Abstract
Objective
To evaluate whether a customized exercise tolerance testing (ETT) protocol based on an individual’s habitual gait speed (HGS) on level ground would be a valid mode of exercise testing older adults. Although ETT provides a useful means to risk-stratify adults, age-related declines in gait speed paradoxically limit the utility of standard ETT protocols for evaluating older adults. A customized ETT protocol may be a useful alternative to these standard methods, and this study hypothesized that this alternative approach would be valid.
Design
We performed a cross-sectional analysis of baseline data from a randomized controlled trial of older adults with observed mobility problems. Screening was performed using a treadmill-based ETT protocol customized for each individual’s HGS. We determined the content validity by assessing the results of the ETTs, and we evaluated the construct validity of treadmill time in relation to the Physical Activity Scale for the Elderly (PASE) and the Late Life Function and Disability Instrument (LLFDI).
Setting
Outpatient rehabilitation center.
Participants
Community-dwelling, mobility-limited older adults (N = 141).
Interventions
Not applicable.
Main Outcome Measures
Cardiac instability, ETT duration, peak heart rate, peak systolic blood pressure, PASE, and LLFDI.
Results
Acute cardiac instability was identified in 4 of the participants who underwent ETT. The remaining participants (n = 137, 68% female; mean age, 75.3y) were included in the subsequent analyses. Mean exercise duration was 9.39 minutes, with no significant differences in durations being observed after evaluating among tertiles by HGS status. Mean peak heart rate and mean peak systolic blood pressure were 126.6 beats/ min and 175.0mmHg, respectively. Within separate multivariate models, ETT duration in each of the 3 gait speed groups was significantly associated (P<.05) with PASE and LLFDI.
Conclusions
Mobility-limited older adults can complete this customized ETT protocol, allowing for the identification of acute cardiac instability and the achievement of optimal exercise parameters.
Keywords: Aged, Exercise Test, Mobility limitation, Rehabilitation
Standard exercise tolerance testing (ETT) provides an important method by which to stratify older adults for risk associated with cardiovascular disease. When prescribing moderate-to high-intensity exercise programs for older adults, clinical guidelines recommend screening with exercise testing before exercise initiation.1 Also, ETT is a means by which an individual’s aerobic capacity can be measured.1 However, treadmill exercise testing does not always seem practical and is often avoided for older adults, especially those with health conditions (eg, musculoskeletal or neurologic conditions) that result in mobility problems. Additionally, 20% to 67% of older adults are not able to achieve diagnostic thresholds required by most commonly used ETT protocols.2 Some might interpret these reports as a rationale to replace exercise stress testing with other modes of stress testing, such as pharmacologic provocation (chemical stress tests), which are frequently used for older adults with mobility limitations. However, in comparison with exercise testing, chemical stress tests provide more circumscribed findings, especially with regard to provoking ischemia or arrhythmias. Others might suggest that hallway walking tests might be a better means to stratify the elderly.3 However, we hypothesize that treadmill exercise testing protocols may be modified to better respond to the physical limitations of older adults.4
With aging, the accumulation of physical impairments from chronic disease results in worsening mobility skills that limit capacities to complete a standard ETT protocol.2,5 It has been estimated that more than 25% of adults 70 years and older have mobility limitations, which is defined as slowed performance when conducting timed walking or chair rise tasks.5 Tests of mobility are also clinically relevant because scores on these tests are predictive of future mortality and disability.6,7 However, mobility problems directly diminish the utility of standard exercise treadmill protocols. In particular, standard ETT protocols do not account for the slower habitual gait speeds (HGSs) observed among older adults, and these protocols start at treadmill speeds that exceed the capacities of typical older patients.8,9 Gill et al8 advocated for the establishment of more appropriate ETT protocols that account for these common manifestations of mobility limitations, and leaders in cardiology have advocated for the use of individualized protocols that are based on an individual’s capacities.4,10 One possible approach is to customize ETT protocols around older adults’ physical capabilities as well as their clinical and demographic information.4
In contrast to the standard and currently existing customized ETT protocols, an ETT protocol based on the HGS of older adults might be more appropriate because it could accommodate to the individual’s walking strategy from both a mechanical and a physiologic perspective.11 Prior small-scale pilot studies12,13 have used this approach successfully to screen older adults for exercise studies. Pu et al13 used a screening ETT test protocol based on 80% of HGS among 16 older women with class I to III congestive heart failure and 80 older women without heart disease. Unfortunately, no information is provided regarding their protocol’s ability to screen out individuals with undiagnosed heart disease, and details regarding the protocol’s findings (duration of testing) are only presented for the 16 patients with heart failure. Bean et al12 used an identical protocol among 45 older adults with mild to moderate mobility problems and performed submaximal testing. This protocol was well tolerated, but was not evaluated as a primary study aim in that pilot study. In summarizing these studies, although showing promise as a clinical test and demonstrating safety of the protocol, neither study provided adequate information to establish the validity of a customized ETT based on HGS among older adults with mobility problems.
