Abstract
Alloantibodies mediate acute antibody-mediated rejection as well as chronic allograft rejection in clinical transplantation. To better understand the cellular dynamics driving antibody production, we focused on the activation and differentiation of alloreactive B cells in the draining lymph nodes and spleen following sensitization to allogeneic cells or hearts. We used a modified staining approach with a single MHC Class I tetramer (Kd) bound to two different fluorochromes to discriminate between the Class I-binding and fluorochrome-streptavidin-binding B cells with a high degree of specificity and binding efficiency. By day 7-8 post-sensitization, there was a 1.5-3.2-fold increase in the total numbers of Kd-binding B cells. Within this Kd-binding B cell population, approximately half were IgDlow, MHC Class IIhigh and CD86+, 30-45 % expressed a germinal center (Fas+GL7+) phenotype, and 3-12 % were IRF4hi plasma cells. Remarkably, blockade with anti-CD40 or CTLA-4Ig, starting on day 7 post-immunization for 1 or 4 weeks, completely dissolved established GCs and halted further development of the alloantibody response. Thus MHC Class I tetramers can specifically track the in vivo fate of endogenous, Class I-specific B cells, and was used to demonstrate the ability of delayed treatment with anti-CD154 and CTLA-4Ig to halt established allo-B cell responses.
Keywords: Alloreactive B cells, MHC Class I Tetramers, Allograft Rejection, GC, PCs, anti-CD154, CTLA-4Ig
Introduction
It has become increasingly clear that alloantibodies can mediate acute antibody-mediated rejection (AMR) as well as chronic allograft rejection, and as a result, play an important role in determining the outcome of allografts in the clinic (1-5). In non-sensitized transplant recipients, alloantibodies are secreted by plasma cells (PCs) that have differentiate from naïve alloreactive B cells, whereas in sensitized individuals, PCs from a pre-existing long-lived pool or arising from memory B cells are responsible for the de novo alloantibody produced following the re-exposure to alloantigens (6-8).
The characterization of endogenous B cells that participate in a de novo alloantibody response has been technically challenging because of their low frequencies even during active immunization (9). In experimental models, attempts to circumvent this limitation have involved the use BCR-transgenic models where the frequencies of alloreactive B cells are significantly increased (10, 11). However the caveats of this approach are becoming increasingly apparent, since the frequencies of alloreactive B cells in these mice greatly exceed physiological frequencies and observations with a monoclonal population of B cells with a single affinity may not fully reflect alloreactive B cells with a spectrum of affinities (12, 13). Thus, there has been a renewed interest in tracking endogenous alloreactive B cells in experimental models as well as in humans.
The dominant specificity of alloantibodies is for donor MHC Class I and Class II antigens and donor reactive antibodies quantified in the clinic focus primarily on these specificities (5, 14, 15). MHC Class I tetramers have been extensively used to characterize the change in frequencies of antigen-specific CD8 cells following infection and immunization in both humans and mice (16, 17). MHC Class I tetramers have also been used to identify alloreactive B cells in humans, and more recently in mice (9, 18-22). In humans, the frequencies of B cells in the peripheral blood capable of binding specific HLA-tetramers was reported to be significantly higher in individuals who had detectable circulating alloantibodies of the same specificity compared to those that did not (19). While those reports demonstrated feasibility of approach, the access only to B cells in the blood of transplant recipients significantly limited mechanistic investigations that delve into how alloantibody production by these B cells are orchestrated because much of the B cell response occurs in the secondary lymphoid organs (23, 24). Thus complementary studies in mice are important if we are to develop a better understanding of the regulation of alloreactive B cell responses. Indeed, the recent report by Kwun et al. (22) demonstrated that MHC Class I tetramers could be used to identify an emergent IgD− subset of alloreactive B cells, in parallel with increased titers of DSA, in a murine model of chronic cardiac allograft rejection.
Using MHC Class I tetramers, we here demonstrate in qualitative and quantitative detail the presence of alloreactive B cells in naïve mice, and their entry into germinal centers (GC) and commitment into PC fates during allo-sensitization and following allogeneic cardiac transplantation. We further demonstrate the therapeutic efficacy of delayed treatment with anti-CD154 and CTLA-4Ig in dissolving established GCs and halting established alloreactive B cell responses.
Materials and Methods
Mice
C57BL/6 mice and BALB/c mice were purchased from Harlan Sprague Dawley, while BALB.B (C.B10-H2b/LilMcdJ) mice were from Jackson Labs. μMTmice (B6.129S2-Igh-6tm1Cgn/J) were purchased from Jackson Labs and maintained in our animal facility. All mouse procedures described in this study had been approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Chicago.
Tetramers and Monomers
H2Kd- biotin monomers, H2Kb-biotin monomers, H2Kb or H2Kd tetramers conjugated with PE, APC or FITC were from NIH tetramer Core Facility (Atlanta, GA). The peptides bound to H2Kd and H2Kb were SYIPSAEKI (Plasmodium berghei circumsporozoite peptide 252-260) (25) and SSIEFARL (herpes simplex virus (HSV) glycoprotein B peptide 498 to 505) (26), respectively. For the staining 5 × 106 cells, saturating (0.5 μg) concentrations of each tetramer were used.
Antibodies and Flow cytometry
Flow cytometry was performed with an LSRII- blue or LSR-Fortessa (BD) and analyzed with Flowjo (Tree Star, Ashland, OR). All antibodies were purchased from Ebioscience, unless specified. For H2Kd-specific B cells staining, single cells from lymph nodes or spleens were pre-blocked at 4°C with 2.4G2, followed by lineage-specific antibodies (for dump channel: (Anti-CD3 (17A2), anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-F4/80 (BM8, Biolegend), anti-Gr1 (RB6-8C5, Biolegend), anti-CD49b (DX5, Biolegend)) and anti-B220 (RA3-6B.2), anti-IgD (11-26C.2a). Next, H2Kd tetramers together with other antibodies were added and incubated for 30 minutes: anti-MHC Class II (I-A/I-E) (M5/114/15.2) and CD86 (GL1) or anti-Fas (JO2, BD Pharmingen) and anti-GL7 (GL7). For Interferon response factor 4 (IRF4) intracellular staining, cells were pre-incubated with Live/Dead fixable violet dead cell stain kit for 405 nm excitation (Invitrogen, Cat. L34955) prior to incubation with H2Kb- or H2Kd-tetramers, anti-B220 and anti-IgD. The cells were fixed, permeabilized (FoxP3 TF staining buffer kit, EBioscience), and then incubated with the anti-IRF4 antibody (clone 3E4) for 1 hour.
