Abstract
H2O2 produced by extracellular NADPH oxidases regulates tyrosine kinase signaling inhibiting phosphatases. How does it cross the membrane to reach its cytosolic targets? Silencing aquaporin-8 (AQP8), but not AQP3 or AQP4, inhibited H2O2 entry into HeLa cells. Re-expression of AQP8 with silencing-resistant vectors rescued H2O2 transport, whereas a C173A-AQP8 mutant failed to do so. Lowering AQP8 levels affected H2O2 entry into the endoplasmic reticulum, but not into mitochondria. AQP8 silencing also inhibited the H2O2 spikes and phosphorylation of downstream proteins induced by epidermal growth factor. These observations lead to the hypothesis that H2O2 does not freely diffuse across the plasma membrane and AQP8 and other H2O2 transporters are potential targets for manipulating key signaling pathways in cancer and degenerative diseases. Antioxid. Redox Signal. 19, 1447–1451.
Introduction
Reactive oxygen species can be highly cytotoxic. At low concentrations, however, they are essential for cell function and survival. H2O2 inhibits phosphatases and can activate certain kinases (4). How it can diffuse in and between cells is, thus, a fundamental question in pathophysiology. Many pathways can generate H2O2: NADPH oxidases in the plasma membrane, oxidative phosphorylation in mitochondria, and oxidative protein folding in the endoplasmic reticulum (ER) (5). In all cases, H2O2 must cross a membrane to reach its cytosolic targets. H2O2 has long been thought to cross lipid bilayers freely. However, the slightly larger dipole moment of H2O2 makes its simple diffusion through membranes less likely than for water. Its size and electrochemical properties qualify H2O2 as a possible substrate of aquaporins (AQP). In fact, some members of the AQP family (8) have been shown to transport H2O2 (2, 6). In this study, we investigate the intracellular transport of H2O2 exploiting a panel of organelle-targeted ratiometric HyPer sensors (1, 3).
Innovation.
Our observations that efficient transport across the plasma and endoplasmic reticulum membranes requires AQP8, or other membrane proteins, introduce a novel level of regulation in redox signaling. Indeed, in our cell model, AQP8 silencing inhibited not only epidermal growth factor -induced entry of H2O2, but also downstream tyrosine phosphorylation of target proteins. Thus, H2O2 transporters may be potential targets in cancer and degenerative diseases.
Results
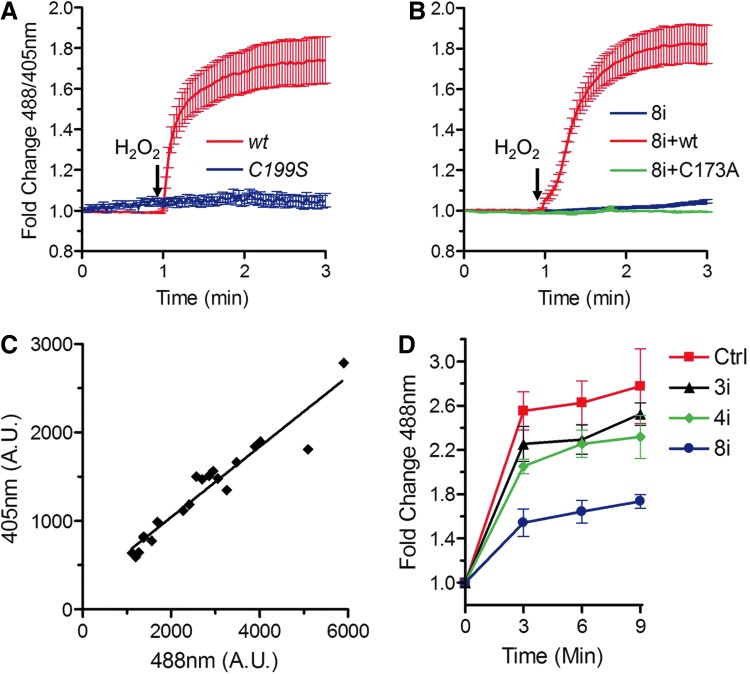
To investigate H2O2 transport across the plasma membrane, we expressed a cytosolic HyPer sensor (1) into HeLa cells. Addition of 50 μM H2O2 clearly activated the sensor, as determined by the profound 488/405 nm shifts observed in live cell imaging (Fig. 1A). A C199S HyPer mutant that has lost redox sensitivity did not shift its fluorescence emission, excluding pH-dependent events. Pretreatment with HgCl2, a compound that inhibits many membrane transporters, prevented HyPer oxidation (data not shown). Given the poor specificity of HgCl2, we performed silencing experiments targeting members of the AQP family, membrane channels involved in the transport of water and other solutes (8), including H2O2 (2, 6). Silencing AQP8 efficiently prevented H2O2 entry (Fig. 