For the purpose of evaluating the validity of a customized ETT based on HGS, we conducted an analysis of baseline data from a recently completed randomized controlled clinical trial among older adults with mobility problems who were screened using this approach. Mobility problems were defined by abnormal scores on the Short Physical Performance Battery (SPPB).6 The first aim of our study was to demonstrate the content validity of the customized ETT protocol through 2 means. First, we hypothesized that the measure could effectively screen out individuals who were at high risk for cardiovascular events and therefore not safe to participate in high-intensity exercise. In this study, we defined high risk for cardiovascular events as any adverse baseline cardiovascular findings during testing, such as ischemic changes or supraventricular tachycardia. Second, we hypothesized that the protocol would facilitate clinically optimal testing durations (8 –12min), as well as physiologically meaningful exercise intensities as summarized by Myers et al.4 For our second aim, we compared customized ETT performance among older adults and examined the construct validity of the customized ETT protocol. Construct validity is defined as the extent to which a measurement corresponds to higher-order concepts that are theoretically related to that measure.14 Physical activity, physical function, and disability are all higher-order concepts that are in part related to aerobic capacity.15–17 Performance on an ETT protocol is dependent on aerobic capacity.16 Therefore, we hypothesized that, after controlling for relevant covariates, ETT duration would be significantly associated with self-reported physical activity, physical function, and disability.
METHODS
This study is a cross-sectional analysis of baseline data from a randomized controlled trial of exercise among community-dwelling older adults with mobility problems. The study was approved by Spaulding Rehabilitation Hospital Institutional Review Board and conforms to the Helsinki Declaration.
Recruitment of Participants
Participants were recruited through advertisements in newspapers, direct mail, referrals from primary care providers, and telephone screenings. These efforts resulted in 493 initial inquiries from which 236 possible subjects were chosen for an initial screening at an outpatient rehabilitation facility. The assessment was completed over 1 to 2 visits. Before administration of the ETT test, 142 subjects met inclusion/exclusion criteria and consented to participate in the study.
Initial Screening Process
Inclusion criteria were community-dwelling older adults (age ≥65y) with mobility limitations, which we defined as a score between 4 and 10 on the SPPB, and who were able to climb 1 flight of stairs with use of the handrail.6 The SPPB assesses standing balance, timed usual-pace 4-m walk, and timed test of 5 repeated chair stands. Each task is scored from 0 (unable) to 4 (highest performance), with a maximum score being 12. Participants underwent a comprehensive examination conducted by the study physician after providing informed consent. Active medical conditions of each participant were recorded. Exclusion criteria included unstable acute or chronic disease, a score of less than 23 on the Folstein Mini-Mental State Examination,18 a neuromusculoskeletal impairment (eg, hemiplegia or amputation) limiting participation in testing or a medical history that indicated a contraindication for exercise according to American College of Sports Medicine guidelines. 16
Performance Testing
Habitual gait speed
HGS was measured using an ultrasonic gait speed monitora as previously described in other reports.13,19 Briefly, to measure HGS, participants were asked to walk at their normal walking speed on level ground, as if they were “walking down the street to go to the store.” Walking speed was recorded after an individual reached HGS. Speeds were recorded to the nearest .01 second, and an average of 2 performance trials was obtained. It is important to note that this reliable and valid method of gait speed measurement20 is different from that used within the SPPB, which incorporates an acceleration phase from a standing position. The HGS measurement, which is generally faster for a given individual than the SPPB gait speed measurement, was considered a more appropriate guide for the treadmill protocol that used steady speeds at each of its different stages.
Customized ETT
Participants underwent a symptom-limited ETT on a treadmill as illustrated in figure 1. For safety purposes, participants were required to hold on to a horizontal bar at the front of the treadmill with at least 1 arm at all times. The purpose of the protocol design was to increase the subject’s perceived exertion to a Borg score of 17 within a clinically relevant time.21 All exercise stages were 3 minutes in duration. For the first 3 stages, the speed was set to participants’ HGS at 0%, 5%, and 10% grades of treadmill inclination, respectively. These percent grades correspond to approximately 0°, 3°, and 6°, respectively. After the third stage (9min into the protocol), treadmill speed was increased to 125% of HGS at 12% treadmill inclination. Finally, the treadmill inclination was increased by 2% at each subsequent stage until the end of the test (see fig 1). This increase in HGS and the lower grade advancement were chosen to optimize completion times within 8 to 12 minutes while at the same time avoiding large inclines on the treadmill for the majority of participants. Subjects were monitored by a 12-lead electrocardiogram throughout the protocol. At the end of each stage, blood pressure was recorded manually, and perceived exertion was measured using the Borg scale.21 Testing was terminated when a participant had achieved a Borg scale rating of 17 or greater, representing perceived exertion of “very hard.”21 Testing was stopped before this point in time if a participant experienced any adverse symptoms or signs, such as shortness of breath or pain.