Adoptive Transfer
Cell suspensions of spleen and lymph nodes from C57BL/6 mice were incubated with H2Kd-PE and H2Kd-APC at 4°C for 1 hour, then anti-APC and anti-PE microbeads (Miltenyi) were added. After 30 mins, the cell-bead suspension was passed through magnetized LS column (Miltenyi), the flow through was collected and adoptively transferred into μMT mice. The other half of the non-depleted spleen and lymph node cells suspension was transferred into another cohort of μMT mice. One day after transfer, mice were immunized with BALB/c splenocytes subcutaneously and intraperitoneally, and at 21 days after cell transfer, sera were collected.
Immunization
For comparison of draining and non-draining lymph nodes, B6 mice were injected with 10 million BALB/c splenocytes in two sites on the flank. On day 7 after immunization, draining LN and non-draining LNs were collected (Inguinal LNs, axillary LNs, brachial LNs) respectively. For comparison of BALB/c or BALB.B immunized mice, separate groups of B6 mice were immunized on their flanks. On day 7 after immunization, the draining LNs were collected and analyzed by flow cytometry. Total cell numbers were counted with a hemotocytometry.
ELISA and Donor Specific Antibody (DSA) Assay
For H2Kd antibody detection, plate were coated with streptavidin overnight at 37°C, after several washes, H2Kd-biotin monomers were added and incubated for 1 hour at room temperature, cells were then blocked with BSA% 1 hour at room temperature, serum were diluted (1:100) and incubated with H2Kd coated plate at room temperature for 2 hours, plates were washed and anti-IgG conjugated to alkaline phosphatase (AP) (Jackson Immunoresearch, Cat. 115-055-071) or anti-IgM-AP (Jackson Immuoresearch, Cat. 115-055-075) were added. The substrate was used according to product information (Sigma, SIGMAFAST™ r-Nitrophenyl phosphate Tablets), and O.D values ere measured at 405 nm by a multiwall plate reader (BioRad). For donor specific antibody detection, serum were diluted (1:25) and incubated with 106 BALB.B splenocytes for 1 hour incubation at 4°C, then the cells were wash and incubated with anti-IgG (Southern Biotech, Cat. 1030-02) or anti-IgM (Southern Biotech, Cat. 1021-09) for 30 minutes. Mean fluorescence intensity were measured by flow cytometry.
Heart Transplantation
Hearts from BALB/c mice were transplanted into C57BL/6 recipients using techniques described previously (27).
Immunosuppression
C57BL/6 mice were immunized subcutaneously on Day 0 using BALB/c splenocytes and divided into 5 groups: (i) MR1 d0: Anti-CD154 (MR1; 500 μg/mouse) starting on Day −1, 0 and 1; (ii) MR1 d7: Anti-CD154 (500 μg/mouse) starting on Day 7, 8, 9; (iii) CTLA-4Ig D0: CTLA-4Ig (500 μg/mouse) starting on Day −1, 0 and 1; (iv) CTLA-4Ig d7 group: CTLA4-Ig (500 μg/mouse) starting on Day 7, 8 and 9. Mice then received MR1 or CTLA-4Ig (i.p.) every three days till day 35 post-immunization. (v) Immunized but no treatment. Mice were bled every week and on the day of sacrifice, and the spleen, inguinal LNs, brachial LNs and axillary LNs were collected for analysis of GC B cells.
Statistical analysis
The statistical significance of differences in mean values, cell numbers, percentages were analyzed by student's T test (unpaired) or ANOVA using Prism 5 (Graphpad, San Diego, CA). P values ≤ 0.05 were considered statistically significant.
Results
Visualizing allospecific B cells with MHC Class I tetramers
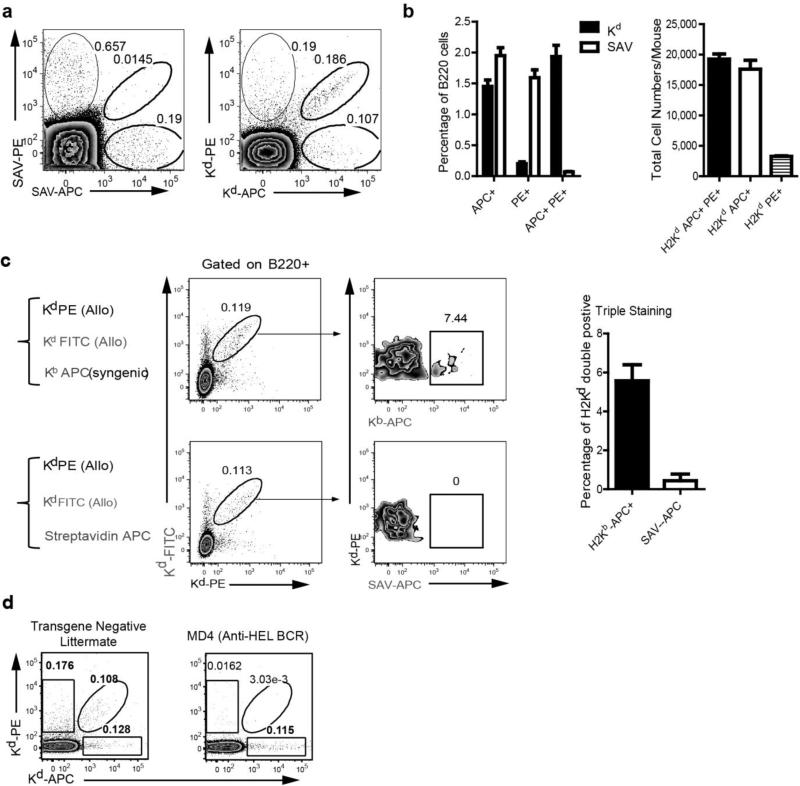
To enhance the detection of Kd-reactive B cell clonotypes, the Kd molecule is tetramerized to increase the avidity and thus half-life of the interaction. This is accomplished by combining purified MHC class I Kd possessing a single biotin molecule at the carboxy terminus with streptavidin (SAV) at a 4 to 1 molar ratio, respectively. Visualization is then possible if the SAV is conjugated to a fluorochrome (16). Early studies by Mulder A et al. (2003) observed high background staining of human B cells to SAV-phycoerythrin (PE), and recent studies by Pape et al. (13) reported on a detectable population of B cells in naïve mice that bound specifically to PE. Thus, B cells binding to fluorescently labeled MHC Class I tetramers should comprise of at least two distinct populations of B cells - those recognizing the MHC Class I molecule directly or those recognizing the fluorochome-coupled SAV. Indeed, we observed that approximately 0.2% of B cells from naive C57BL/6 mice bound to SAV-PE or to SAV-allophycocyanin (SAV-APC) in the absence of the MHC Class I (Fig 1a).
