Abstract
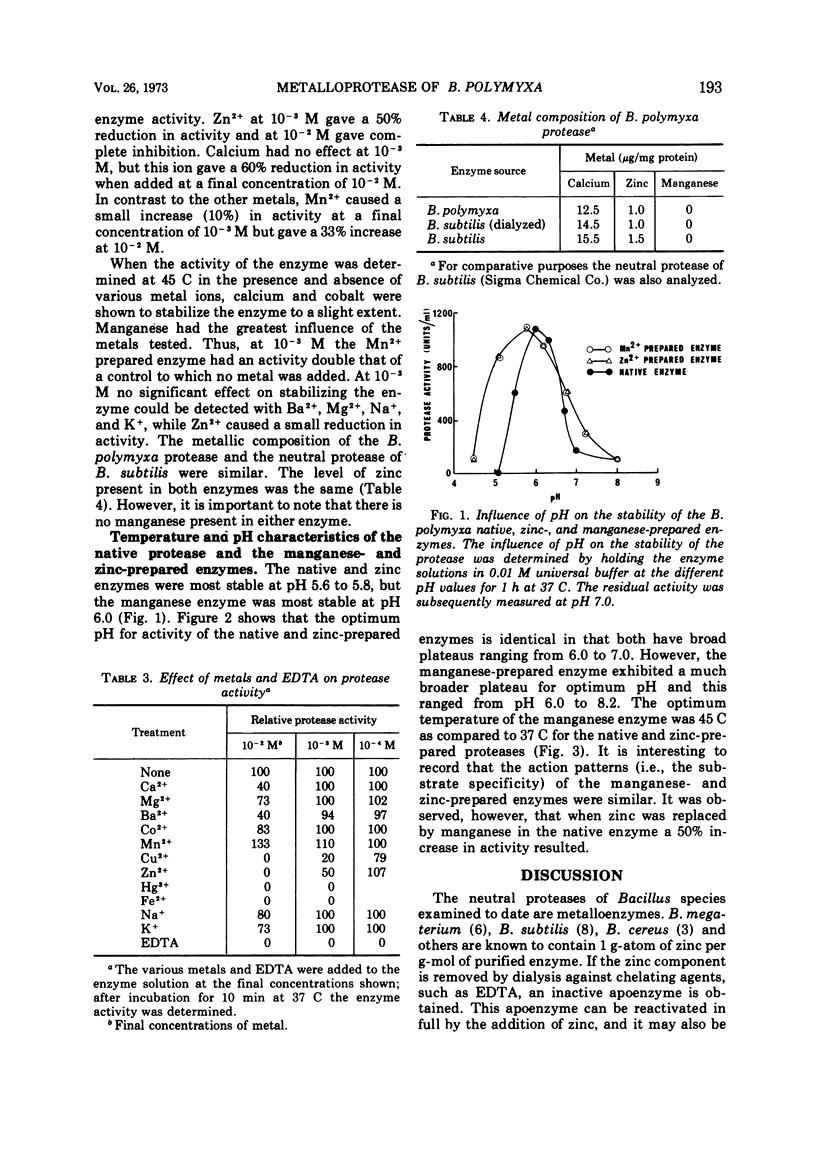
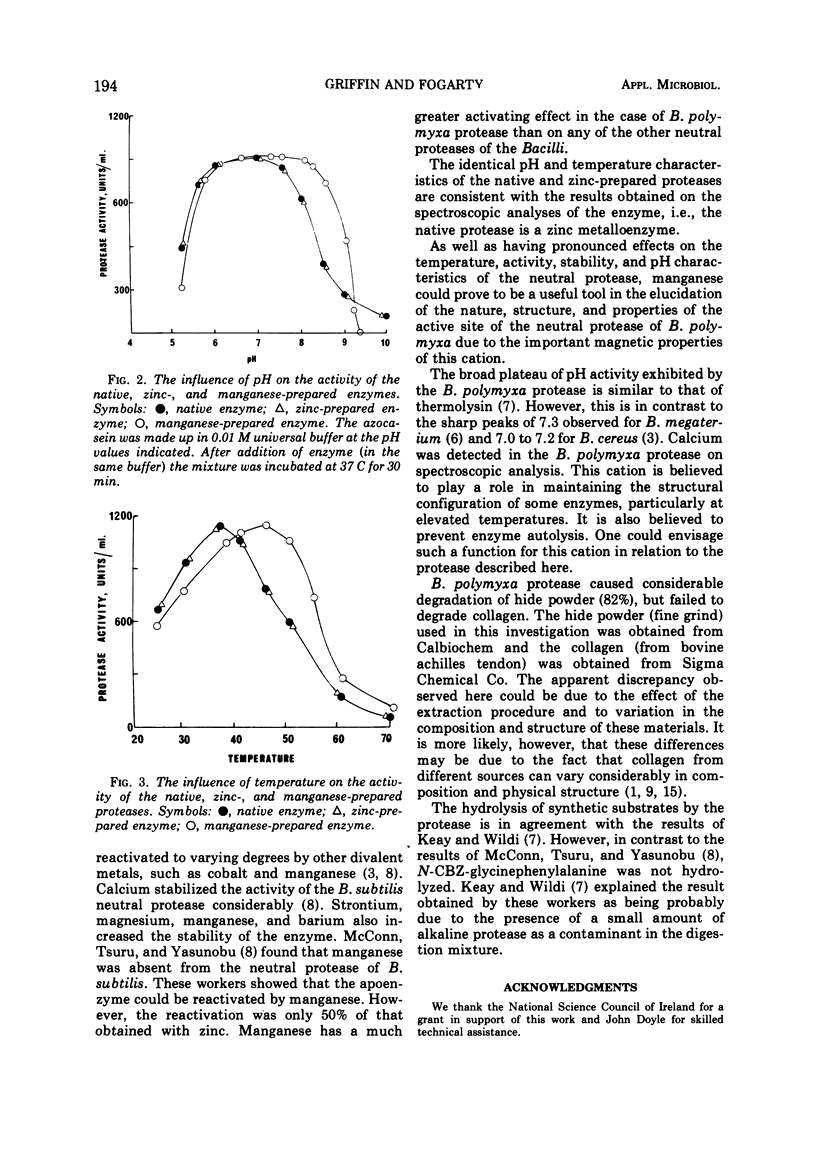
The neutral protease of Bacillus polymyxa had a broad pH optimum (6.0 to 7.2) for activity at 37 C. The enzyme was most stable at pH 5.6 to 5.8. The protease had an optimum temperature of 37 C and was quite thermostable up to 35 C, but at higher temperatures the stability decreased rapidly. The substrate specificity of the protease was similar to that of the neutral proteases of other members of the genus Bacillus. The enzyme was shown to be a zinc metalloprotease. However, manganous ions had a greater activating and stabilizing influence on the activity of this enzyme than zinc. Replacement of zinc in the native enzyme by manganese resulted in a 50% increase in activity. In addition, the prepared manganese metalloprotease had higher temperature and more alkaline pH optima than the native enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butzow J. J., Eichhorn G. L. Physical chemical studies on the age changes in rat tail tendon collagen. Biochim Biophys Acta. 1968 Jan 22;154(1):208–219. doi: 10.1016/0005-2795(68)90273-0. [DOI] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr Purification and properties of an alpha-amylase from facultative thermophilic bacteria. Arch Biochem Biophys. 1955 Jan;54(1):154–161. doi: 10.1016/0003-9861(55)90018-7. [DOI] [PubMed] [Google Scholar]
- Feder J., Keay L., Garrett L. R., Cirulis N., Moseley M. H., Wildi B. S. Bacillus cereus neutral protease. Biochim Biophys Acta. 1971 Oct;251(1):74–78. doi: 10.1016/0005-2795(71)90061-4. [DOI] [PubMed] [Google Scholar]