Fig 1.
Graphical depiction of the submaximal ETT protocol based on HGSs.
Physical Activity
The level of habitual physical activity was measured via the Physical Activity Scale for the Elderly (PASE).22 The PASE is a reliable and valid self-report measure of habitual physical activity among older adults of varying health status.22
Late Life Function and Disability Instrument
The Late Life Function and Disability Instrument (LLFDI) provides a self-reported assessment of distinct function and disability domains in older adults.23–26 The disability component of the LLFDI is organized into 2 domains: assessing the person’s limitation in his/her capability to perform 16 life tasks, and the frequency with which the person performs the same 16 tasks. Participants were scored on a transformed scale of 1 to 100 in which higher numbers denoted higher levels of function or disability.23 For the purpose of this analysis, we evaluated the LLFDI-function and the LLFDI-limitation as outcomes. It has been validated among diverse populations of older adults.23–26
Additional Measures
Potential adjustment variables included age, sex, body mass index (BMI), number of medications, β-blockers, pain medications, number of chronic medical conditions, and falls self-efficacy, which was measured by a version of the Falls Efficacy Scale that scores individuals from 10 to 40, with higher values being worse.27,28 Additionally, we evaluated the number of cardiovascular conditions, the number of musculoskeletal conditions, and the presence of certain common medical conditions (neurologic disease, diabetes mellitus, pulmonary disease, or depression). Height and weight were measured using a calibrated scale and stadiometer. BMI was calculated by the following formula: mass (kg)/height (m)2. BMI categories were characterized using standard National Heart, Lung, and Blood Institute categories. Medications were recorded and classified by indication. β-blockers were placed into their own classification because of their effect on heart rate during exercise.29
Statistical Analysis
Means, SDs, and ranges were calculated for continuous variables, and frequencies were calculated for categorical variables. Then, HGS was categorized into tertiles, ensuring that groupings were equal in size and consistent with clinically meaningful differences in gait speed.30 Differences among groups were determined using analysis of variance for continuous variables and chi-square analysis for categorical variables. Kaplan-Meier survival analysis was conducted evaluating ETT duration for each HGS category. For the purposes of this analysis, we used data from ETTs with durations of up to 16 minutes. As a result, 1 participant was censored in each subgroup because their ETT durations exceeded 16 minutes (18min). To further evaluate whether group differences influenced these findings, iterative models were constructed using Cox proportion hazard ratios, and we evaluated those variables that were statistically different among HGS subgroups and those that were clinically indicated. The following covariates were used in these models: age, sex, BMI status, medications, number of chronic conditions, β-blockers, neurologic disease (presence or absence), and falls efficacy score. Separate multivariate regression models were performed to predict PASE, LLFDI-function and LLFDI-disability domains. For these models, ETT duration was included as a continuous variable. HGS, age, sex, and medications were included as covariates. An α level of .05 was used to determine statistical significance. Statistical analyses were performed using SAS/STAT software version 9.1.b
RESULTS
ETT Findings and Participant Characteristics (Content Validity)
One participant from the parent study did not undergo a baseline ETT. That individual had a normal result from a maximal ETT completed within 1 month of their study enrollment date. Data from that participant were excluded from this analysis. That individual was excluded from this analysis. Among the 141 individuals undergoing the customized ETT testing, overt cardiac instability was induced in 4 individuals. Two of these participants showed ischemic changes on an electrocardiogram during ETT, 1 participant sustained supraventricular tachycardia with hypertension, and another showed complex ventricular ectopy on ETT. These 4 individuals did not differ from the remaining 137 participants in terms of age, physical or medical status. As a result of these findings, and consistent with the protocol of the intervention study from which this analysis was derived, these participants were excluded from any subsequent testing. The remaining 137 subjects were included in the subsequent analyses, and their baseline characteristics are presented in table 1. A full description of the entire cohort is included in prior reports,31,32 and descriptive baseline characteristics of the participants within each mobility subgroup are shown in table 1.
Table 1.