Fig 1.
Visualizing Kd-specific B cells in naïve C57BL/6 mice with fluorescent Kd-tetramers. (a) The percentage of B220+ B cells binding to Streptavidin-phycoerythrin (SAV-PE), Streptavidin-allophycocyanin (SAV-AP), or both (Left), compared to binding of Kd tetramer-PE or Kd tetramer-APC or both (Right). (b) To enhance detection of alloreactive B cells, single-cell suspensions of spleen and lymph node cells of naïve C57BL/6 mice were stained with either the combination of Kd-PE and Kd-APC or SAV-PE and SAV-APC, and then enriched by anti-PE and anti-APC microbead positive selection. The percentage (following enrichment) and total numbers of B220+ B cells in a naïve C57BL/6 mouse binding only to Kd tetramer-PE or Kd tetramer-APC or to both (Kd Tet-DP) were determined. (N=4). (c) Kd Tet-DP (Kd-PE and Kd-FITC tetramers) B cells display minimal binding to irrelevant molecules, Kb Tet+ APC or SAV-APC. Data are the mean and SEM of 4 different individuals. (d) Kd Tet DP B cells are reduced in MD4 HEL BCR transgenic mice. Inguinal LNs from MD4 BCR transgenic mice or transgene negative littermates were collected and stained for Kd Tet DP B cells.
To reduce false positive detection of fluorochrome-binding B cells, we used MHC Class I (Kd) tetramers conjugated to PE or APC simultaneously during the staining process. This dual fluorochrome approach has been shown to enable exquisite sensitivity in detecting low frequencies of antigen specific B cells (28). B cells from naïve C57BL/6 mice binding to Kd-PE and Kd-APC could be segregated into three populations: those that only bound to Kd-PE or Kd-APC and those that bound to both the Kd-PE and Kd-APC tetramers; we refer to the latter population as Tet-DP cells for the remainder of this manuscript (Fig 1a). The total numbers of Tet-DP cells, PE-binding and APC-binding B cells in a naïve C57BL/6 mouse was approximately 19,241, 17,604 and 3,270 respectively. To further probe the specificity of the Tet-DP B cells, single-cell suspensions of spleen and lymph node cells of naïve C57BL/6 mice were stained with either the combination of Kd-PE and Kd-APC or SAV-PE and SAV-APC, and then enriched by anti-PE and anti-FITC microbead positive selection. A significantly higher percentage of Kd Tet-DP B cells (1.93%) was observed compared to the SAV-DP B cells (0.07%), even after Kd Tet enrichment (Fig 1b). In addition, non-enriched single-cell suspensions of spleen and lymph node cells of naïve C57BL/6 mice were co-stained with Kd-PE, Kd-APC and an irrelevant ligand, H-2Kb-APC or SAV-APC. Only 5% of the Kd Tet-DP cells bound to H-2Kb-APC while <1% bound to the SAV-APC, confirming that the Kd Tet-DP cells in naïve mice were highly specific for Kd-reactive B cells (Fig 1c). Similar results were obtained with spleen cells isolated from a C57BL/6 mouse 14 days after receiving a BALB/c heart transplant (Supplemental Fig 1). Finally we confirmed that the Kd Tet-binding was BCR specific, by demonstrating significantly fewer Kd Tet-DP cells in MD4 mice that express a transgenic BCR specific for hen egg lysozyme, compared to non-transgenic littermate controls (Fig 1d).
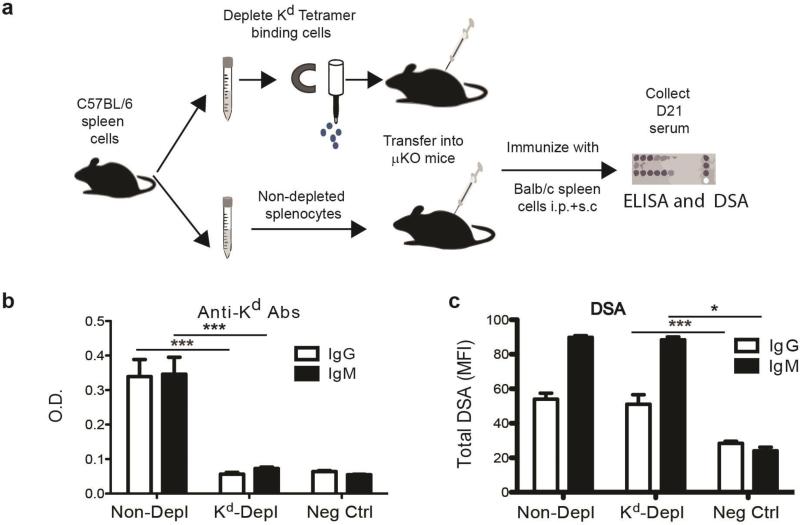
To determine the efficiency to which the Kd tetramers bound to the B cells that produce anti-Kd antibodies, we used a sensitive functional assay, as described by Pape et al. (13), that enabled the presence of these cells to be analyzed following immunization. Kd tetramer-binding cells were depleted from splenocytes isolated from naïve C57BL/6 mice, transferred into B cell deficient mice and then immunized with BALB/c (H2d) splenocytes; the control group consisted of transferring non-depleted cells (Fig. 2a). Three weeks after immunization, the serum was collected and assessed for circulating anti-Kd antibodies as well as for anti-BALB/c DSA. Mice receiving Kd tetramer-depleted spleen cells did not produce detectable anti-Kd antibodies, confirming in this sensitive assay that the Kd tetramers efficiently bound to virtually all Kd-specific B cells (Fig 2b). Although Kd-specific antibody responses were voided, the remaining allo-specificities were intact as they were able to produce DSA specific for other BALB/c antigens that were expressed and detected on BALB.B targets, comparable to mice receiving non-depleted spleen cells (Fig 2c) and significantly above negative control with no serum added or naïve serum (Supplemental Fig 2).
Fig 2.
