Abstract
Aims: The mitochondrial respiratory chain is recognized today to be arranged in supramolecular assemblies (supercomplexes). Besides conferring a kinetic advantage (substrate channeling) and being required for the assembly and stability of Complex I, indirect considerations support the view that supercomplexes may also prevent excessive formation of reactive oxygen species (ROS) from the respiratory chain. In the present study, we have directly addressed this issue by testing the ROS generation by Complex I in two experimental systems in which the supramolecular organization of the respiratory assemblies is impaired by: (i) treatment either of bovine heart mitochondria or liposome-reconstituted supercomplex I-III with dodecyl maltoside; (ii) reconstitution of Complexes I and III at high phospholipids to protein ratio. Results: The results of our investigation provide experimental evidence that the production of ROS is strongly increased in either model, supporting the view that disruption or prevention of the association between Complex I and Complex III by different means enhances the generation of superoxide from Complex I. Innovation: Dissociation of supercomplexes may link oxidative stress and energy failure in a vicious circle. Conclusion: Our findings support a central role of mitochondrial supramolecular structure in the development of the aging process and in the etiology and pathogenesis of most major chronic diseases. Antioxid. Redox Signal. 19, 1469–1480.
Introduction
Evidence accumulated in the last ten years has demonstrated that a large proportion of the mitochondrial respiratory chain complexes in a variety of organisms is arranged in supramolecular assemblies called supercomplexes or respirasomes (48, 51, 66, 70). Supercomplex association is not just a mere structural feature but has deep functional implications on the properties of the respiratory chain.
First of all, supercomplex association confers a kinetic advantage by maximizing vicinity between consecutive complexes and thus facilitating the electron transfer through partner redox components (substrate channeling) more efficiently than any process based on diffusion and random collision of the acting partners (2, 8). Available evidence arguing in favor or against substrate channeling has been critically reviewed in previous articles by the authors (42, 45). In brief, reconstitution studies (37) showed that electron transfer between Complexes I and III is possible by both diffusion of the ubiquinone pool (cf. 41) and direct transfer (substrate channeling) by contact of the complexes, depending on their supramolecular organization as individual enzymes embedded in the membrane or as supercomplex I-III, respectively. Most evidence points out that substrate channeling takes place in the ubiquinone region, that is the one we have explored in this study, at least in State 3 or uncoupled conditions, whereas random diffusion might be operative in State 4 mitochondrial respiration (cf. 56). The few studies dealing with biological systems also appear to indicate that disruption of respiratory supercomplex association is accompanied by a pronounced decrease of integrated electron transfer in spite of normal activity of the individual complexes (cf. 64, 77). Three-dimensional (3D) models of the structure of the mitochondrial respirasome were determined by single particle electron microscopy and indicated the possible pathway along which the small electron carriers ubiquinone (CoQ) and cytochrome c travel to shuttle electrons through the supercomplex (3, 22). However, particular uncertainty exists concerning cytochrome c channeling because experimentation about the functional role of supercomplexes is still scant. Kinetic evidence in favor of Complex III and Complex IV forming a functional supercomplex is mostly obtained in yeast, whereas many conclusions for cytochrome c channeling in mammalian mitochondria are based on assumptions driven by the observation that a fraction of molecules appears physically associated in the respirasome. On the contrary, some studies by metabolic flux control (8) and time-resolved analysis of the respiratory chain (76) have questioned the functional role of mammalian supercomplexes in the transfer of electrons to Complex IV and supported the model of random collisions of free diffusing molecules of cytochrome c despite the presence of Complex IV units bound in the respirasome.
In addition, several studies demonstrated that supercomplex association is required for the stability and assembly of the individual complexes, in particular Complex I, as shown mainly by the effect of mutations in Complex III and Complex IV on the structure and activity of Complex I (1, 17, 21, 69, 74).
Innovation.
This is the first demonstration that dissociation of the supercomplex I1III2 in the mitochondrial membrane is a cause of oxidative stress from Complex I. We have previously demonstrated that lipid peroxidation can dissociate the supramolecular assemblies (27); thus, here we confirm our conclusion that primary causes of oxidative stress may perpetuate reactive oxygen species (ROS) generation by a vicious circle involving supercomplex dissociation as a major determinant. It is easy to foresee the implications of these findings in human diseases and in aging, where oxidative stress plays a major etiologic and pathogenic role. Moreover, we can comment that the postulated dynamic structure of supercomplexes (2) and the modulation of their association may represent a physiological means to control the production of ROS as redox signals from mitochondria to the cell.
The forces responsible for supercomplex association appear to strongly depend on the lipid content and composition of the inner mitochondrial membrane (45, 50, 54, 79); in particular previous studies from our laboratory have shown that reconstitution of a binary Complex I/Complex III mitochondrial protein fraction at a high lipid to protein ratio prevents formation of the supercomplex I1III2; moreover, peroxidation of the lipids prior to reconstitution prevents the formation of the supercomplex even under the optimal condition of low lipid to protein ratio (27). This finding may have important pathological implications (29), since in several disease conditions characterized by oxidative stress the normal supercomplex association was found to be lost.
Different sources of reactive oxygen species (ROS) that may be of importance in signal transduction and in pathological conditions exist in the cell, and many evidences demonstrate the relevance of the mitochondrial respiratory chain to the production of ROS (40, 42, 60); the main redox components responsible for univalent oxygen reduction to superoxide are situated in Complex I and in Complex III. In particular, conditions limiting the electron flow within Complex I induce large excess of superoxide production. This may take place in physiological conditions [Complex I dephosphorylated (59)] or in pathological conditions in which Complex I is affected [for example, in genetic diseases such as Leber Hereditary Optic Neuropathy (44), in Parkinson's disease (24), etc.] or as a consequence of the action of selective Complex I inhibitors (such as rotenone, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, etc.) (23). The site of electron escape to oxygen is controversial and either FMNH2 or ubisemiquinone or iron sulfur cluster N2 have been suggested as the possible sites (23, 26, 30, 52, 55).
Since dissociation of the supercomplex I1III2 limits electron transfer between Complex I and III and in addition destabilizes Complex I, a reasonable question is whether such condition is also bound to enhance and/or modify the normal ROS production from the Complex I.
Indirect circumstantial evidence suggests that supercomplex assembly may limit the extent of superoxide generation by the respiratory chain. Panov et al. (53) reason that respirasome helps in maintaining redox components of the complexes in the oxidized state through the facilitation of electron flow by channeling, thus limiting ROS formation. Similarly, Seelert et al. (72) also suggest that facilitation of electron transfer by channeling may limit the detrimental generation of ROS.
The controversial results on the site of electron escape to oxygen to form superoxide obtained from different laboratories working either on isolated Complex I or on mitochondrial membranes (cf. above) generally indicate that N2 as a source of ROS would be predominant in membrane particles (23, 30), whereas FMN might become available after Complex I isolation (26, 55). A reasonable hypothesis is that FMN becomes exposed to oxygen only when Complex I is dissociated from Complex III (48), which can be the case of both purified Complex I and individual enzyme particles embedded in the membrane as occurring in vitro or under specific in vivo conditions due to supercomplex disassembling (2, 46).
Following this line of thought we have decided to directly investigate ROS production by Complex I under conditions in which the complex is arranged as a component of the supercomplex I1III2 or it is dissociated as an individual enzyme. The study has been conducted both in bovine heart mitochondrial membranes and in reconstituted proteoliposomes composed of complexes I and III. The results of this investigation support the view that disruption or prevention of the association between Complex I and Complex III by different means enhances the generation of ROS from Complex I.