Characteristics of Participants Based on Tertile Groups of HGS
| Characteristic or Outcome | Total Participants | High HGS (≥1.2m/s) |
Moderate HGS (>1.0m/s and<1.2m/s) |
Low HGS (≤1.0m/s) |
P |
|---|---|---|---|---|---|
| N | 137 | 47 | 44 | 46 | N/A |
| Age (y) | 75.3±6.8 | 73.2±5.2 | 73.7±5.9 | 78.9±7.6 | <.001 |
| BMI (kg/m2) | 27.7±4.9 | ||||
| <25 | 49 (35.8) | 17 (36.2) | 15 (34.1) | 17 (37.0) | |
| 25.0–29.9 | 51 (37.2) | 18 (38.3) | 15 (34.1) | 18 (39.1) | |
| ≥30 | 37 (27.0) | 12 (25.5) | 14 (31.8) | 11 (23.9) | .89 |
| Sex (female) | 93 (68) | 28 (60) | 31 (70) | 34 (74) | .14 |
| Medications (total) | 4.3±2.7 | 3.4±2.3 | 4.0±2.8 | 5.3±2.8 | .003 |
| β-Blocker | 39 (28.5) | 7 (14.9) | 12 (27.3) | 20 (43.5) | .002 |
| Pain | 41 (29.9) | 12 (29.8) | 11 (25.0) | 16 (34.8) | .60 |
| Chronic medical conditions | 5.6±2.4 | 5.0±1.8 | 5.5±2.5 | 6.2±2.8 | .04 |
| No. of cardiovascular conditions | |||||
| 0 | 35 (25.6) | 13 (27.7) | 11 (25.0) | 11 (23.9) | |
| 1 | 46 (33.6) | 16 (34.0) | 13 (29.6) | 17 (37.0) | |
| 2 | 33 (24.1) | 14 (29.8) | 10 (22.7) | 9 (19.6) | |
| ≥3 | 23 (16.8) | 4 (8.5) | 10 (22.7) | 9 (19.6) | .46 |
| No. of musculoskeletal conditions | |||||
| 0 | 19 (13.9) | 6 (12.8) | 8 (18.2) | 5 (10.9) | |
| 1 | 35 (25.6) | 14 (29.8) | 11 (25.0) | 10 (21.7) | |
| 2 | 42 (30.7) | 15 (31.9) | 12 (27.3) | 15 (32.6) | |
| ≥3 | 41 (29.9) | 12 (25.5) | 13 (29.6) | 16 (34.8) | .33 |
| Neurologic | 35 (25.6) | 8 (17.0) | 9 (20.5) | 18 (39.1) | .02 |
| Diabetes | 18 (13.1) | 5 (10.6) | 7 (15.9) | 6 (13.0) | .73 |
| Pulmonary | 13 (9.5) | 5 (10.6) | 3 (6.8) | 5 (10.9) | .73 |
| Depression* | 16 (13.6) | 6 (14.6) | 5 (12.2) | 5 (13.9) | .91 |
| Falls Efficacy Scale† | 12.9±4.5 | 11.3±1.9 | 12.4±3.3 | 15.0±6.3 | <.001 |
| PASE | 94.0±50.5 | 106.7±45.5 | 93.0±52.6 | 81.9±51.2 | .06 |
| LLFDI-function‡ | 64.3±10.8 | 68.6±10.2 | 66.0±9.6 | 58.3±9.9 | <.001 |
| LLFDI-limitation§ | 78.1±16.29 | 80.6±15.4 | 83.2±16.4 | 70.9±14.7 | <.001 |
| LLFDI-frequency‖ | 52.7±5.6 | 53.3±5.3 | 53.1±4.7 | 51.8±6.6 | .37 |
| ETT duration (min) | 9.39±3.2 | 9.24±2.9 | 9.60±2.83 | 9.33±3.76 | .86 |
| Baseline resting HR (beats/min) | 68.1±19.8 | 71.4±11.6 | 65.1 | 67.6±11.2 | .02 |
| Maximum HR (beats/min) | 126.6±16.8 | 135.1±17.8 | 127.8±18.0 | 117.0±19.6 | <.001 |
| Baseline resting SBP (mmHg) | 136.5±11.1 | 134.4±16.5 | 138.8±18.5 | 136.5±15.3 | .46 |
| Maximum SBP (mmHg) | 175.0±21.5 | 173.4±20.0 | 181.7±20.2 | 170.1±22.9 | .03 |
| Maximum RPP | 22,283±4842.5 | 23,491.9±4271.6 | 23,240.2±4321.9 | 20,132.4±5217.4 | <.001 |
| Maximum RPE | 16.8±1.3 | 16.9±1.4 | 16.9±1.5 | 16.7±.9 | .86 |
NOTE. Values are mean ± SD, n (%), or as otherwise indicated.