1B, blue trace), suggesting that this protein plays a major role in our cell model. Transport of H2O2 was rescued by expression of a Halo-AQP8 chimeric protein, in which the third bases of triplets 12–16 were mutated (red trace). In this way, transgene transcripts were not targeted by silencing oligonucleotides and yet encoded the same amino acids. A similarly engineered C173A-AQP8 mutant was inactive, excluding off-target effects (green trace). Having confirmed that the 488/405 nm ratio remained constant independently from the sensor expression levels (Fig. 1C), we analyzed cells at the population level using single wavelength-automated Typhoon imaging (see Notes). Measuring the increase in 488 nm fluorescence in wells containing >105 cells confirmed that AQP8 promotes H2O2 membrane transport. In contrast, silencing AQP3 or AQP4 had only marginal effects (Fig. 1D). The above experiments provided additional evidence that H2O2 cannot freely permeate through the plasma membrane and identified AQP8 as an efficient transporter.
FIG. 1.
Entry of exogenous H2O2 into HeLa cells requires AQP8. (A) Time course response of wt cytosolic HyPer (red line) or a redox-insensitive mutant (C199S, blue line) to 50 μM H2O2. (B) Kinetics of H2O2-dependent HyPerCyto activation in HeLa cells silenced for AQP8 expression (8i), and then transfected with silencing-resistant constructs driving the expression of wt (8i+wt) or mutant AQP8 (8i+C173A, see Methods for details). Graphs in A and B show mean fold change±SEM of the 488/405 nm ratio measured by confocal laser scanning. (C) The HyPer 488/405 nm ratio is not influenced by the expression levels of the probe. Transiently transfected HeLa cells were analyzed by confocal laser scanning. Despite cells being varied in the extent of probe expression, the ratio remained rather constant, as demonstrated by the linear correlation of 405 and 488 nm light excitation (r=0.92). (A.U. stands for Arbitrary Units). (D) H2O2-dependent activation of HyPerCyto was analyzed in HeLa cells treated with specific silencing oligos for AQP8, AQP4, or AQP3 by Typhoon scanning (see Methods). Data are expressed as fold increase in the 488 nm fluorescence±SEM, relative to cells that were not treated with H2O2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
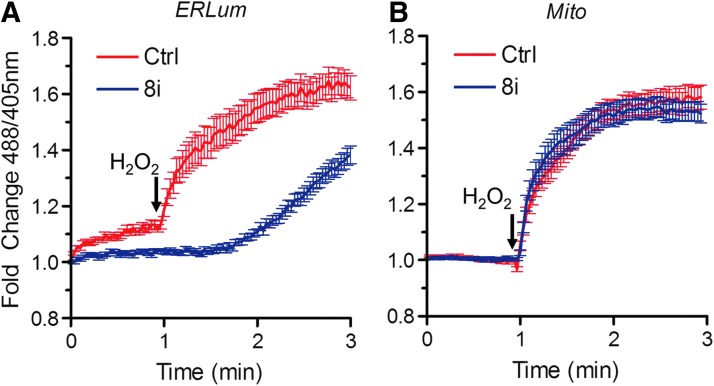
Next, we investigated whether AQP8 is needed for H2O2 transport across the mitochondria and ER membranes. Since the silencing of this protein prevented H2O2 passage across the plasma membrane (Fig. 1B), we treated cells expressing specific sensors in mitochondria or ER (HyPerMito or HyPerERlum) with digitonin, a detergent that permeabilizes the cholesterol-enriched plasma membrane without significantly affecting the integrity of intracellular membranes (7), and followed the import of added H2O2. AQP8 silencing perturbed H2O2 transport into the ER lumen, but had no significant effects on mitochondria (Fig. 2). The causes and potential physiological significance of the delayed H2O2 entry into the ER of AQP8-silenced cells are presently unclear. Taken together, the above observations suggest that AQP8 is important for H2O2 to cross the plasma and ER membranes, but dispensable for mitochondrial import.