Results
Effects of supercomplex dissociation in mitochondrial membranes
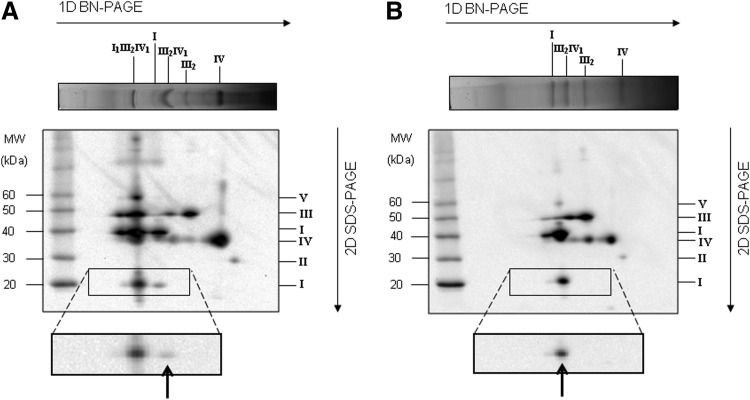
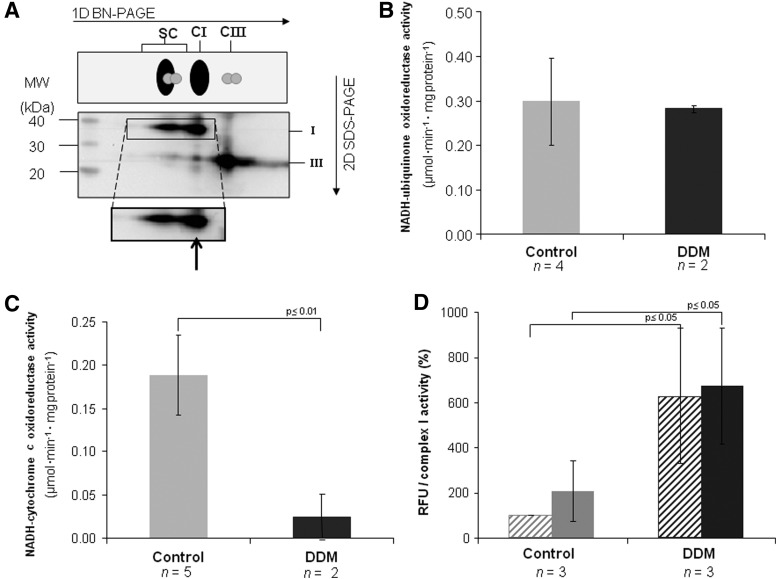
It is known that detergent treatment with dodecyl-β-D-maltoside (DDM) dissociates supercomplexes into individual complexes (67). We have exploited DDM treatment in bovine heart mitochondria (BHM) confirming the complete dissociation of Complex I from the other respiratory complexes (free CI=98%) (Fig. 1 and Table 1).
FIG. 1.
Supercomplex disassembling in bovine heart mitochondria (BHM). Respiratory supercomplexes and complexes from (A) digitonin-solubilized BHM with a detergent to protein ratio (w:w) of 8 and from (B) DDM-solubilized BHM with a detergent to protein ratio (w:w) of 2.6 were resolved by western blotting after 2D-BN/SDS-PAGE. Arrows point to monomeric Complex I. Monoclonal antibodies specific for single subunits of each OXPHOS complex are as follows: NDUFB8 (20 kDa) and NDUFA9 (39 kDa) of Complex I, SDHB (30 kDa) of Complex II, Core protein 2 (47 kDa) of Complex III, and COX-I (57 kDa, apparent 35 kDa) of Complex IV and alfa subunit (53 kDa) of ATP synthase. The images shown in the picture were obtained in camera by double overlaying exposures (not postproduction computer-graphic overlay) to the antibody against the NDUFA9-subunit and, in sequence, to a mixture of the remaining antibodies listed above. DDM, dodecyl-β-D-maltoside; OXPHOS, oxidative phosphorylation system.
Table 1.
Production of Reactive Oxygen Species by Mitochondrial Complex I in Different Situations Where Supercomplexes Are Maintained or Disassembled
| ROS production (%) | Relative amount of Complex I (free/total) | |
|---|---|---|
| R4B 1:1 | 224±83a [6] | 0.08±0.07 [3] |
| R4B 1:1+DDM 4.3 g/g | 672±257a** [3] | 0.79±0.08* [3] |
| R4B 1:30 | 851±243a** [3] | 0.76±0.03* [2] |
| SMP | 223±56b [5] | 0.23 [1] |
| SMP+0.2 M KSCN | 662±120b§ [5] | 0.39 [1] |
| BHM | 121±20c [5] | 0.13±0.07 [3] |
| BHM+DDM 2.6 g/g | 223±86c## [5] | 0.98±0.02# [3] |
The samples were assayed in the presence of 1.8 μM mucidin and 4 μM rotenone as described in the section “Materials and Methods.” The production of ROS is expressed as the relative fluorescence intensity of dichlorofluorescein normalized against Complex I activity; data are shown as percentage values of the corresponding reference sample (aR4B1:1; bSMP; and cBHM respectively assayed in the presence of 1.8 μM mucidin only). The ratio of free Complex I versus total Complex I was determined by densitometric analysis of immunoblots obtained after 2D BN/SDS-PAGE as described in the section “Materials and Methods.” Values in square brackets indicate the number of independent samples. Numbers followed by asterisks are significantly different from their respective control (*p<0.001 and **p<0.005 vs. R4B 1:1; #p<0.001 and ##p<0.05 vs. BHM; §p<0.001 vs. SMP) according to the Student's t-test.
KSCN, potassium thiocyanate; BHM, bovine heart mitochondria; DDM, dodecyl-β-D-maltoside; ROS, reactive oxygen species; R4B, mitochondrial fraction enriched in Complex I and Complex III; SDS, sodium dodecyl sulphate; SMP, submitochondrial particles.
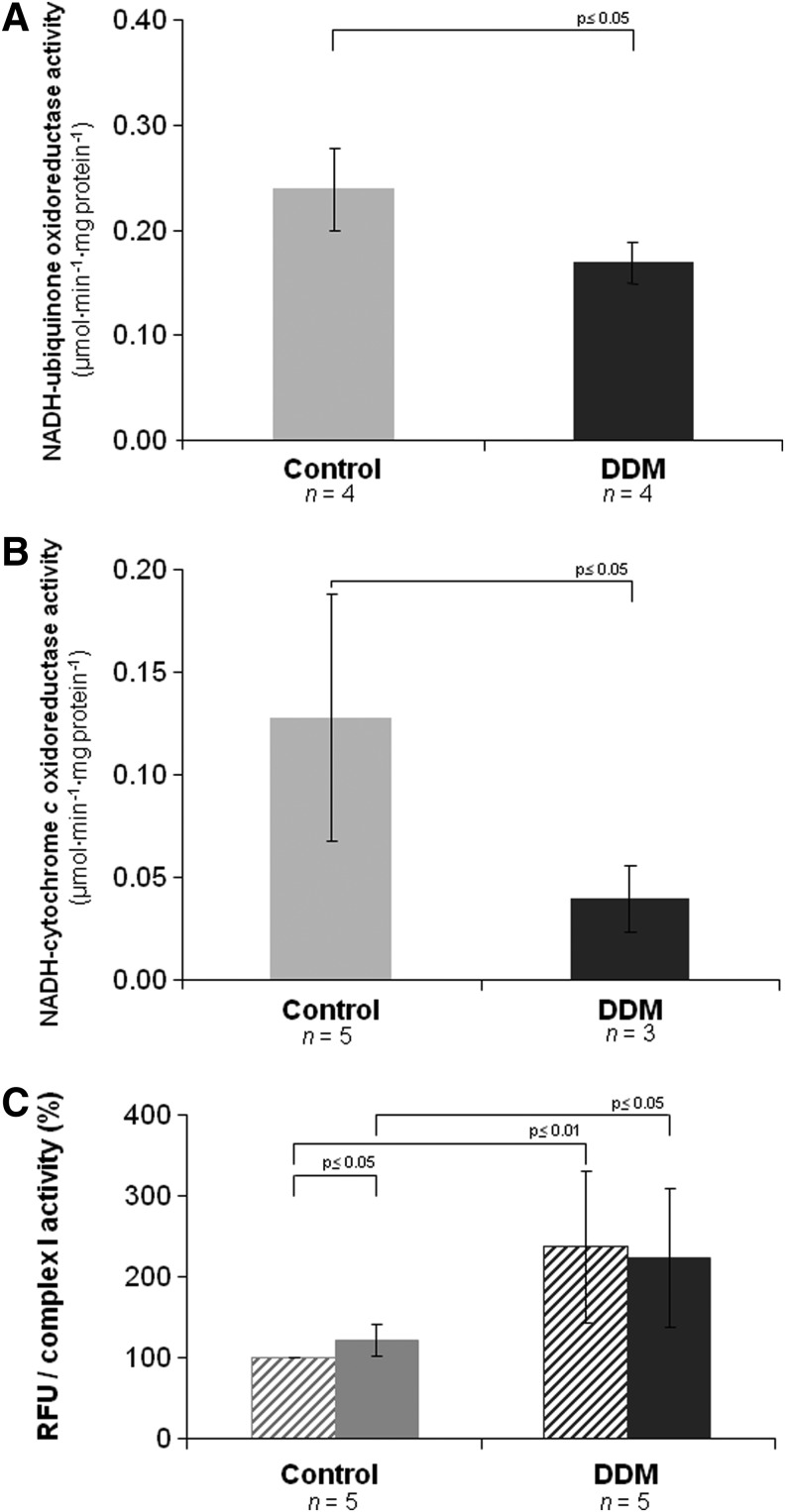
As shown in Figure 2A, Complex I activity was decreased by 30% in the detergent-treated sample, indicating only a marginal effect on the activity of the enzyme, whereas the activity of NADH-cytochrome c reductase, comprising the integrated function of complexes I and III, was decreased by 70% indicating a less efficient electron transfer between the two enzymes (Fig. 2B). The antimycin A-insensitive residual rates of NADH-cytochrome c activity were <10% of the total rates shown in the figure. On the other hand, oxygen uptake was almost completely lost after addition of DDM, probably due to the removal of endogenous cytochrome c (not shown).