Abbreviations: HR, heart rate; N/A, not applicable; RPE, rating of perceived exertion; SBP, systolic blood pressure.
Depression is defined as 16 or more on Center for Epidemiology–Depression scale.
Maximum score is 40.
Function portion of the LLFDI questionnaire.
Limitation portion of the LLFDI questionnaire.
Frequency portion of the LLFDI questionnaire.
The mean SPPB score for participants was 8.7, and 16% of participants used an assistive device (cane) for walking (data not shown). The average HGS for all participants was 1.1m/s. HGS tertiles resulted in the characterization of 47 participants as high (HGS ≥1.2m/s), 44 participants as moderate (1.2m/s> HGS >1.0m/s), and 46 participants as low (HGS ≤1.0m/s).30
Evaluation of ETT Duration (Content Validity)
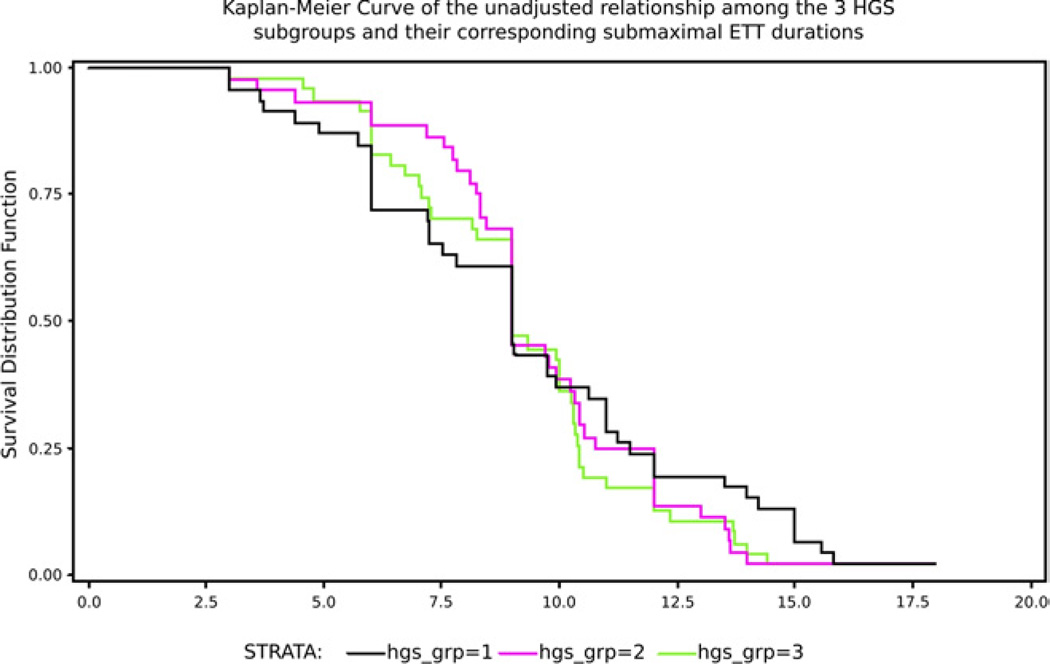
Mean ETT duration ± SD for those with high, moderate, and low HGS was 9.24±2.9, 9.60±2.83, and 9.33±3.76 minutes (P= .86), respectively. There were significant differences in maximal heart rate, systolic blood pressure, and rate-pressure product (RPP) across the 3 groups (P<.001, P=.03, and P<.001, respectively). Kaplan-Meier curves depict the unadjusted relationship among the 3 HGS subgroups and their corresponding submaximal ETT durations as demonstrated in figure 2. No significant difference in ETT duration was found among HGS subgroups within any of the multivariate models.
Fig 2.
Survival analysis between the duration of the ETT results and the 3 groups classified by HGS (N = 137).
Multivariate Analysis to Determine Construct Validity
Three separate multivariate linear regression models predict PASE, LLFDI-function, and LLFDI-limitation scores as shown in table 2. All 3 models were statistically significant (P<.05), describing 20%, 39%, and 20% of the variance (R2) in PASE, LLFDI-function, and LLFDI-limitation, respectively. ETT duration was a significant predictor in all 3 of the multivariate models (P<.05). Within the model predicting PASE, ETT duration explained 6% of the variance, age explained 9% of the variance, and HGS explained 5% of the variance. No other adjustment variables were statistically significant. ETT duration, HGS, and sex explained 14%, 20%, and 4% of the variance, respectively, for LLFDI-function, and none of the other covariates were significantly associated with the outcome. Lastly, for LLFDI-limitation, ETT duration and HGS explained 9% and 10% of the variance, respectively, and no other covariates were statistically significant.