FIG. 2.
H2O2 transport into the endoplasmic reticulum (ER) is facilitated by AQP8. HeLa cells expressing HyPerERLum (A) or HyperMito (B) were silenced with AQP8-specific (blue line) or irrelevant (red line) oligos before semipermeabilization with 40 μg/ml digitonin (7) to bypass the plasma membrane and allow access to ER and mitochondria. The kinetics of H2O2 entry into the two organelles were measured by confocal laser scanning. Graphs show mean fluorescence±SEM. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
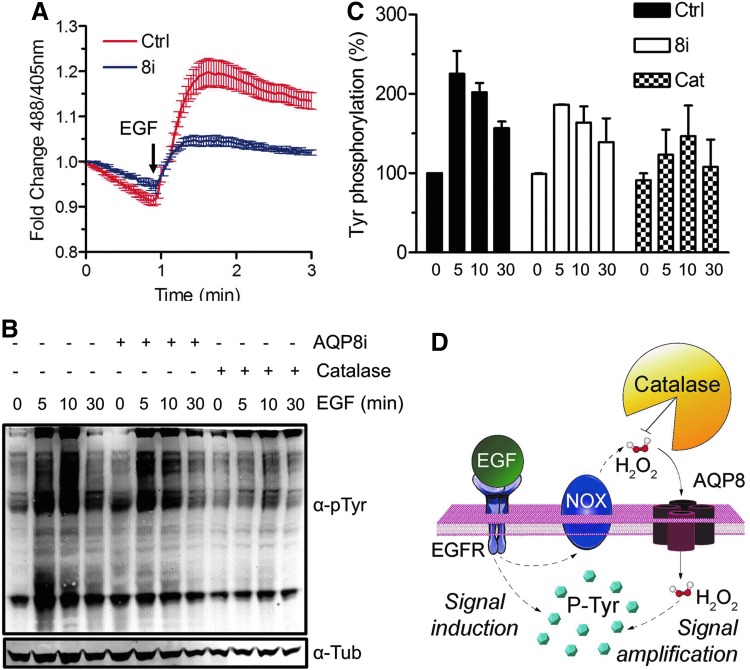
Epidermal growth factor (EGF)-induced H2O2 inhibits phosphatases through sulfenylation of their active-site cysteines, potentiating kinase signaling (4). Accordingly, EGF induced a transient spike of cytosolic H2O2 in our cells (Fig. 3A, red trace). This increase was strongly diminished in AQP8-silenced cells (blue trace), suggesting that part of the H2O2 measured derives from extracellular sources. Hence, when catalase was added to the cell medium before EGF stimulation, the intensity of bands corresponding to tyrosine-phosphorylated proteins was decreased (Fig. 3B, C). Silencing AQP8 expression diminished tyrosine phosphorylation to a level similar to that obtained with catalase. In this way, our results corroborate that, during EGF signaling, AQP8 allows entry of exogenously generated H2O2, amplifying signal transduction.
FIG. 3.
Epidermal growth factor (EGF) induces transport of H2O2 through AQP8. (A) Time course analysis of H2O2 uptake by HeLa cells expressing HyPerCyto by confocal laser scanning after treatment with 10 μg/ml EGF. Color code as in Figure 2; (B). Western blot analysis with the indicated antibodies showing changes in phosphorylation of total cell tyrosines on whole HeLa cell extracts that were either silenced for AQP8 (8i) or treated with catalase before treatment with EGF. (C) The intensity of protein phosphotyrosine signals in the blots shown in B were quantified by densitometry and normalized to Tubulin. Bars show average band intensities relative to untreated control cells±SEM. (D) A schematic view of H2O2 generation and transport during EGF signaling. NOX and DuOX enzymes on the cell surface are activated to produce H2O2. As demonstrated by the inhibitory effects of catalase, extracellularly generated H2O2 is important for efficient amplification of signaling and enters the cell through AQP8, as indicated by silencing (B, C). The residual activity in silenced cells could reflect incomplete AQP8 downregulation, the presence of additional H2O2 transporters in the plasma membrane and/or its generation in mitochondria. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Discussion
Our results indicate that H2O2 cannot freely diffuse through the plasma and ER membranes and identify AQP8 as an efficient transporter. In our HeLa cell model, entry of exogenous H2O2 is largely sustained by AQP8. The efficient rescue of H2O2 entry by AQP8 re-expression excludes off-target effects. Presumably, AQP8 directly transports H2O2; a less likely possibility is that it controls the expression and/or activity of other transporter(s). Silencing AQP3, another family member thought to mediate H2O2 transport (6), had minor effects, possibly reflecting lower expression levels in our cells. If both AQP3 and AQP8 can allow H2O2 entry into cells, a differential tissue expression of the two molecules may reflect other metabolic requirements, like glycerol or ammonia transport (8).
