian X., Li J. & Wang X. Anisotropic liquid penetration arising from a cross-sectional wettability gradient. Soft Matter 8, 2633–2637 (2012). [Google Scholar]
- Zhou H., Wang H., Niu H., Gestos A. & Lin T. Robust, Self-Healing Superamphiphobic Fabrics Prepared by Two-Step Coating of Fluoro-Containing Polymer, Fluoroalkyl Silane, and Modified Silica Nanoparticles. Advanced Functional Materials 23, 1664–1670 (2013). [Google Scholar]
- Michielsen S. & Lee H. J. Design of a Superhydrophobic Surface Using Woven Structures. Langmuir 23, 6004–6010 (2007). [DOI] [PubMed] [Google Scholar]
- Feng L. et al. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 24, 4114–4119 (2008). [DOI] [PubMed] [Google Scholar]
- Bhushan B. & Nosonovsky M. The rose petal effect and the modes of superhydrophobicity. Phil. Trans. R. Soc. A 368, 4713–4728 (2010). [DOI] [PubMed] [Google Scholar]
- Bhushan B. & Her E. K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir 26, 8207–8217 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information