FIG. 2.
Functional analysis of supercomplex I1III2 and Complex I in detergent-solubilized BHM. Detergent dependence of (A) NADH-ubiquinone oxidoreductase activity and (B) NADH-cytochrome c oxidoreductase activity. Activity rates are expressed in μmoles of NADH·min−1·mg protein−1. (C) The production of hydrogen peroxide was measured in the presence of 1.8 μM mucidin (dashed bars) and 1.8 μM mucidin plus 4 μM Rotenone (solid bars). Values are expressed as relative fluorescence units (RFU) by DCF normalized against Complex I activity and shown as percentage values of the reference sample (i.e., control BHM in the presence of mucidin only). The corresponding percentage values of RFU·mg protein−1 in the presence of mucidin plus rotenone are 121±20 and 158±61, respectively in control and in DDM-treated samples. DDM sample treated with 2.6 g dodecylmaltoside/g of protein; n indicates the number of independent samples. Data are given as mean±S.D. p-values were calculated using the Student's t-test. DCF, 2′,7′-dichlorofluorescein.
After DDM addition, the production of ROS detected by the 2′,7′-dichlorofluorescein (DCF) assay is enhanced (Fig. 2C and Table 1). Data were expressed against Complex I activity to normalize the NADH-dependent leakage of electrons by Complex I to the total number of electrons flowing through the fraction of catalytically active molecules of the enzyme in the control and in the detergent-treated mitochondria. The assays were performed both in presence of 1.8 μM mucidin plus 4 μM rotenone and of mucidin only; previous studies from our laboratory had shown that under these conditions ROS production by Complex III is prevented and the addition of rotenone enhances generation of superoxide by Complex I that represents the only source of ROS (cf. 23). Consequently, we can consider that mucidin functionally isolates Complex I from downstream segments of the respiratory chain. Thus, samples pretreated only with 1.8 μM mucidin were used as control for all assays (23, 30). However, it is worth mentioning that in our DDM-treated samples rotenone had no further effect over the conspicuous increase of ROS production above the nontreated sample (Fig. 2C).
Another mean shown to disrupt supercomplex association is addition of chaotropic agents. Boumans et al. (10) found loss of pool behavior for CoQ and cytochrome c in Saccharomyces cerevisiae mitochondria treated with trichloroacetic acid (TCA), suggesting dissociation of a supramolecular unit in which succinate dehydrogenase and cytochrome c oxidase physically interacted with the bc1 complex. In our studies, we have also employed either 100 mM TCA (data not shown) or 0.2 M KSCN (Table 1) to weaken hydrophobic interactions inside the supercomplex I-III-IV in submitochondrial particles from BHM, but the treated samples showed incomplete disassembling of the supercomplex and partial loss of Complex I activity; therefore, the results were difficult to interpret; nevertheless, under these experimental conditions we can observe enhanced ROS generation (Table 1).
Effects of supercomplex dissociation in the reconstituted supercomplex I1III2
A mitochondrial fraction enriched in Complex I and Complex III (R4B) was reconstituted with different amounts of phospholipids (PLs) and CoQ10. Our previous results (cf. 47, 43) demonstrated that the experimentally determined NADH-cytochrome c oxidoreductase activity overlaps with the values expected by theoretical calculation applying the pool equation of Kröger and Klingenberg (41) at 1:30 (w:w) PL dilution, whereas at 1:1 (w:w) ratio pool behavior was not effective any more. The proteoliposomes were open vesicles not retaining respiratory control and permeable to substrates (NADH and cytochrome c). In fact, neither the addition of a chemical uncoupler of oxidative phosphorylation (carbonyl cyanide p-trifluoromethoxy-phenyl hydrazone [FCCP]) did change the activity rates nor the addition of progressive amounts of detergent (cholate and DDM) to the vesicles did enhance NADH oxidation or cytochrome c reduction (data not shown), indicating that we were observing the maximal possible rates of the system in the absence of substrate compartmentalization constraints; a threshold additional detergent started to inhibit activity. In the two experimental models of reconstitution, kinetic testing according to the metabolic flux control analysis validated the hypothesis of a random organization in the 1:30-model and of a functional association between Complex I and Complex III in the 1:1-model (27).
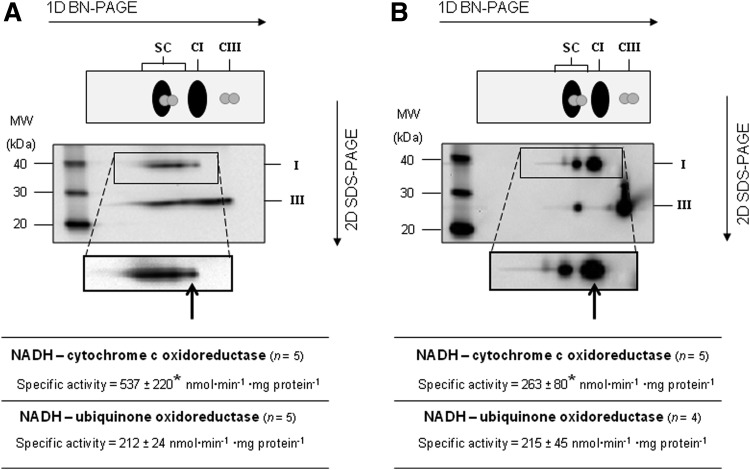
Structural analysis performed in the present study by Blue-Native polyacrylamide gel electrophoresis (BN-PAGE) confirms that the fraction having high protein to lipid ratio is strongly enriched in the supercomplex I1III2, whereas the relative amount of bound complexes compared to their free form appears drastically diminished in the fraction having high PL content (i.e., Complex I free/total=0.08 vs. 0.76, respectively in the 1:1 and 1:30 samples), as suggested by densitometric results for selected paired spots in the gel patterns (Fig. 3 and Table 1).
FIG. 3.
Supramolecular organization of respiratory Complex I and Complex III in R4B 1:1 and R4B 1:30 proteoliposomes. (A) R4B 1:1 and (B) R4B 1:30 samples were separated by 2D BN/SDS-PAGE after solubilization with digitonin at a detergent to protein ratio of 8 (w:w) and resolved by western blotting followed by immunodetection using monoclonal antibodies specific for single respiratory subunits. The images shown in the picture were obtained in camera by double overlaying exposures (not postproduction computer-graphic overlay) to the antibody against the NDUFA9 (39 kDa) subunit of Complex I and, in sequence, to the antibody against the Rieske protein (22 kDa) of Complex III. Arrows point to monomeric Complex I. The upper panel of the figures schematically shows the position of supercomplex I1III2 (SC), Complex I (CI) and dimeric Complex III (CIII) in the 1D BN-gel prior to second dimension electrophoresis (2D SDS-PAGE). NADH-cytochrome c and NADH-ubiquinone oxidoreductase activities are expressed in nmoles of NADH·min−1·mg protein−1. n indicates the number of independent samples. Data are given as mean±S.D. Numbers followed by asterisks are significantly different according to the Student's t-test (*p<0.05). R4B, mitochondrial fraction enriched in Complex I and Complex III.