Efficient binding and depletion of alloreactive Kd-specific B cells with Kd-tetramers. (a) Experimental design where Kd tetramer-depleted or non-depleted spleen cells from naïve C57BL/6 mice were adoptively transferred into B cell-deficient (μMT) mice. Depletion resulted in a 4% reduction of total spleen cells and a 1.25% reduction in total B cells. One day later these mice were immunized with BALB/c spleen cells, and 21 days later the serum was harvested and assessed for the presence of anti-Kd antibodies by (b) ELISA and for (c) DSA using flow cytometry and BALB.B targets. Negative controls were the optical densities (O.D.) or mean channel fluorescence (MFI) of wells or cells not receiving serum. All data are the mean and SEM of 3-4 mice, and statistically significant differences are indicated (*p<0.05; ***p<0.005).
Quantifying the expansion of allospecific B cells following immunization
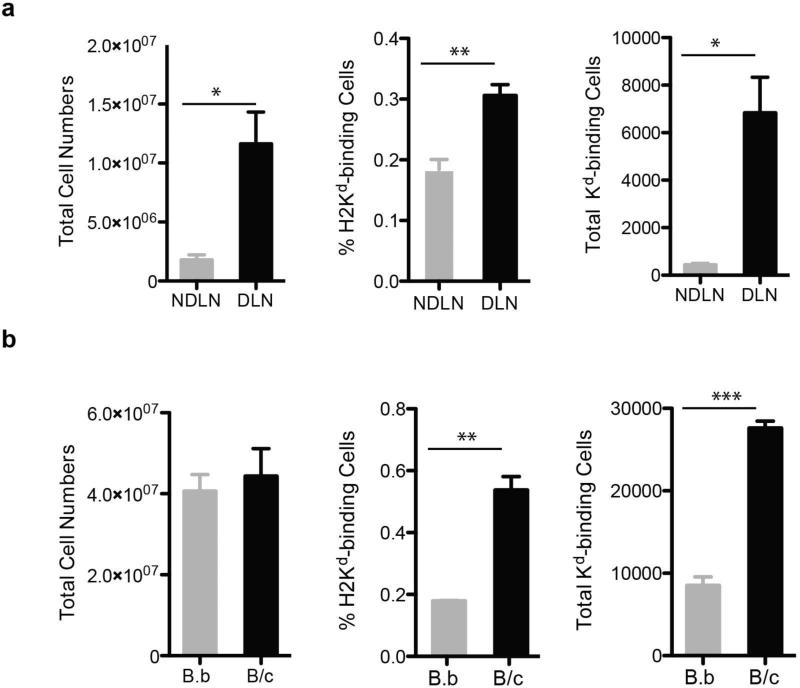
We quantified the expansion in the numbers of Kd Tet-DP B cells in the draining (DLN) lymph nodes following a sub-cutaneous injection in the right flank with BALB/c spleen cells (Fig 3a). The negative control consisted of cell in the contralateral non-draining (NDLN) lymph node of the same immunized mice. At 7 day post-immunization, we observed an approximately 6-fold increase in the total number of cells in the DLN versus the NDLN, and a 16-fold increase in total number of Kd Tet-DP B cells.
Fig 3.
Quantifying the expansion of Kd-binding B cells at day 7 after immunization with BALB/c (B/c) or BALB.B (B.b) splenocytes. The total number of cells recovered, percentage of Kd Tet-DP B cells of B220+ cells, and total number of Kd Tet-DP B cells from (a) the draining lymph node (DLN) compared to the contralateral non-DLN (NDLN); or (b) from the DLN of mice immunized with BALB/c or BALB.B splenocytes. Data are the mean and SEM of three mice per group. In (a) only one side of the mouse was immunized with BALB/c splenocytes to allow the retrieval of draining and non-draining lymph nodes, whereas in (b) both sides of the mouse were immunized with BALB/c or BALB.B splenocytes.
Because activated B cells can become sticky and prone to non-specific binding of the Kd-tetramers, we additionally compared the Kd Tet-DP B cell response in C57BL/6 mice immunized to BALB/c versus BALB.B spleen cells that are MHC-matched to C57BL/6 but minor-mismatched. Following both immunizations, the total numbers of cells in the DLNs were comparable (Fig 3b) and significantly increased over the NDLN (Fig 3a). However, the percentage and total numbers of Kd Tet-DP B cells were significantly increased by three-fold only in mice immunized with BALB/c splenocytes but not in mice immunized with BALB.B (Fig 3b). Thus collectively, these results confirm the specificity of the dual fluorochrome-single tetramer approach in tracking the fate of alloreactive B cells, and also provide insights into the magnitude of the B cell response to a single MHC Class I antigen in the context of an alloimmune response elicited to complete MHC and minor antigen mismatch BALB/c splenocytes.