Since AQP8 folds in the ER, it is not surprising that downregulation of this channel impacts H2O2 transport across the membrane of this organelle. The observation that mitochondrial entry was not affected reinforces the view that no functional AQP8 resides in this organelle (9). It will be of interest to determine whether other H2O2 transporters be present in mitochondrial membranes. The above observations have important functional implications, since both the ER and mitochondria can be sources of abundant H2O2. During intense metabolic activity or hypoxia, generation of H2O2 from electron leakage in mitochondria (4) might act as a signaling rheostat. Likewise, it is conceivable that exuberant disulfide bond formation in the ER could modify cytosolic H2O2 levels with anti- or proapoptotic effects (5). The observation that devoted transporters are needed also for inter-organellar transport delineates yet another possible level at which eukaryotes can exert homeostatic control.
Elegant work from many laboratories demonstrated that H2O2 increases the strength of tyrosine kinase signaling [(4) and references therein]. AQP8 silencing strongly reduced the H2O2 fluxes observed soon upon EGF stimulation. Importantly, also, downstream tyrosine phosphorylation was inhibited. AQP8 silencing was almost as efficient as extracellular catalase, confirming that most physiologically EGF-related H2O2 entered through the AQP8 channels. Thus, the regulation of H2O2 entry could be an additional means to tune signal transduction (Fig. 3D).
Notes
Methods
Cell culture, plasmids, siRNAs, and transfection
HyPer sensors targeted to the cytosol (Cyto) or mitochondria (Mito) and the HyPerC199S mutant (5) were generous gifts of Dr. V. Belousov (IBCh, Moscow, Russian Federation), while HyPer ER luminal (ERLum) plasmid (3) was kindly provided by Drs. E. Margittai and M. Geistz (Semmelweis University, Budapest, Hungary). Human AQP8 cDNA was amplified from pCMV6AC-GFP-AQP8 (NM_001169; Origene) and cloned into HaloTag® vector pHTN (Promega). Silencing-resistant Halo-AQP8 was generated by introducing five silent point mutations in the 21 bp AQP8-siRNA target sequence, to obtain 5′ TTCGGGAACGATAAA 3′, and used to create the C173A mutant. All constructs and mutations were validated by sequencing. Reagents to silence AQP3 (5′ GGG UCGUCACUCCUUUAAUU 3′), AQP4 (5′ GAUCAGCAU CGCCAAGUCU 3′), and AQP8 (5′ UUUGGCAAUGACA AGGCCA 3′), and an unrelated control (Block-it™) were purchased from Ambion (Life Technologies).
Two or 10×104 HeLa cells were grown overnight in 24- or 6-well plates, transfected with 18 or 90 pmol of siRNA, respectively, using RNAiMAX lipofectamin (Life Technologies) and analyzed after 72 h. Transient transfections were performed by Polyethylenimine as described (7).
Imaging HyPer-oxidation
Cells were equilibrated in Ringer buffer (140 mM NaCl, 2 mM CaCl2, 1 mM MgSO4, 1.5 mM K2HPO4, 10 mM glucose, pH 7.3) for 15 min at RT and images taken using a Typhoon FLA 9000 fluorescence scanner (GE Healthcare) equipped with a 488 nm excitation filter 0, 3, 6, or 9 min after treatment with or without 50 μM H2O2 (Sigma-Aldrich). Untransfected HeLa cells were used to calibrate laser sensitivity. Images were analyzed using ImageJ and results presented as fold change increase in 488 nm intensity relative to control cells.