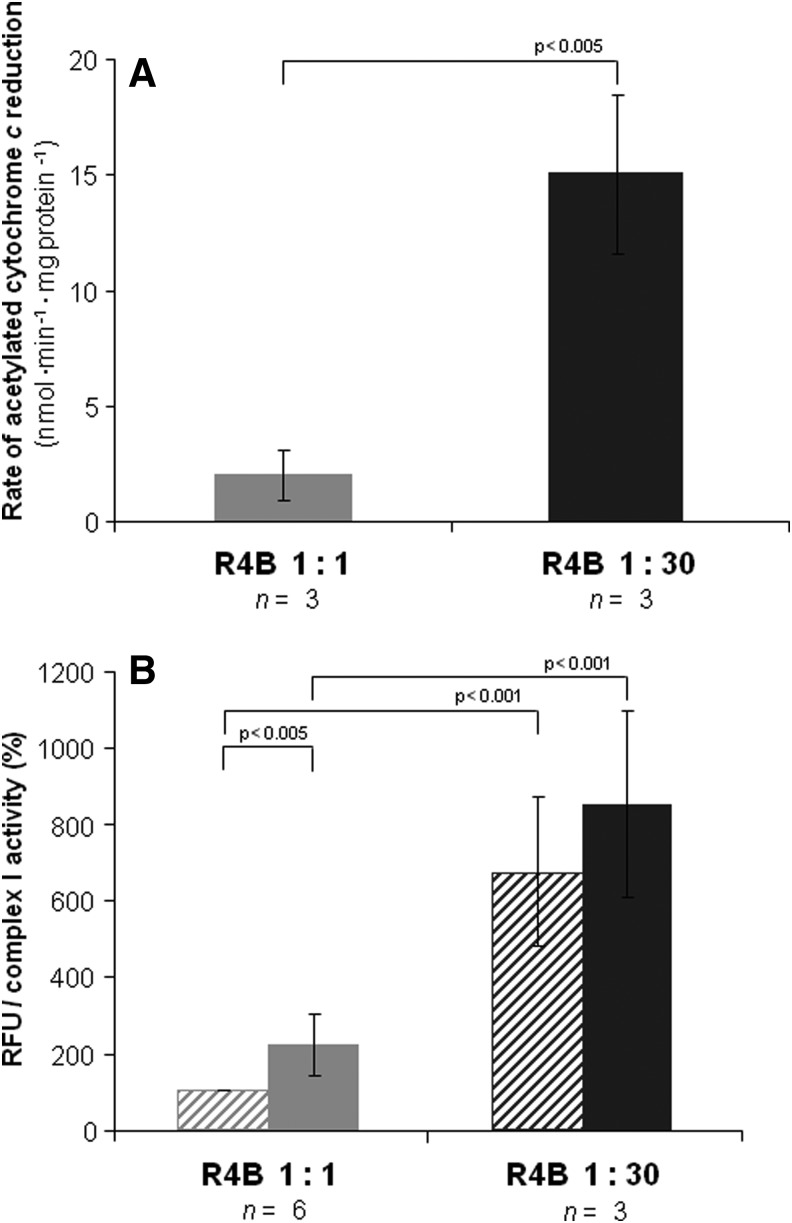
We also confirmed that the Complex I activity was the same at both PL dilutions, whereas the integrated activity of NADH cytochrome c reductase was about half in the 1:30 sample with respect to the 1:1 sample (Fig. 3). The production of ROS was evaluated both by the superoxide dismutase (SOD)-sensitive reduction of acetylated cytochrome c for the detection of superoxide and by the DCF assay; both assays showed a dramatic increase of ROS production in the R4B 1:30 with respect to the R4B 1:1 sample (Fig. 4 and Table 1). The fraction of SOD-insensitive NADH-acetylated cytochrome c reductase activity that may possibly due to direct, not superoxide-mediated reduction of acetylated cytochrome c in the R4B 1:30 sample is low (<12%), showing unchanged absolute values compared to the SOD-insensitive activity in the 1:1 proteoliposomes (1.7±1.0 and 1.9±1.7 nmol·min−1·mg protein−1, respectively in the 1:30 vs. 1:1 samples).
FIG. 4.
ROS production mediated by Complex I in R4B 1:1 and R4B 1:30 proteoliposomes. (A) The rate of superoxide formation is determined as the superoxide dismutase (SOD)-sensitive rate of acetylated cytochrome c reduction in the presence of 1.8 μM mucidin and 4 μM rotenone. The corresponding rates of SOD-insensitive activity are: 1.9±1.7 and 1.7±1.0 nmol·min−1·mg protein−1, respectively in the 1:1 versus 1:30 samples. (B) The production of hydrogen peroxide was measured in the presence of 1.8 μM mucidin (dashed bars) and 1.8 μM mucidin plus 4 μM Rotenone (solid bars). Values are expressed as relative fluorescence units (RFU) by DCF normalized against Complex I activity and shown as percentage values of the reference sample (i.e., R4B 1:1 in the presence of mucidin only). n indicates the number of independent samples. Data are given as mean±S.D. p-values were calculated using the Student's t-test. ROS, reactive oxygen species.
A further proof of our interpretation of the higher values of ROS production due to dissociation of the supercomplex I1III2 was given by the effect of the DDM treatment in the R4B 1:1 sample (Fig. 5A and Table 1). The ratio of free to total Complex I increased to 0.79 after detergent addition, compared to a reference value of 0.08 in the control; conversely, the integrated NADH-cytochrome c reductase activity dramatically decreased to less than 15% of the control, while Complex I activity was not altered (Fig. 5C, B). Again, ROS production was increased more than threefold when the 1:1 proteoliposomes were treated with DDM (Fig. 5D and Table 1).
FIG. 5.
Disassembling of supercomplex I1III2 in R4B1:1 proteoliposomes after detergent solubilization. (A) Respiratory complexes were separated by 2D BN/SDS-PAGE after solubilization with DDM with a detergent to protein ratio (w:w) of 4.3 and resolved by western blotting using monoclonal antibodies specific for single respiratory subunits. The images shown in the picture were obtained in camera by double overlaying exposures (not postproduction computer-graphic overlay) to the antibody against the NDUFA9 (39 kDa) subunit of Complex I and, in sequence, to the antibody against the Rieske protein (22 kDa) of Complex III. The arrow points to monomeric Complex I. The upper panel of the figure schematically shows the position of supercomplex I1III2 (SC), Complex I (CI), and dimeric Complex III (CIII) in the 1D BN-gel prior to second dimension electrophoresis (2D SDS-PAGE). The detergent dependence of (B) NADH-ubiquinone oxidoreductase and (C) NADH-cytochrome c oxidoreductase activities in the DDM-treated samples was estimated by comparison to the corresponding values in R4B 1:1 samples without DDM (control). (D) The production of hydrogen peroxide was measured in the presence of 1.8 μM mucidin (dashed bars) and 1.8 μM mucidin plus 4 μM Rotenone (solid bars). Values are expressed as relative fluorescence units (RFU) by DCF normalized against Complex I activity and shown as percentage values of the reference sample (i.e., control R4B 1:1 in the presence of mucidin only). DDM sample treated with 4.3 g dodecylmaltoside/g of protein. NADH-ubiquinone oxidoreductase and NADH-cytochrome c oxidoreductase activities are expressed in μmoles of NADH·min−1·mg protein−1. n shows the number of independent samples. Data are given as mean±S.D. p-values were calculated using the Student's t-test.
Discussion
Disassembling of respiratory supercomplexes has several functional consequences on mitochondrial respiration. The most striking and obvious consequence is loss of enzymatic channeling. We have shown this effect in the CoQ region by demonstrating that the integrated activity of NADH-cytochrome c oxidoreductase is decreased when Complex I and Complex III are present as individual enzymatic entities; in that case the kinetics approaches the values predicted by CoQ pool behavior (7, 41), that is, according to the random collision model (34). The decreased overall activity is not merely the result of enzyme damage, since the individual activities of Complex I and Complex III can be not affected or only marginally decreased (cf. 7).
Another functional consequence previously described from disruption of the supercomplex (SC) core structure I1III2 is loss of stability of Complex I, as appeared from dissociation of Complex I under the conditions of BN-PAGE and loss of NADH-ubiquinone oxidoreductase activity in cell models lacking the respiratory supercomplexes (1, 17, 21, 69, 74). However, this was not observed in our present study, since the activity of Complex I does not appear to be dramatically decreased upon SC dissociation, and free Complex I stably appears in the BN-gels. Indeed, Complex I upon isolation and purification was shown to be sufficiently stable to allow high turnover number (35). Nevertheless, in this free form it becomes more susceptible to free radical attack and loses stability (20).