Quantifying the activation of allospecific B cells following immunization
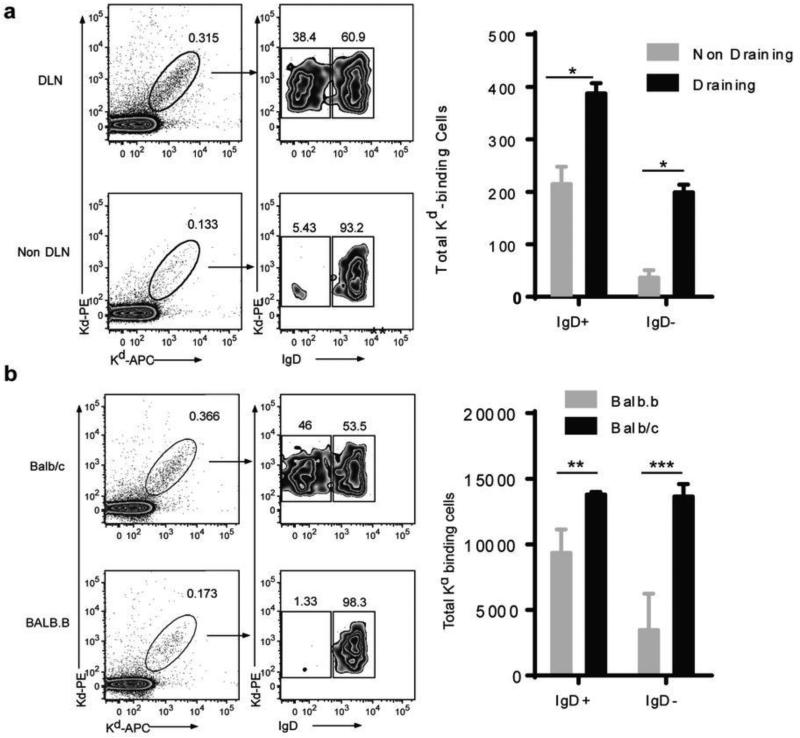
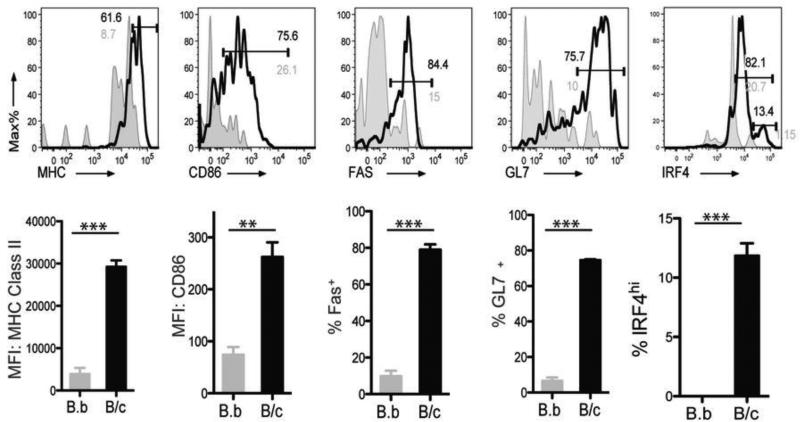
Within hours of antigen encounter by the B cell receptor, B cells rapidly down regulate surface IgD, up regulate MHC Class II and CD86, and migrate toward the T-B cell interface, where these B cells present antigen and provide co-stimulatory signals to CD4+ T cells (reviewed in (23)). These activated T cells then provide pro-survival and instructive signals, comprising CD154-CD40, Icos-IcosL and cytokines, to B cells. After 2-4 days at the T-B zonal interface, the proliferating B cells commit to three different fates – extrafollicular PCs, memory B cells or GC B cells. In the GC, B cells undergo repeated interactions with follicular dendritic cells (FDC) and T follicular helper cells (Tfh), and then further B cell proliferation, somatic hypermutation and class switch recombination. High affinity, class-switched cells emerge from the GC as a second wave of PCs and quiescent memory B cells. We used the Kd-tetramer system to track the activation of alloreactive B cells, and observed that at 7 days after exposure to BALB/c splenocytes cells, 38.4-46.0% of Kd Tet-DP B cells had down-regulated their surface IgD compared to only 1.3-5.4% of the mice immunized with BALB.B splenocytes or in the NDLD (Fig 4). The total numbers of Kd Tet-DP B cells in the DLN was 13,933 and 688 in the BALB/c versus BALB.B immunized mice, reflecting an 18.9-fold increase upon exposure to specific alloantigen (Table 1).
Figure 4.
Emergence of IgD− Kd Tet-DP B cells at day 7 after immunization with BALB/c or BALB.B splenocytes: (a) comparing draining versus non-draining lymph nodes, and (b) comparing BALB/c versus BALB.B immunization. Detailed gating strategy is provided in Supplemental Fig 4. All data are the mean and SEM of 3-4 mice, and statistically significant differences are indicated (*p<0.05; **p<0.01; ***p<0.005).
Table 1.
Magnitude of the anti-Kd B cell Response in the draining lymph node of mice immunized with BALB/c or BALB.b Spleen Cells
| Total LN | Tet+ | IgDhi | IgDlo | MHCIIhi | CD86hi | Fas+GL7+ | IRF4hi | |
|---|---|---|---|---|---|---|---|---|
| BALB.B | 40.7 × 106 | 8537 | 7,727 | 688 | 4,122 | 1,310 | 361 | 0.0 |
| BALB/c | 44.4 × 106 | 27,633 | 13,933 | 13,033 | 16,231 | 12,621 | 12267 | 3,275 |
| Fold Increase | 1.1 | 3.2 | 1.8 | 18.9 | 3.9 | 9.6 | 33.9 | >3275 |
When Kd Tet-DP IgD− B cells from BALB/c-immunized mice were further analyzed, the majority had up regulated MHC Class II and CD86, indicative of their ability to enter into cognate interactions with T cells. Furthermore the majority (70-80%) of Kd Tet-DP IgD− B cells expressed the GC markers, Fas and GL7 (29, 30). Approximately 13% of the IgD− Kd Tet-DP were committed into the PC lineage; expressing high levels of the transcription factor, IRF4 (Fig 5) (31) (32) (33-35). We further confirmed that majority of the IRF4hi B cells co-expressed CD138, another marker of PCs (Supplemental Fig 3). When the total cell numbers were quantified (Table 1), we observed a 4-10-fold increase in IgD− Kd Tet-DP B cells that had increased expression of MHC Class II/CD86, and a 30-40-fold increase in cells that had acquired the GC phenotype. Finally, committed PCs were only observed in mice immunized with BALB/c and not in the negative control mice immunized with BALB.B splenocytes.
Fig 5.
Tracking the changes in phenotype of Kd Tet-DP B cells at day 7 after immunization with BALB/c or BALB.B splenocytes. Increased percentages (top) of IgD− Kd Tet-DP B cells expressing MHC Class II and CD86 and their mean fluorescence intensities (MFI) (bottom), as well as increased percentages of IgD− Kd tetramer-binding B cells expressing Fas, GL7 and IRF4 in the DLN of mice immunized with BALB/c (black) compared to BALB.B (grey) splenocytes. All data are the mean and SEM of 3-4 mice, and statistically significant differences are indicated (**p<0.01; ***p<0.005).