Table 2.
Multivariate Linear Regression Models Predicting PASE, LLFDI–Function, and LLFDI–Limitation
| PASE Multivariate Analysis Total Model R2=.20 Model P<.001 |
LLFDI–Function Multivariate Analysis Total Model R2=.39 Model P<.001 |
LLFDI–Limitation Multivariate Analysis Total Model R2=.20 Model P<.001 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Parameter Estimate (SE) |
P | Squared Semipartial Correlation |
Parameter Estimate (SE) |
P | Squared Semipartial Correlation |
Parameter Estimate (SE) |
P | Squared Semipartial Correlation |
| ETT duration | 3.29 (1.32) | .01 | .06 | 1.22 (0.25) | <.001 | .14 | 1.53 (0.43) | .005 | .09 |
| HGS | 22.9 (18.3) | .21 | .05 | 17.0 (3.41) | <.001 | .20 | 17.6 (5.87) | .003 | .10 |
| Age | −2.40 (0.69) | <.001 | .09 | −0.07 (0.13) | .60 | .<01 | −0.26 (0.22) | .25 | .01 |
| Sex | 4.72 (9.62) | 62 | <.01 | −5.79 (1.80) | .001 | .043 | 0.56 (3.11) | .86 | <.01 |
| Weight | −0.08 (0.29) | .80 | <.01 | −0.06 (0.05) | .26 | .01 | −0.03 (0.09) | .73 | <.01 |
| Medications | 0.42 (9.18) | .96 | <.01 | −1.00 (1.71) | .56 | <.01 | −2.48 (2.98) | .41 | <.01 |
DISCUSSION
This study has produced several important observations. First, consistent with our first hypothesis, the results indicate that this ETT protocol was able to identify cardiac instability among older adults with baseline mobility limitations. Four participants (3%) were found to have high risk for cardiovascular disease. The success of this screening test is further corroborated by a prior report that described the supervised exercise program in which the 137 participants were included. None of these participants dropped out because of cardiovascular events despite participation in 16 weeks of moderate to high intensity exercise.32 Second, participants achieved the optimal range of 8 to 12 minutes4 of treadmill time despite baseline mobility limitations. Participants consistently achieved substantive changes in heart rate, systolic blood pressure, and RPP in response to this progressive exercise program (see table 1) in accordance with the second hypothesis of aim 1.
The overall responses in cardiovascular parameters are consistent with those observed by Myers,4 who conducted customized exercise tolerance tests among a larger and younger cohort. To develop their customization, Myers developed a questionnaire, validated among veterans, to predict exercise tolerance and used a nomogram to predict ramp rates. HGS measurement may be an even simpler and more expeditious method of determining ramp rates for customizing ETT protocols.
Significant differences among the 3 tertiles of HGS with regard to the observed maximal heart rate, systolic blood pressure, and RPP may be due to the significantly higher frequency of β-blocker use in the slower groups. Importantly, β-blocker use was not a significant predictor of ETT duration within the multivariate model, and therefore, did not prevent most individuals on these medications from having ETT durations within the clinically recommended range of 8 to 12 minutes.
Our analysis based on baseline HGS has statistical relevance because it allowed us to divide subjects into tertiles. Additionally, HGS measurement is predictive of adverse outcomes,33 and thus our cut points have clinical relevance as well. For example, a clinically meaningful difference in HGS has been identified as 0.1m/s.30 Our categories represented differences of 0.2m/s, which correspond to meaningfully different risk profiles for subsequent disability. Additionally, the cut point of HGS less than 1.0m/s that categorizes our lowest performance group has also been characterized as a clinically meaningful group at risk for subsequent morbidity.7
Consistent with the definition provided in the introduction and in the hypothesis of aim 2, the construct validity of our protocol was confirmed. ETT duration was a significant factor within multivariate models predicting performance on the PASE and on both the function and disability components of the LLFDI. These outcomes are all age-related, higher-order measures that have been validated among older adults. It is also important to note that ETT duration was a significant factor even when HGS was included in the models. This supports the argument that ETT duration represents a measure consistent with exercise capacity and independent of gait speed. Physical activity and functional outcomes are complex domains in which performance is attributable to many physiologic and behavioral factors besides ETT endurance. The magnitude of variance described by ETT performance (6%–14%) is consistent with what is expected when such relationships are evaluated.19,31
Conceptually, our chosen protocol represents a comparison of individuals at relative exercise workloads based on HGS. This is an important contrast to the more established standardized protocols that are designed around progressive increases in absolute exercise workloads based on levels of oxygen consumption. 4,16,34 Typical protocols progress aerobic power output at various intensities (combination of speed and inclination) and rates using metabolic equivalents (METs) as the basis for these increases. This is problematic because the physiologic basis of METs is defined according to a typical younger, seventy-kilogram adult male.16,34 The majority of adults 65 years or older are women with smaller muscle mass and lower biomechanical efficiency as a result of accumulated neuromusculoskeletal impairments, making conduct and completion of standard protocols very challenging. Similar protocols to ours have been used successfully in pilot studies.12,13 Others have advocated for use of hallway walking tests as modes of determining aerobic capacity,3 but our approach may be more appropriate when there are space limitations, the need for close physiologic monitoring, or both. All of our participants achieved Borg scale ratings and age-predicted maximum heart rates exceeding levels at which vigorous exercise or physical activity would commonly be recommended.17,35 The ability to achieve high heart rates under testing conditions is clinically important given that more than 25% of our participants were prescribed β-blocker medications.