The possibility that SC assembly prevents excessive ROS generation from Complex I has been advanced on theoretical grounds (49, 53, 72) but no direct experimental study has been yet addressed to this issue. Indeed, in a complex biological system it is difficult to ascertain a clear relationship of cause-effect between SC alteration and ROS production since the two phenomena may influence each other. On the contrary, in this study we induce a primary condition in the samples by means able to enhance free Complex I with respect to SC-bound Complex I, and we obtain the first direct demonstration that loss of supercomplex organization causes an enhancement of ROS generation by Complex I itself. This demonstration is clearly evident (Fig. 6) both in reconstituted proteoliposomes, where the dissociation of Complex I from the respirasome is accompanied by a three/four-fold increase of ROS generation, and in mitochondrial membranes. In the latter case, the existence of endogenous systems operating to reduce ROS levels in the mitochondrial sample (i.e., mitochondrial gluthatione peroxidase, Mn-SOD, and nonenzymatic endogenous antioxidants) might have counteracted the dramatic effects of the complete dissociation of Complex I, thus leading only to a two-fold increase of the measured ROS production.
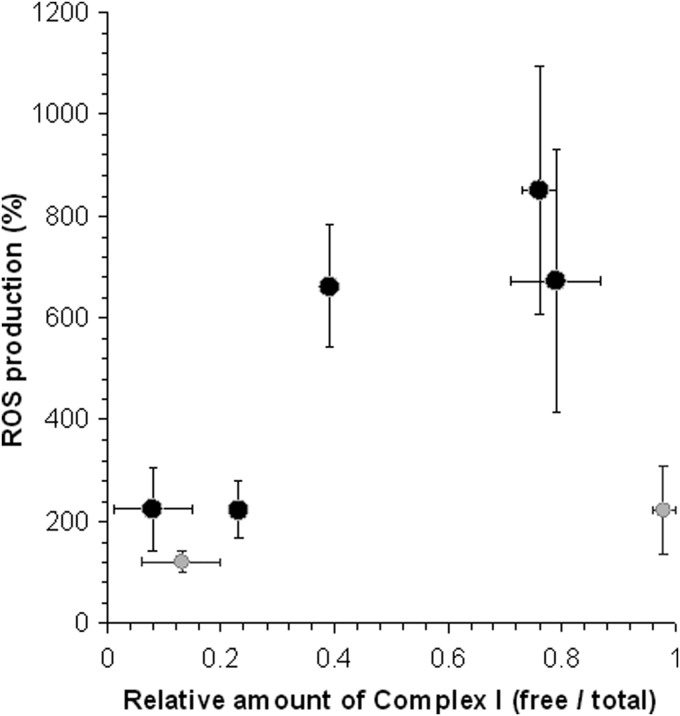
FIG. 6.
Production of ROS by mitochondrial Complex I in different situations where supercomplexes are maintained or disassembled. The percent value of ROS production measured in all the samples listed in Table 1 is plotted in the graph against the corresponding ratio of free Complex I versus total Complex I. The statistical analysis of the data using the Pearson's parametric test indicates a positive correlation (r=0.884, p<0.05) between ROS generation and relative amount of free Complex I in the R4B and in the SMP samples (black symbols). The BHM samples (gray symbols) were not included in the correlation analysis because the existence of endogenous antioxidant systems operating to reduce ROS levels in the mitochondria might have counteracted the dramatic effects of the complete dissociation of Complex I, thus leading to a two-fold only increase of the measured ROS production. SMP, submitochondrial particles.
The ROS increase does not appear to be the mere result of Complex I damage by the treatment of the samples in the presence of detergent, since the NADH-ubiquinone redox activity is slightly depressed, though Complex I is mostly in its free form, whereas the integrated respiration is dramatically decreased. Other means, such as chaotropic agents, were previously found to dissociate the SC in yeast mitochondria (10). However, we found that SCN− was only partially effective in bovine mitochondrial membranes and induced some damage to Complex I; nevertheless, we have seen that the increase in the amount of free Complex I is accompanied by enhanced levels of ROS (Fig. 6).
A further demonstration of the enhanced ROS generation due to SC disruption was obtained in a model system of reconstituted binary Complex I/Complex III at high lipid to protein ratio (30:1) where formation of the supercomplex I1III2 is prevented; likewise, the generation of superoxide is several fold higher than in a R4B system reconstituted at a 1:1 ratio, which is rich in SC. In agreement with this finding, dissociation with DDM of the SC present in R4B 1:1 induces a strong enhancement of ROS generation (Fig. 6). Further results from our laboratory suggest that a similar increase of ROS production in R4B 1:1 can be also observed when SC dissociation is induced by treatment with the chaotropic agent SCN− (data not shown).
In conclusion, as we summarize in Table 1 and in Figure 6, our results emphasize that when we induce the dissociation of the respiratory supercomplexes, no matter how, we observe enhanced ROS production by Complex I. However, the hypothetical reasoning that facilitation of electron flow by substrate channeling within the respirasome helps maintaining the redox components of the complexes in the oxidized state, thus limiting ROS formation, cannot be the only explanation. In fact, in the experiments reported in this study, ROS production is investigated in the presence of inhibitors (mucidin and rotenone) that prevent electron transfer to any possible acceptor, so that we can guess that the redox centers in Complex I are maximally reduced both in the situations where supercomplexes are maintained and in the situations where Complex I is free. Two potential sites for oxygen reduction exist in Complex I, represented by FMN and iron-sulfur cluster N2, which might distinctly prevail in generating ROS under different conditions. N2 would be a predominant source of ROS in membrane particles containing super-assembled Complex I whereas FMN would become exposed to oxygen only when Complex I is dissociated from Complex III (23, 38, 48). Although the molecular structure of the individual complexes does not allow to envisage a close apposition of the matrix arm of Complex I, where FMN is localized, with either Complex III or IV, the actual shape of the I1III2IV1 supercomplex from bovine heart (65) suggests a slightly different conformation of Complex I in the supercomplex, showing a smaller angle of the matrix arm with the membrane arm and a higher bending toward the membrane (and presumably Complex III). Moreover, the observed destabilization of Complex I in the absence of supercomplex may render the 51 kDa subunit containing the FMN more “loose” allowing it to interact with oxygen (cf. 58).
It is true that this is an in vitro study, but it is supported by several observations in cellular and animal models linking together supercomplex dissociation and enhanced ROS production. It is noteworthy that our results are compatible with very recent observations by Diaz et al. (20) showing that diminished stability of SC and Complex I is associated with increased levels of ROS in mouse lung fibroblasts lacking the Rieske iron-sulfur protein of Complex III and hence devoid of the supercomplexes containing Complex I. Also in mouse fibroblasts expressing the activated form of the k-ras oncogene we had previously observed a strong decrease of high molecular weight supercomplexes correlating with higher ROS generation in comparison with wild-type fibroblasts (6, 43). Moreover, enhanced ROS generation and oxidative stress were found in yeast mutants lacking the supercomplex assembly factor Rcf1 and thus devoid of supercomplexes III-IV (15, 75, 78); since the yeast S. cerevisiae lacks Complex I (CI), in this case we may consider the origin of the extra ROS being presumably Complex III (CIII). Numerous pathological states are accompanied by enhanced generation of ROS (13) and both mitochondria and other systems, such as plasma membrane NADPH oxidase, have been implicated as the sources of ROS. In an experimental model of heart failure, the decrease of oxidative phosphorylation has been associated with a decrease of respiratory supercomplexes (62, 63, 64). Lymphoblasts from patients affected by Barth Syndrome, due to genetic loss of tafazzin, an enzyme involved in cardiolipin remodeling, have altered mitochondrial supercomplexes (50); likewise, in a yeast experimental model of tafazzin mutation, Chen et al. (14) observed an increased oxidative stress in response to ethanol. Aging is also accompanied by a decline of supercomplex association (25, 32); despite some uncertainties and challenges, aging is generally associated with increased ROS and oxidative damage (for a recent review cf. 16). Gomez and Hagen (31) reason that age-associated destabilization of respiratory supercomplexes may be important for the development of the mitochondrial aging phenotype by means of impaired bioenergetics and enhanced ROS production; in addition Frenzel et al. (25) on the basis of the 3D-structures of supercomplexes and the close spatial arrangement of the respective electron carrier binding sites (65) conclude that less superoxide radical formation is expected to occur in supercomplexes than in randomly distributed individual complexes. These latter studies, however, fail to show which is the causing event (i.e., supercomplex dissociation causing ROS increase or, alternatively, ROS increase causing supercomplex dissociation) or even if they are independent phenomena. In this perspective our findings contribute to a better understanding of the observations made in cellular and animal systems and represent a starting point to further elucidate the intricate relationship between the supramolecular organization of the respiratory chain and the production of ROS, which can modulate some signaling pathways from mitochondria to the cell and also act as damaging agents, depending on the amounts produced (4, 39, 61).