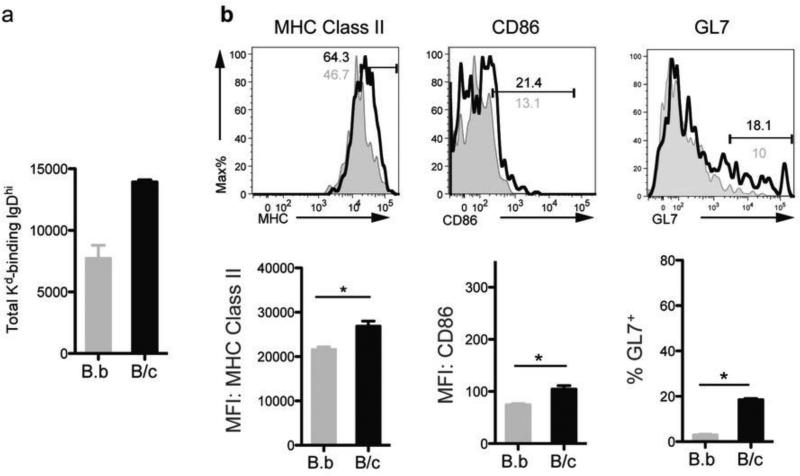
Kwun J et al. (22) focused on the IgD− Kd Tet-DP B cells that emerge following encounter with alloantigen, but did not comment on the IgD+ Kd Tet-DP B cells that are present in both naïve and immunized mice because of concerns of a lack of specificity of the MHC Class I tetramers for the IgD+ B cells. Because our approach of using the dual fluorochrome-single Kd tetramer approach results in the specific identification of Kd-binding B cells, we were able to extend our analysis to the IgD+ Kd Tet-DP B cells cells in BALB/c and BALB.B immunized mice (Figs 4 & 6). The total number of these IgD+ B cells increased 2-fold (Fig 4), and exhibited modest signs of activation (increased MHC Class II, CD86, and GL7 expression) that were nevertheless statistically significant. These data suggest that at least some of the IgD+ Kd Tet-DP B cells had encountered antigen, but it remains to be determined whether these cells go on to develop into GC B cells or early memory B cells as has been recently described (36).
Fig 6.
Tracking the changes in the total number and phenotype of IgD+ Kd Tet-DP B cells at day 7 after immunization with BALB/c or BALB.B splenocytes. Modest but statistically significant increased percentages of IgD+ Kd Tet-DP B cells expressing MHC Class II and CD86 (top) and their mean fluorescence intensities (MFI) (bottom), as well as the percentage of IgD+ Kd Tet-DP B cells expressing GL7+ cells in the DLN of mice immunized with BALB/c (black) compared to BALB.B (grey) splenocytes. Detailed gating strategy is provided in Supplemental Fig 4. All data are the mean and SEM of 3-4 mice, and statistically significant differences are indicated (*p<0.05).
Tracking alloreactive B cells in the spleen following BALB/c heart transplantation
The above experiments involving immunization with BALB/c splenocytes provide important proof-of-principle that we can specifically detect Kd Tet-DP B cells in the DLN. We extended those findings to demonstrate that we can also track alloreactive B cells in the spleen following heterotopic BALB/c cardiac transplantation into C5BL/6 mice (Table 2). Overall the fate of the Kd-binding B cells resembled that observed with subcutaneous immunization with BALB/c spleen cells, with the emergence of IgD− cells that expressed increased MHC Class II, CD86. At the time of rejection, day 7 post-transplantation, approximately 65% of these IgD− Kd Tet-DP B cells were GC B cells (Fas+GL7+) and 10% were IRF4hi PCs. By day 14 post-transplantation, a significantly increased number and percentage of Kd Tet-DP B cells demonstrated signs of antigen encounter, namely downregulated IgD and upregulated MHC Class II. While the total number of Kd Tet-DP B cells that acquired the GC phenotype remained the same as on day 7, the number of IRF4high PCs on day 14 post-transplantation doubled compared to day 7, consistent with a further maturation of the alloreactive B cell response (Table 2). Finally, while there were significant numbers of B cells in the heart at the time of acute rejection, the percentage of Kd Tet-DP B cells were not further enriched compared to the spleen (data not shown).
Table 2.
Magnitude of the anti-Kd B cell Response in the Spleen of C57BL/6 recipients at day 7 and day 14 post-BALB/c heart transplantation
| Total Spl | Tet+ | IgDhi | IgDlo | MHCIIhi | CD86hi | Fas+GL7+ | IRF4hi | |
|---|---|---|---|---|---|---|---|---|
| None | 81 × 106 | 23,121 | 17,607 | 5,021 | 460 | 137 | 168 | 0 |
| BALB/c HT× D7 | 157 × 106 | 35,419 | 18,522 | 16,133 | 9,611 | 592 | 10,460 | 962 |
| Fold Increase | 1.9 | 1.5 | 1.1 | 3.2 | 20.9 | 4.3 | 62.3 | >962 |
| BALB/c HT× D14 | 135 × 106 | 44,027 | 18,865 | 24,435 | 12,305 | 305 | 10,548 | 1,905 |
| Fold Increase | 1.7 | 1.9 | 1.1 | 4.9 | 26.8 | 2.2 | 62.8 | >1905 |
Delayed treatment with anti-CD154 or CTLA4-Ig attenuated ongoing GC responses
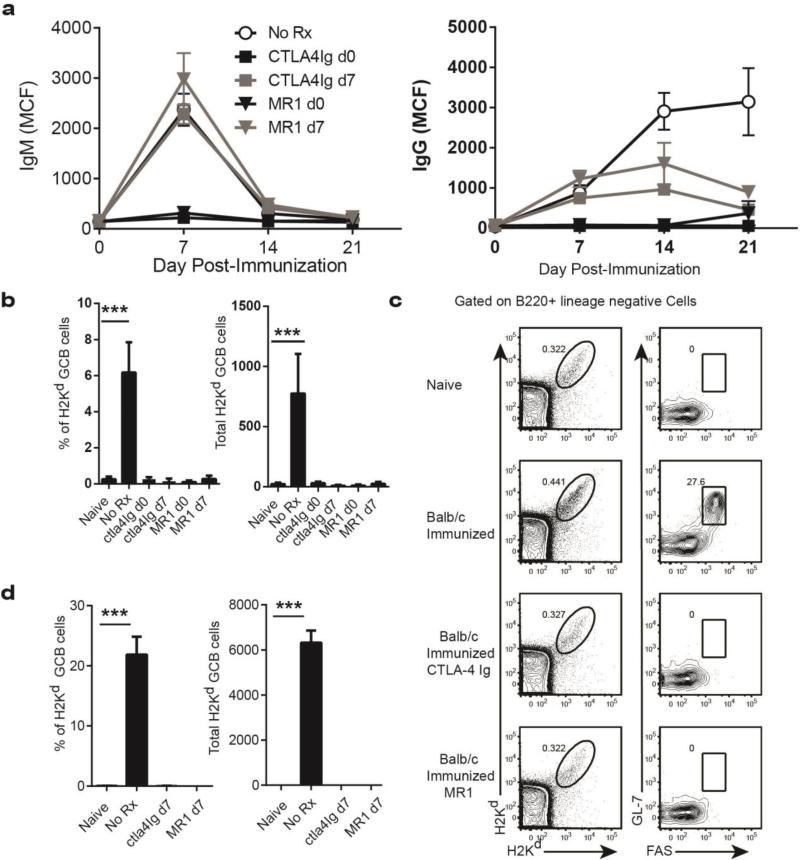
Recent observations by Taylor et al (36) and Kaji et al. (37) indicated that CD40-mediated interactions are necessary for sustaining GC reactions, and that delayed initiation of anti-CD154 treatment at day 5-6 post-immunization resulted in a dramatic reduction of GC B cells and post-GC B cells. Because these observations could have therapeutic implications in regards to the inhibition of an ongoing antibody response, we defined the effects of delayed administration of anti-CD154 antibodies at 7 days post-sensitization, when the GC response was clearly developed (Table 1). By analyzing serum titers of anti-Kd over time, we observed that anti-CD154 treatment significantly inhibited the ongoing allo-IgG response (Days 14 and 21) but not the initial wave of antigen specific antibodies (Day 7) demonstrating that delayed anti-CD154 treatment prevented the expansion but not the priming of plasma cell precursors. Importantly, this inhibition was comparable to anti-CD154 administered on day 0 (Fig 7a) suggesting that this treatment effectively blocks T cell dependent maintenance of Kd-specific B cells that would have progressed to plasma cell differentiation. This possibility was investigated by enumerating the numbers of Kd Tet-DP B cells 4 weeks after treatment. Indeed, the percentage and total numbers of GC Kd Tet-DP B cells were significantly reduced compared to untreated sensitized mice and comparable to naïve mice and (Fig 7b). Remarkably, delayed intervention with CTLA-4Ig had comparable effects as anti-CD154 (Fig 7a&b).