Study Limitations
The results of this study should be interpreted in light of the following limitations. In figure 2 depicting the survival analysis, a large drop-off of subjects is observed after stage 3 among all 3 groups. This is likely due to the increase in gait speed that occurred at that point of the protocol. An ideal ETT protocol would produce a more gradual drop-off of participants. In retrospect, maintaining HGS throughout subsequent stages and continuing to increase the incline by a 5% grade might prevent this occurrence and still be well tolerated. Also, this was an ancillary study of an investigation with different aims and hypotheses. As a result, we did not have direct measures of oxygen consumption and measurements of other physical factors such as balance, strength, and range of motion. Although we have addressed both content and construct validity with this investigation, we were not able to compare our measure to an established criterion standard, which leaves the question of criterion validity unaddressed. Additionally, we used perceived exertion as the primary endpoint for testing and not the achievement of age-predicted maximal heart rate. However, greater limitations of using heart rate as an endpoint have recently been emphasized, especially for older adults.10 The participants in this study may not be a true representation of an older population. However, our study sample was relatively diverse functionally, and did include individuals characterized with mild to severe mobility status as defined by our inclusion criteria of SPPB scores ranging from 4 to 10.6
Despite these limitations, our findings represent pilot work justifying a study comparing different customized ETT protocols among mobility-limited older adults. This information would help establish the criterion validity of our ETT protocol and clarify whether individuals of varying mobility status were, in fact, exercising at the same relative workloads.
CONCLUSIONS
Our investigation demonstrated that a symptom-limited customized ETT protocol based on HGS is a valid means of risk-stratifying older adults. This may be a useful method of customizing ETT testing and is worthy of future investigation.
Acknowledgments
Supported by the Dennis W. Jahnigen Scholars Career Development Award, the American Geriatrics Society/Hartford Foundation, an NIH Mentored Clinical Scientist Development Award (grant no. K23AG019663-01A2), the Department of PM&R, Harvard Medical School, and the Association of Academic Physiatrist’s Rehabilitation Research Experience for Medical Students.
List of Abbreviations
- BMI
body mass index
- ETT
exercise tolerance testing
- HGS
habitual gait speed
- LLFDI
Late Life Function and Disability Instrument
- METs
metabolic equivalents
- PASE
Physical Activity Scale for the Elderly
- RPP
rate-pressure product
- SPPB
Short Physical Performance Battery
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Suppliers
Ultratimer; DCPB Electronics, Glasgow, Glasgow, G11 6NT Scotland.
SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513.