Materials and Methods
2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) was purchased from Molecular Probes Invitrogen (product code n. D-399). Stock solutions were prepared 5 mM in dimethyl sulfoxide; and stored at −20°C for no longer than 3 months.
Mucidin was a kind gift from Dr. F. Nerud of the Academy of Sciences in Prague, Czech Republic. All other chemicals were purchased from Sigma-Aldrich unless specifically indicated.
Preparation of mitochondria and proteoliposomes
Bos taurus heart mitochondria (BHM) were prepared by a large scale procedure as described elsewhere (73). The BHM were opened membranes devoid of substrate permeability barriers due to three cycles of ultrasound irradiation at 10-s periods with 50-s intervals at high power in a sonifier (Labsonic U2000, B. Braun Biotech International GmbBH) under a nitrogen flux in an ice-water bath.
Crude mitochondrial fractions enriched in Complex I and Complex III (R4B) were prepared from BHM as described by Hatefi and Rieske (36).
Complex I and Complex III proteoliposomes preparations were obtained as follows.
PL vesicles were prepared according to a modified version of Degli Esposti et al. (19). In brief, dry soybean phosphatidylcholine (Sigma-Aldrich P5638) was suspended in petroleum ether at 0.3 g·ml−1 and mixed with Coenzyme Q10 (Sigma-Aldrich C9538) in absolute ethanol at 30 nmol·mg−1 PL. The suspension was evaporated to dryness under nitrogen stream. Multilamellar vesicles form spontaneously when the dry PL-ubiquinone film swells in excess aqueous buffer (50 mM KCl, 10 mM Tris, and 1 mM EDTA, pH 7.4) at 30 mg PL·ml−1 under vigorous mixing. To produce small unilamellar vesicles, the mixture was subjected to 30-s periods with 30-s intervals of ultrasonic irradiation for 10 min at high power in a sonifier (Labsonic U2000; B. Braun Biotech International GmbH). To prevent overheating and lipid peroxidation, sonication was performed under a nitrogen flux in an ice-water bath.
The vesicles were stored at 4°C and used within 2 days from preparation. R4B and PL-Coenzyme Q10 vesicles were mixed at protein:PL ratios of 1:1 or 1:30 w/w in the presence of 1.5% v/v final concentration of 1.7 M n-octyl-D-glucopiranoside (Fluka 75083). After 10 min of incubation at 0°C, the samples were diluted 40-fold with buffer solution (cf. above) to induce proteoliposome formation according to the detergent-mediated and dilution method of Racker et al. (57).
Protein content in the samples was determined by the biuret method (33) after treatment with 10% Na-deoxycholate.
Dissociation of supercomplex with dodecylmaltoside
When indicated, the samples (BHM or R4B 1:1) were supplemented with 10% w/v solution of n-DDM (Sigma-Aldrich D5172) to adjust variable detergent:protein ratios, as shown in the legend of the figures, and incubated in ice-cold bath for 10 min before assay (70).
Enzymatic assays
All the kinetic measurements employed protein samples diluted in buffer solution (BHM, 40 μg·ml−1 and R4B proteoliposomes, 14 μg·ml−1). The catalytic activities were measured at 30°C using a dual wavelength spectrophotometer (V550 extended model; Jasco Europe) according to (28). In brief, NADH-ubiquinone oxidoreductase activity were measured under saturating substrate conditions by the rotenone-sensitive oxidation of 75 μM NADH (Sigma-Aldrich N8129) respectively in the absence and presence of 2 μM Antimycin and 60 μM decylubiquinone (DB) (Sigma-Aldrich D7911) as electron acceptor. The reaction was monitored at λ=340 minus 380 nm. NADH-cytochrome c oxidoreductase activity was assayed by the reduction of 50 μM equine heart cytochrome c (Sigma-Aldrich C7752), using 75 μM NADH as electron donor in the presence of 1 mM KCN, the reaction was monitored at λ=550 minus 540 nm. Residual rates of antimycin-insensitive activity were <10% of the total rates shown in the figures. The rates of the enzymatic activities are given as mean±S.D. of at least three independent determinations.
Assay of hydrogen peroxide and superoxide production by Complex I
Hydrogen peroxide production was measured in a fluorescence 96-well plate reader (Wallac Victor2 1420 Multilabel counter; PerkinElmer) at an excitation/emission wavelength of 485/535 nm at 25°C using 5 μM DCFDA and 0.5 mg·ml−1 of BHM or R4B proteoliposomes in the presence of 1.8 μM mucidin and 4 μM rotenone when indicated. The reaction was started with the addition of a large excess of electron donor (150 μM NADH) aimed to guarantee not to consume all the substrate during the 45 min of the assays (18, 23).
The fluorescence change was followed at different times, and the results are calculated as the difference of fluorescence units at 45 min minus zero time and given as mean±S.D. of at least three independent determinations. Interference of NADH, rotenone, mucidin, and DDM with the background fluorescence of DCF was evaluated in blank samples. Preliminary laboratory tests included the direct effect of enhancing the fluorescence of the probe by added H2O2 (5–50 μM) to exclude the possibility that the fluorescence signal produced by the samples was underestimated by a lack of DCF (data not shown).
According to (9), the fluorescence emission is due to the reaction of hydrogen peroxide with the deacetylated probe (dichlorofluorescein). It was previously demonstrated that DCFDA is spontaneously deacetylated in mitochondria preparations (18). On the contrary, our R4B 1:1 and R4B 1:30 samples required chemical hydrolysis of DCFDA prior to assay. In detail, 10 μl of DCFDA stock solution were mixed with 40 μl of 0.01 N NaOH for 30 min in the dark at room temperature (12). The mixture was neutralized with 200 μl of 10 mM KCl, 25 mM Tris, and 1 mM EDTA, pH 7.4, kept on ice in the dark and immediately used.
Superoxide production in the R4B proteoliposomes was measured at 30°C in a dual wavelength spectrophotometer (V550 extended model; Jasco Europe) by monitoring the reduction of 50 μM acetylated cytochrome c (Sigma-Aldrich C4186) at λ=550 minus 540 nm and using 75 μM NADH as electron donor; all samples were diluted in buffer solution (50 mM KCl, 10 mM Tris, and 1 mM EDTA, pH 7.4) to the final concentration of 14 μg protein/ml in the presence of 1.8 μM mucidin and 4 μM rotenone. Conforming to the method (5, 11), data are shown as the rate of SOD–sensitive and–insensitive reduction of acetylated cytochrome c [2.7 I.U. SOD(CuZn)/ml] and given as mean±S.D.
Electrophoretic analysis
Separation of supercomplexes and single respiratory complexes was achieved by 2D BN/SDS-PAGE according to (68, 71).
Protein solubilization of BHM and R4B samples was obtained by resuspension in 50 mM NaCl, 50 mM imidazole, and 5 mM 6-aminohexanoic acid, pH 7.0 in the presence of digitonin or DDM when indicated.
1D-electrophoresis was made using precast 3%–12% T gradient gels (Native-PAGE Bis-Tris gel system; Invitrogen). After 2D-electrophoresis, the gels (precast 4%–12% T, Nu-PAGE Bis-Tris gel system; Invitrogen) were blotted onto nitrocellulose membranes (GE-Healthcare) and exposed to monoclonal antibodies (MitoSciences, Inc., Eugene, OR) specific for single subunits of each respiratory complex: a first exposure of the membrane to the MS-111 antibody directed against the NDUFA9 (39 kDa) subunit of Complex I was followed by double overlaid exposure to a cocktail of antibodies (MS-604) against subunits of complexes I, II, III, IV, and V or to the MS-305 antibody against the Rieske protein (22 kDa) of Complex III, respectively as indicated in the legend of the figures. Detection of primary antibodies was achieved using a goat anti-mouse IgGH+L secondary antibody labeled with horseradish peroxidase (Molecular Probes, Invitrogen) with a chemiluminescent technique (Amersham ECL Western Blotting Reagent Pack; GE Healthcare). Digital images were captured in a Fluor-S Max MultiImager (Bio-Rad Laboratories) after 60–120 s exposition and were processed by densitometric analysis of the spots of interest according to the manufacturer's indications (Quantity-One Analysis Software, Bio-Rad Laboratories). Closely spaced or overlapped spots were resolved using the 3D-viewer tool in the software application that renders the original 2D-spots as 3D-peaks. The profile of the basal area of selected peak-volumes was determined assuming that the distribution of the clustered pixels in the 3D-objects was conform to a Gaussian model.