- Griffin P. J., Fogarty W. M. Some properties of a protease from Bacillus polymyxa. Biochem J. 1971 Dec;125(4):109P–109P. doi: 10.1042/bj1250109pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay L., Feder J., Garrett L. R., Moseley M. H., Cirulis N. Bacillus megaterium neutral protease, a zinc-containing metalloenzyme. Biochim Biophys Acta. 1971 Mar 23;229(3):829–835. doi: 10.1016/0005-2795(71)90302-3. [DOI] [PubMed] [Google Scholar]
- Keay L., Wildi B. S. Proteases of the genus Bacillus. I. Neutral proteases. Biotechnol Bioeng. 1970 Mar;12(2):179–212. doi: 10.1002/bit.260120205. [DOI] [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- Mechanic G., Tanzer M. L. Biochemistry of collagen crosslinking. Isolation of a new crosslink, hydroxylysinohydroxynorleucine, and its reduced precursor, dihydroxynorleucine, from bovine tendon. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1597–1604. doi: 10.1016/0006-291x(70)90571-1. [DOI] [PubMed] [Google Scholar]
- Millet J., Acher R., Aubert J. P. Biochemical and physiological properties of an extracellular protease produced by Bacillus megaterium. Biotechnol Bioeng. 1969 Nov;11(6):1233–1246. doi: 10.1002/bit.260110617. [DOI] [PubMed] [Google Scholar]
- Schneider A., Henson E., Blumenfeld O. O., Gallop P. M. The presence of lysinal (2,6-diaminohexanal) in tropocollagen. Biochem Biophys Res Commun. 1967 Jun 9;27(5):546–551. doi: 10.1016/s0006-291x(67)80022-6. [DOI] [PubMed] [Google Scholar]