Fig 7.
Effect of delayed anti-CD154 or CTLA-4Ig administration on established GCs and developing alloantibody response. Anti-CD154 (500 μg/mouse) or CTLA-4Ig (500 μg/mouse) was administered starting on the day before (d0) or 7 days (d7) after immunization with BALB/c splenocytes. Positive controls were immunized, untreated (No Rx) mice. All groups receiving anti-CD154 or CTLA-4Ig demonstrated reduced (a) donor-specific antibodies and (b) reduced percentages and total numbers of GC Kd Tet-DP B cells at the end of the 28 day treatment period, compared to the immunized, untreated group. (c & d) Effect of delayed treatment with anti-CD154 or CTLA-4Ig from day 7-14 post-immunization on the percentage and total numbers of GC Kd-Tet DP cells. Untreated (No Rx) group were sacrificed on day 7 post-immunization. All data are the mean and SEM of 4-5 mice, and statistically significant differences are indicated (***p<0.001).
To gain better insight into the kinetics of the inhibition, we analyzed a cohort of sensitized mice that underwent only 7 days of anti-CD154 or CTLA-4Ig treatment. We did not detect Kd Tet-DP B cells with the GC phenotype in both treatment groups, confirming that delayed anti-CD154 or CTLA-4Ig therapy was able to rapidly prevent further increase in circulating antibody titers by dissolving established GCs and inhibiting the generation of new GCs. These flow-cytometry data were confirmed by immunofluorescence-based staining of tissue sections performed on the draining LN following treatment with CTLA-4Ig for 7 days (from day 7-14 post-immunization) and demonstrating that the GCs (IgD−PNA+) after treatment with CTLA4-Ig were significantly smaller even when compared to day 7 post-immunization controls (Table S5).
Discussion
The ability to track endogenous alloreactive B cells at resting state and also following antigen exposure provided us with an unprecedented opportunity to investigate the cellular basis of the alloantibody response. Recent reports of MHC or HLA Class I tetramers being used to detect allospecific B cells by flow cytometry in mice and in humans have used a single tetramer conjugated to a single fluorochrome, which simultaneously identifies B cells that recognize Class I and the SAV-fluorochrome. Molecules comprising the SAV-conjugated fluorochrome are large proteins (475 D - 240 kD) derived from bacteria and algae, thus it is not surprising that significant numbers of B cells in naive C57BL/6 mice display specificities to these structures. With the single tetramer-single fluorochrome approach, approximately half of the B cells identified may actually not be allospecific but binding to the SAV-fluorochrome. To address the problem of SAV-fluorochrome binding B cells we used the approach of a single Kd tetramer bound to two different fluorochromes, which allowed the identification of alloreactive, MHC Class I-specific B cells in a highly specific manner. We have been cognizant of the issue of false positive detection and have validated the use of the Kd-tetramer in identifying the vast majority of allo-specific B cells by showing (i) their accumulation in response to sensitization (Fig. 1&3) (ii) their acquisition of expected phenotypes; GC B cells, plasma cells, and activation markers in response to sensitization (Fig. 4-6) (iii) negligible binding to antigen-irrelevant (anti-HEL) transgenic B cells (Fig. 1d) and (iv) ≤ 10% binding by irrelevant fluorochrome-conjugated reagents (Fig. 1c). With respect to the last observation, it is unclear whether the “contaminant” population reflects true biological cross-reactivity or idiosyncratic interactions of isolated proteins and cells in a tube and the ultra-sensitive fluorescence detection methods employed with this approach. We were also able to demonstrate that the Kd tetramers bound to essentially all the B cells capable of producing anti-Kd antibodies, as the elimination of B cells binding to Kd tetramers resulted in a remaining B cell population that could no longer produce anti-Kd antibodies but were able to produce DSA of other specificities. These data confirm both specificity and efficiency of the MHC Class I tetramer approach to identify endogenous alloreactive B cells.
This approach has enabled us to quantify the total number of Kd-specific B cells in a naive C57BL/6 mouse to be approximately 20,000, comparable to the total number of APC-binding B cells. Single MHC Class I molecules can associate with a wide array of peptides, and T cell receptors have exquisite ability to discriminate between these peptide-MHC Class I complexes (16). In contrast the allo-determinants recognized by anti-Class I monoclonal antibodies have mapped predominantly to the polymorphic first and/or second external domains of the MHC Class I molecule (14, 38, 39). While alloantibodies and the corresponding BCR can discriminate between peptide-MHC Class I complexes (40, 41) the contribution of the peptide to the ability of the antibody to bind to the peptide Class I complex is relatively subtle. In these studies we used Kd tetramers bound to the Plasmodium berghei circumsporozoite peptide 252-260 (SYIPSAEKI) as a proof of principle of the technique; however, it remains to be confirmed experimentally whether and to what degree Kd tetramers bound to self-peptides would alter the absolute numbers of Kd–specific B cells identified.