Abbreviations Used
- BHM
Bos taurus heart mitochondria
- BN-PAGE
blue-native polyacrylamide gel electrophoresis
- CI
Complex I, NADH-ubiquinone oxidoreductase
- CIII
dimeric Complex III, ubiquinol-cytochrome c oxidoreductase
- CIV
Complex IV, cytochrome c oxidase
- CoQ
coenzyme Q, ubiquinone
- DB
decylubiquinone
- DCF
2′,7′-dichlorofluorescein
- DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- DDM
dodecyl-β-D-maltoside
- FCCP
carbonyl cyanide p-trifluoromethoxy-phenyl hydrazone
- OXPHOS
oxidative phosphorylation system
- PL
phospholipid
- R4B
mitochondrial fraction enriched in Complex I and Complex III
- RFU
relative fluorescence unit
- ROS
reactive oxygen species
- SC
supercomplex I1III2
- SDS
sodium dodecyl sulphate
- SMP
submitochondrial particles
- SOD
superoxide dismutase
- TCA
trichloroacetic acid
Acknowledgments
This work was supported by MIUR (grant number PRIN2008LSHCFC_005) and also by the University of Bologna, which provided financial support for G.B.'s research contract (Assegno di ricerca—RFO2008). E.M. was awarded a scholarship grant from the European Commission (EADIC-Erasmus Mundus–ECW Consortium Lot 16), which supported her PhD program in Bologna.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Acín-Perez R. Bayona-Bafaluy MP. Fernández-Silva P. Moreno-Loshuertos R. Perez-Martos A. Bruno C. Moraes CT. Enriquez JA. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell. 2004;13:805–815. doi: 10.1016/s1097-2765(04)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acín-Pérez R. Fernández-Silva P. Peleato ML. Pérez-Martos A. Enriquez JA. Respiratory active mitocondrial supercomplexes. Mol Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Althoff T. Mills DJ. Popot JL. Kühlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J. 2011;30:4652–4664. doi: 10.1038/emboj.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anilkumar N. Sirker A. Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 5.Azzi A. Montenecucco C. Richter C. The use of acetylated ferricytochrome c for the detection of superoxide produced in biological membranes. Biochem Biophys Res Commun. 1975;65:597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- 6.Baracca A. Chiaradonna F. Sgarbi G. Solaini G. Alberghina L. Lenaz G. Mitochondrial Complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta. 2010;1797:314–323. doi: 10.1016/j.bbabio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi C. Fato R. Genova ML. Parenti Castelli G. Lenaz G. Structural and functional organization of Complex I in the mitochondrial respiratory chain. Biofactors. 2003;18:3–9. doi: 10.1002/biof.5520180202. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi C. Genova ML. Parenti Castelli G. Lenaz G. The mitochondrial respiratory chain is partially organized in a supercomplex assembly. J Biol Chem. 2004;279:36562–36569. doi: 10.1074/jbc.M405135200. [DOI] [PubMed] [Google Scholar]
- 9.Black MJ. Brandt RB. Spectrofluorometric analysis of hydrogen peroxide. Anal Biochem. 1974;58:246–254. doi: 10.1016/0003-2697(74)90464-3. [DOI] [PubMed] [Google Scholar]
- 10.Boumans H. Grivell LA. Berden JA. The respiratory chai in yeast behaves as a single functional unit. J Biol Chem. 1998;273:4872–4877. doi: 10.1074/jbc.273.9.4872. [DOI] [PubMed] [Google Scholar]
- 11.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 12.Cathcart R. Schwiers E. Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen K. Keaney JF., Jr Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr Atheroscler Rep. 2012;14:476–483. doi: 10.1007/s11883-012-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S. He Q. Greenberg ML. Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol. 2008;68:1061–1072. doi: 10.1111/j.1365-2958.2008.06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YC. Taylor EB. Dephoure N. Heo JM. Tonhato A. Papandreou I. Nath N. Denko NC. Gygi SP. Rutter J. Identification of a protein mediating respiratory supercomplex stability. Cell Metab. 2012;15:348–360. doi: 10.1016/j.cmet.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui H. Kong Y. Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 20122012:13. doi: 10.1155/2012/646354. Article ID 646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Aurelio M. Gajewski CD. Lenaz G. Manfredi G. Respiratory chain supercomplexes set the threshold for respiration defects in human mtDNA mutant cybrids. Hum Mol Genet. 2006;15:2157–2169. doi: 10.1093/hmg/ddl141. [DOI] [PubMed] [Google Scholar]
- 18.Degli Esposti M. Measuring mitochondrial reactive oxygen species. Methods. 2002;26:335–340. doi: 10.1016/S1046-2023(02)00039-7. [DOI] [PubMed] [Google Scholar]
- 19.Degli Esposti M. Bertoli E. Parenti Castelli G. Fato R. Mascarello S. Lenaz G. Incorporation of ubiquinone homologs into lipid vesicles and mitochondrial membranes. Arch Biochem Biophys. 1981;210:21–32. doi: 10.1016/0003-9861(81)90159-4. [DOI] [PubMed] [Google Scholar]
- 20.Diaz F. Enríquez JA. Moraes CT. Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV. Mol Cell Biol. 2012;32:415–429. doi: 10.1128/MCB.06051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz F. Fukui H. Garcia S. Moraes CT. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol Cell Biol. 2006;26:4872–4881. doi: 10.1128/MCB.01767-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudkina NV. Kudryashev M. Stahlberg H. Boekema EJ. Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proc Natl Acad Sci U S A. 2011;108:15196–15200. doi: 10.1073/pnas.1107819108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fato R. Bergamini C. Bortolus M. Maniero AL. Leoni S. Ohnishi T. Lenaz G. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta. 2009;1787:384–392. doi: 10.1016/j.bbabio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fato R. Bergamini C. Leoni C. Strocchi P. Lenaz G. Generation of reactive oxygen species by mitochondrial complex I: implications in neurodegeneration. Neurochem Res. 2008;33:2487–2501. doi: 10.1007/s11064-008-9747-0. [DOI] [PubMed] [Google Scholar]
- 25.Frenzel M. Rommelspacher H. Sugawa MD. Dencher NA. Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp Gerontol. 2010;45:563–572. doi: 10.1016/j.exger.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Galkin A. Brandt U. Superoxide radical formation by pure complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica. J Biol Chem. 2005;280:30129–30135. doi: 10.1074/jbc.M504709200. [DOI] [PubMed] [Google Scholar]
- 27.Genova ML. Baracca A. Biondi A. Casalena G. Faccioli M. Falasca AI. Formiggini G. Sgarbi G. Solaini G. Lenaz G. Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Biochim Biophys Acta. 2008;1777:740–746. doi: 10.1016/j.bbabio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Genova ML. Castelluccio C. Fato R. Parenti Castelli G. Merlo Pich M. Formiggini G. Bovina C. Marchetti M. Lenaz G. Major changes in complex I activity in mitochondria from aged rats may not be detected by direct assay of NADH: coenzyme Q reductase. Biochem J. 1995;311:105–109. doi: 10.1042/bj3110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genova ML. Lenaz G. New developments on the functions of coenzyme Q in mitochondria. Biofactors. 2011;37:330–354. doi: 10.1002/biof.168. [DOI] [PubMed] [Google Scholar]
- 30.Genova ML. Ventura B. Giuliano G. Bovina C. Formiggini G. Parenti Castelli G. Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001;505:364–368. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 31.Gómez LA. Hagen TM. Age-related decline in mitochondrial bioenergetics: Does supercomplex destabilization determine lower oxidative capacity and higher superoxide production? Semin Cell Dev Biol. 2012;23:758–767. doi: 10.1016/j.semcdb.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez LA. Monette JS. Chavez JD. Maier CS. Hagen TM. Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys. 2009;490:30–35. doi: 10.1016/j.abb.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gornall AG. Bardawill CJ. David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 34.Hackenbrock CR. Chazotte B. Gupte SS. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr. 1986;18:331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- 35.Hatefi Y. Haavik AG. Griffiths DE. Studies on the electron transfer system. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962;237:1676–1680. [PubMed] [Google Scholar]
- 36.Hatefi Y. Rieske JS. The preparation and properties of DPNH-cytochrome c reductase (Complex I-III of the respiratory chain) Methods Enzymol. 1967;10:225–231. [Google Scholar]