HeLa transfectants were seeded on glass coverslips. Forty-eight hours after transfection, coverslips were equilibrated in Ringer buffer for 10 min at RT before H2O2 exposure or stimulation with 10 μg/ml of EGF (Sigma-Aldrich) at 37°C. Confocal images were collected every 2 s for 3 min by excitation with 488 nm argon and 405 nm violet diode lasers on an Ultraview confocal laser scanning microscope using a 40× oil-immersion lens (Perkin Elmer). The 488/405 nm ratios were calculated by ImageJ software in triplicate wells, always averaging in each ≥10 cells, and plotted against time. HaloAQP8-expressing cells were identified by labeling with 5 μM HaloTag TMR Ligand (Promega) and analyzed at 532 nm.
To measure intracellular H2O2 fluxes, transfectants expressing HyPerMito or HyPerERLum were treated and silenced as above. Coverslips were placed in a live cell chamber at RT, equilibrated in Ringer Buffer for 5 min, and then treated for 5 min with 40 μg/ml of digitonin (Sigma-Aldrich). The integrity of the ER and mitochondrial membranes was assessed as described previously (7).
Antibodies and western blotting
Mouse anti-PhosphoTyrosine (4G10), mouse anti-tubulin, goat anti-mouse IgG-HRP, or AlexaFluor-647 were from Millipore, Sigma-Aldrich, Zymed, and Invitrogen, respectively. Images were acquired by Chemidoc-it Imaging System (UVP) or Typhoon FLA 9000, processed with ImageJ and densitometrically quantified by ImageQuant 5.2 (Molecular Dynamic).
To follow EGF responses biochemically, subconfluent HeLa cells were cultured in the FCS-free DMEM for 3 h before incubation with or without 5000 U/ml of catalase and EGF addition. Cells were then washed with ice-cold PBS containing 0.4 mM Na3VO4 to block phosphatases, lysed in sample buffer and analyzed by electrophoresis and western blot.
Further details will be made available upon request (milena.bertolotti@hsr.it)
Abbreviations Used
- AQP
aquaporins
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- SEM
standard error of the mean
Acknowledgments
We apologize to several colleagues whose seminal work could not be cited due space limitations. We thank Drs. V. Belousov, E. Margittai, M. Geistz, T. Dick, and M. Toledano for providing reagents and/or advice, Claudio Fagioli for technical help, and Cesare Covino and colleagues at the ALEMBIC facility of University Vita-Salute San Raffaele Scientific Institute (Milan) for assistance in imaging. This work was supported by grants from Associazione Italiana Ricerca Cancro (AIRC; IG and 5×1000 programs), Ministero della Salute, Regione Lombardia (ASTIL), and Telethon (GGP11077). The authors declare no conflict of interests.
References
- 1.Belousov VV. Fradkov AF. Lukyanov KA. Staroverov DB. Shakhbazov KS. Terskikh AV. Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Meth. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 2.Bienert GP. Moller AL. Kristiansen KA. Schulz A. Moller IM. Schjoerring JK. Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 3.Enyedi B. Varnai P. Geiszt M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid Redox Signal. 2010;13:721–729. doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- 4.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakihana T. Nagata K. Sitia R. Peroxides and peroxidases in the endoplasmic reticulum: integrating redox homeostasis and oxidative folding. Antioxid Redox Signal. 2012;16:763–771. doi: 10.1089/ars.2011.4238. [DOI] [PubMed] [Google Scholar]
- 6.Miller EW. Dickinson BC. Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nerini-Molteni SN. Fassio A. Ciriolo MR. Filomeni G. Pasqualetto E. Fagioli C. Sitia R. Glutathione limits Ero1-dependent oxidation in the endoplasmic reticulum. J Biol Chem. 2004;279:32667–32673. doi: 10.1074/jbc.M404992200. [DOI] [PubMed] [Google Scholar]
- 8.Verkman AS. Aquaporins. Curr Biol. 2013;23:R52–R55. doi: 10.1016/j.cub.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B. Zhao D. Verkman AS. Evidence against functionally significant aquaporin expression in mitochondria. J Biol Chem. 2006;281:16202–16206. doi: 10.1074/jbc.M601864200. [DOI] [PubMed] [Google Scholar]