References
- 1.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1995;91:580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 2.Huggett DL, Connelly DM, Overend TJ. Maximal aerobic capacity testing of older adults: a critical review. J Gerontol A Biol Sci Med Sci. 2005;60:57–66. doi: 10.1093/gerona/60.1.57. [DOI] [PubMed] [Google Scholar]
- 3.Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 4.Myers J, Bader D, Madhavan R, Froelicher V. Validation of a specific activity questionnaire to estimate exercise tolerance in patients referred for exercise testing. Am Heart J. 2001;142:1041–1046. doi: 10.1067/mhj.2001.118740. [DOI] [PubMed] [Google Scholar]
- 5.Kramarow E, Lenttzner H, Rooks R, Weeks J, Saydah S. Health United States, 1999. Hyattsville: National Center for Health Statistics; 1999. Health and aging chartbook. [Google Scholar]
- 6.Guralnik JM, Simonsick EM, Ferucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 7.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, DiPietro L, Krumholz HM. Role of exercise stress testing and safety monitoring for older persons starting an exercise program. JAMA. 2000;284:342–349. doi: 10.1001/jama.284.3.342. [DOI] [PubMed] [Google Scholar]
- 9.Morey MC, Pieper CF, Cornoni-Huntley J. Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults? Med Sci Sports Exerc. 1998;30:1223–1229. doi: 10.1097/00005768-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Wackers FJ. Customized exercise testing. J Am Coll Cardiol. 2009;54:546–548. doi: 10.1016/j.jacc.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Fisher SV, Gullickson G., Jr Energy cost of ambulation in health and disability: a literature review. Arch Phys Med Rehabil. 1978;59:124–133. [PubMed] [Google Scholar]
- 12.Bean JF, Kiely DK, Leveille SG, et al. The six-minute walk test in mobility limited elders: what is being measured? J Gerontol A Biol Sci Med Sci. 2002;57A:M751–M756. doi: 10.1093/gerona/57.11.m751. [DOI] [PubMed] [Google Scholar]
- 13.Pu CT, Johnson MT, Forman DE, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol. 2001;90:2341–2350. doi: 10.1152/jappl.2001.90.6.2341. [DOI] [PubMed] [Google Scholar]
- 14.Pugh MB. 27th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. Stedman’s medical dictionary. [Google Scholar]
- 15.National Institutes of Health. Physical activity and cardiovascular health. National Institutes of Health Consensus Statement. 1995;13:1–33. [PubMed] [Google Scholar]
- 16.American College of Sports Medicine. 5th ed. Baltimore: Williams and Wilkins; 1995. Guidelines for exercise testing and prescription. [Google Scholar]
- 17.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85:S31–S42. doi: 10.1016/j.apmr.2004.03.010. quiz S43-4. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SF, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Bean J, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility limited elders. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 20.Sayers SP, Bean JF, Cuoco A, Lebrasseur NK, Jette AM, Fielding RA. Changes in function and disability after resistance training: does velocity matter? A pilot study. Am J Phys Med Rehabil. 2003;82:605–613. doi: 10.1097/01.PHM.0000078225.71442.B6. [DOI] [PubMed] [Google Scholar]
- 21.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377. [PubMed] [Google Scholar]
- 22.Washburn R, Smith K, Jette A. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;48:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Dubuc N, Haley S, Ni P, Kooyoomjian J, Jette A. Function and disability in late life: comparison of the Late-Life Function and Disability Instrument to the Short-Form-36 and the London Handicap Scale. Disabil Rehabil. 2004;26:362–370. doi: 10.1080/09638280410001658667. [DOI] [PubMed] [Google Scholar]
- 24.Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57:M217–M222. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 25.Jette AM, Haley SM, Coster WJ, et al. Late Life Function and Disability Instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57:M209–M216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 26.Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004;52:1554–1559. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 27.Harada N, Chiu V, Damron-Rodriguez J, Fowler E, Siu A, Reuben DB. Screening for balance and mobility impairment in elderly individuals living in residential care facilities. Phys Ther. 1995;75:462–469. doi: 10.1093/ptj/75.6.462. [DOI] [PubMed] [Google Scholar]
- 28.Jorstad EC, Hauer K, Becker C, Lamb SE. Measuring the psychological outcomes of falling: a systematic review. J Am Geriatr Soc. 2005;53:501–510. doi: 10.1111/j.1532-5415.2005.53172.x. [DOI] [PubMed] [Google Scholar]
- 29.Brawner CA, Ehrman JK, Schairer JR, Cao JJ, Keteyian SJ. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am Heart J. 2004;148:910–914. doi: 10.1016/j.ahj.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 31.Bean J, Kiely D, LaRose S, Leveille S. Which impairments are most associated with high mobility performance in older adults? Implications for a rehabilitation prescription. Arch Phys Med Rehabil. 2008;89:2278–2284. doi: 10.1016/j.apmr.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Bean J, Kiely D, LaRose S, O’Neill E, Goldstein R, Frontera W. Increased velocity exercise specific to task training versus the National Institute on Aging’s strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci. 2009;64:983–991. doi: 10.1093/gerona/glp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers JN, Froelicher VF. Exercise testing and prescription. Phys Med Rehabil Clin N Am. 1995;6:117–136. [Google Scholar]
- 35.Fiatarone-Singh MA. Exercise comes of age: rationale and reccomendations for a geriatric exercise prescription. J Gerontol A Biol Sci Med Sci. 2002;57:M262–M282. doi: 10.1093/gerona/57.5.m262. [DOI] [PubMed] [Google Scholar]