- 37.Heron C. Ragan CI. Trumpower BL. The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol cytochrome c oxidoreductase. Restoration of ubiquinone-pool behaviour. Biochem J. 1978;174:791–800. doi: 10.1042/bj1740791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirst J. King MS. Pryde KR. The production of reactive oxygen species by complex I. Biochem Soc Trans. 2008;36(Pt 5):976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 39.Ide T. Tsutsui H. Kinugawa S. Suematsu N. Hayashidani S. Ichikawa K. Utsumi H. Machida Y. Egashira K. Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 40.Jezek P. Hlavatá L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Kröger A. Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem. 1973;34:358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 42.Lenaz G. Mitochondrial and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol. 2012;942:93–136. doi: 10.1007/978-94-007-2869-1_5. [DOI] [PubMed] [Google Scholar]
- 43.Lenaz G. Baracca A. Barbero G. Bergamini C. Dalmonte ME. Del Sole M. Faccioli M. Falasca A. Fato R. Genova ML. Sgarbi G. Solaini G. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim Biophys Acta. 2010;1797:633–640. doi: 10.1016/j.bbabio.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 44.Lenaz G. Baracca A. Carelli V. D'Aurelio M. Sgarbi G. Solaini G. Bioenergetics of mitochondrial diseases associated with mtDNA mutations. Biochim Biophys Acta. 2004;1658:89–94. doi: 10.1016/j.bbabio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Lenaz G. Genova ML. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: Random collisions versus solid state electron channeling. Am J Physiol Cell Physiol. 2007;292:C1221–C1239. doi: 10.1152/ajpcell.00263.2006. [DOI] [PubMed] [Google Scholar]
- 46.Lenaz G. Genova ML. Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly. Int J Biochem Cell Biol. 2009;41:1750–1772. doi: 10.1016/j.biocel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Lenaz G. Genova ML. Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563–573. doi: 10.1016/j.bbabio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Lenaz G. Genova ML. Structure and organization of mitochondrial respiratory complexes: A new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- 49.Lenaz G. Genova ML. Supramolecular organisation of the mitochondrial respiratory chain: A new challenge for the mechanism and control of oxidative phosphorylation. Adv Exp Med Biol. 2012;748:107–144. doi: 10.1007/978-1-4614-3573-0_5. [DOI] [PubMed] [Google Scholar]
- 50.McKenzie M. Lazaou M. Thorburn DR. Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J Biol Mol. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Lastres D. Fontanesi F. García-Consuegra I. Martín MA. Arenas J. Barrientos A. Ugalde C. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012;15:324–335. doi: 10.1016/j.cmet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnishi ST. Shinzawa-Itoh K. Ohta K. Yoshikawa S. Ohnishi T. New insights into the superoxide generation sites in bovine heart NADH-ubiquinone oxidoreductase (Complex I): the significance of protein-associated ubiquinone and the dynamic shifting of generation sites between semiflavin and semiquinone radicals. Biochim Biophys Acta. 2010;1797:1901–1909. doi: 10.1016/j.bbabio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Panov A. Dikalov S. Shalbuyeva N. Hemendinger R. Greenamyre JT. Rosenfeld J. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol. 2007;292:C708–C718. doi: 10.1152/ajpcell.00202.2006. [DOI] [PubMed] [Google Scholar]
- 54.Pfeiffer K. Gohil VM. Stuart RA. Hunte C. Brandt U. Greenberg ML. Schägger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 55.Pryde KR. Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem. 2011;286:18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quarato G. Piccoli C. Scrima R. Capitanio N. Variation of flux control coefficient of cytochrome c oxidase and of the other respiratory chain complexes at different values of protonmotive force occurs by a threshold mechanism. Biochim Biophys Acta. 2011;1807:1114–1124. doi: 10.1016/j.bbabio.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Racker E. Violand B. O'Neal S. Alfonzo M. Telford J. Reconstitution, a way of biochemical research; some new approaches to membrane-bound enzymes. Arch Biochem Biophys. 1979;198:470–477. doi: 10.1016/0003-9861(79)90521-6. [DOI] [PubMed] [Google Scholar]
- 58.Radermacher M. Ruiz T. Clason T. Benjamin S. Brandt U. Zickermann V. The three-dimensional structure of complex I from Yarrowia lipolytica: a highly dynamic enzyme. J Struct Biol. 2006;154:269–279. doi: 10.1016/j.jsb.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raha S. Myint AT. Johnstone L. Robinson BH. Control of oxygen free radical formation from mitochondrial complex I: roles for protein kinase A and pyruvate dehydrogenase kinase. Free Radic Biol Med. 2002;32:421–430. doi: 10.1016/s0891-5849(01)00816-4. [DOI] [PubMed] [Google Scholar]
- 60.Raha S. Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 61.Ray PD. Huang BW. Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosca MG. Hoppel CL. New aspects of impaired mitochondrial function in heart failure. J Bioenerg Biomembr. 2009;41:107–112. doi: 10.1007/s10863-009-9215-9. [DOI] [PubMed] [Google Scholar]
- 63.Rosca MG. Hoppel CL. Mitochondria in heart failure. Cardiovasc Res. 2010;88:40–50. doi: 10.1093/cvr/cvq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosca MG. Vazquez EJ. Kerner J. Parland W. Chandler MP. Stanley W. Sabbah HN. Hoppel CL. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schäfer E. Dencher NA. Vonck J. Parcej DN. Three-dimensional structure of the respiratory chain supercomplex I1III2IV1 from bovine heart mitochondria. Biochemistry. 2007;46:12579–12585. doi: 10.1021/bi700983h. [DOI] [PubMed] [Google Scholar]
- 66.Schägger H. Respiratory chain supercomplexes. IUBMB Life. 2001;52:119–128. doi: 10.1080/15216540152845911. [DOI] [PubMed] [Google Scholar]
- 67.Schägger H. Blue native electrophoresis. In: Hunte C, editor; von Jagow G, editor; Schägger H, editor. Membrane Protein, Purification and Crystallization. A Practical Guide. San Diego, CA: Academic Press; 2003. pp. 105–130. [Google Scholar]
- 68.Schägger H. Cramer WA. von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- 69.Schägger H. de Coo R. Bauer MF. Hofmann S. Godinot C. Brandt U. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem. 2004;279:36349–36353. doi: 10.1074/jbc.M404033200. [DOI] [PubMed] [Google Scholar]
- 70.Schägger H. Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schägger H. von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 72.Seelert H. Dani DN. Dante S. Hauss T. Krause F. Schäfer E. Frenzel M. Poetsch A. Rexroth S. Schwassmann HJ. Suhai T. Vonck J. Dencher NA. From protons to OXPHOS supercomplexes and Alzheimer's disease: structure-dynamics-function relationships of energy-transducing membranes. Biochim Biophys Acta. 2009;1787:657–671. doi: 10.1016/j.bbabio.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 73.Smith AL. Preparation, properties, and conditions for assay of mitochondria: Slaughterhouse material small—scale. Methods Enzymol. 1967;10:81–86. [Google Scholar]
- 74.Stroh A. Anderkas O. Pfeiffer K. Yagi T. Finel M. Ludwig B. Schägger H. Assembly of respiratory complexes I, III, and IV into NADH oxidase supercomplex stabilizes complex I in Paracoccus dinitrificans. J Biol Chem. 2004;279:5000–5007. doi: 10.1074/jbc.M309505200. [DOI] [PubMed] [Google Scholar]
- 75.Strogolova V. Furness A. Robb-McGrath M. Garlich J. Stuart RA. Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Mol Cell Biol. 2012;32:1363–1373. doi: 10.1128/MCB.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trouillard M. Meunier B. Rappaport F. Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of therespiratory chain. Proc Natl Acad Sci U S A. 2011;108:E1027–E1034. doi: 10.1073/pnas.1109510108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Raam BJ. Sluiter W. de Wit E. Roos D. Verhoeven AJ. Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS One. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vukotic M. Oeljeklaus S. Wiese S. Vögtle FN. Meisinger C. Meyer HE. Zieseniss A. Katschinski DM. Jans DC. Jakobs S. Warscheid B. Rehling P. Deckers M. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab. 2012;15:336–347. doi: 10.1016/j.cmet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Wenz T. Hielscher R. Hellwig P. Schägger H. Richers S. Hunte C. Role of phospholipids in respiratory cytochrome bc1 complex catalysis and supercomplex formation. Biochim Biophys Acta. 2009;1787:609–616. doi: 10.1016/j.bbabio.2009.02.012. [DOI] [PubMed] [Google Scholar]