We traced the activation of Kd–specific B cells following alloantigen exposure, by examining the down-regulation of surface IgD, and the up regulation of MHC Class II and CD86 (Table 1). The expression of the latter two sets of molecules on approximately 45% of the Kd Tet+ B cells imply involvement of these cells in alloantigen presentation and co-stimulation via the indirect pathway. In addition, approximately 43% of Kd Tet+ B cells were Fas+GL7+ GC cells, while about 12% were committed to the PC lineage (IRF4hi). Thus at day 7 post-immunization, we observed that most of the alloreactive B cells were in a GC reaction, consistent with our understanding of alloantibody production being T cell-dependent and class-switched to IgG (42, 43). Importantly, it is the products of the GC that will go on to constitute the pathogenic effects of B cells in sensitized patients – high affinity long lived PCs and memory B cells. While we are able to trace the B cells committed to the PC lineage, a potential limitation of this cell-surface staining approach is inefficient detection of fully-differentiated PCs that are known to down-regulate their BCRs.
While the majority of B cells that expressed the activation markers were in the IgD− population, we observed a modest 2-fold increase in the numbers of Kd Tet+ IgD− B cells in the DLN following immunization with allogeneic spleen cells, with a small but detectable subset of these cells exhibiting increased MHC Class II, CD86 and GL7. While more detailed time course and cell tracking experiments are necessary, we speculate that these cells are most likely poised cells at the earliest stages of antigen encounter or differentiated memory B cells (13).
A current challenge in clinical transplantation is finding immunosuppressive agents that effectively halt an ongoing alloreactive antibody responses. Here we showed that a delayed administration of anti-CD154 to day 7 post-immunization, when GC had already been established, was able to rapidly halt further increase in circulating allo-IgG titers and dissolve established GCs. Also because anti-CD154 is not approved for human use whereas a high affinity mutant of CTLA-4Ig, belatacept, has recently received FDA approval for use in clinical renal transplantation (44), we also demonstrated that CTLA-4Ig was able to comparably halt an ongoing alloantibody response. This latter observation is surprising because CD28 signaling has been reported to be necessary for the initiation of GC development but is not required for T cell-dependent B cell proliferation within established GCs (45). Thus our demonstration of the ability of CTLA-4Ig to rapidly halt a developing antibody response bodes well for its use in the clinic, and provides insights into the requirement for sustained CD28-B7 interactions in GC maintenance. One caveat is that these studies involved allogeneic spleen cell transfusion, and a theoretical possibility exists that GC activity following solid organ transplantation may be more resistant to these therapies.
The studies with subcutaneous injection of BALB/c splenocytes allowed us to define the precision of this approach in tracing the fate of alloreactive B cells in the draining lymph nodes, but a significant percentage of alloreactive immune responses in the clinic are elicited in the context of solid organ transplantation. Thus we also provide data confirming that this approach can be used to visualize the Kd-specific B cell response in the spleen of mice receiving heterotopically transplanted hearts (BALB/c into C57BL/6 recipients). We report that the magnitude of the Kd-specific B cell response at day 7 post-transplantation in the spleen, namely their entry into the GC and commitment into the PC fate was comparable, albeit reduced in magnitude, to that observed in the DLN following allosensitization with subcutaneous injection with BALB/c spleen cells. At day 14, there is a further increase in the percentage and total number of Kd Tet-DP B cells, and of those committed to the PC fate. Interestingly, Kd-specific B cells were not enriched in the acutely rejecting hearts.
The role of antibodies in mediating acute or chronic transplant rejection in the clinic is now widely accepted (4, 42, 46-48). Furthermore, the consensus is that current immunosuppression is most effective for controlling T cell responses, and is less effective at controlling alloantibody production in pre-sensitized individuals awaiting transplantation (7, 8, 49, 50). Both naïve and memory B cells can differentiate into short-lived or as long-lived PCs that produce circulating alloantibodies. Here we describe in detail the use of MHC Class I tetramers to trace the fate of all MHC Class I-reactive B cells responsible for the systemic production of alloantibodies, thereby providing a versatile and a powerful way to analyze the cellular and molecular processes that lead to the production of alloantibody. This ability to specifically monitor the activation, proliferation and differentiation of naive alloreactive B cells in the secondary lymphoid organs will allow us to understand the mechanistic basis for these events and to identify the efficacy and limitations of current immunosuppressive strategies. The challenge will be to apply this approach to monitor changes in alloreactive B cell subsets in the peripheral blood of transplant patients where lymphoid organs are not readily available.
Supplementary Material
Supplemental Fig 1. Splenocytes prepared from naïve C57BL/6 mice or on day 14 after heart transplantation were stained with Kd-PE, Kd-FTIC and Kb-APC, or stained with Kd-PE, Kd FITC and Streptavidin(SAV)-APC. All data are the mean and SEM of 3-4 mice, and no significant differences in the percentage of Kb-APC- or SAV-APC-reactive Kd Tet-DP B cells are observed between the naïve and transplanted mice (p>0.05).
Supplemental Fig 2. Background staining with sera from naïve C57BL/6 mice on BALB/c targets. Sera from naïve mice or 7 days after BALB/c heart transplantation were incubated with BALB/c splenocytes, and then stained with anti-mouse IgG-FITC and anti-mouse IgM-PE. Control samples had no sera added. Data are presented as mean fluorescence intensity ± SEM (3 mice each group).
Supplemental Fig 3. IRF4 and CD138 staining of plasmablasts identifies a B220lo IRF4hi CD138+ plasmablast population. IRF4lo are activated B cells comprising the pre-memory/GC as well as the memory and GC subsets. CD138+B220hi B cells are likely to be activated B cells but not committed PCs.
Supplemental Fig 4. Detailed gating strategy for Fig 5 and Fig 6. After gating on lineage negative Dump−Kd Tet-DP population, ≥ 95% of the cells are B220+, so further B220 gating is not necessary. The Dump−Kd Tet-DP cells are then subdivided into IgDhi and IgDlo populations and their expression of GC (GL7+Fas+) or other markers (not shown) were assessed.
Acknowledgments
This work was supported in part by NIAID/NIH grants, R03 AI069284 and R01AI083452, to ASC. We acknowledge the NIH Tetramer core facility at Emory University for providing the tetramers and monomers used in this project, and Dr. Neal Iwakoshi, Emory University, for initial advice.
Abbreviations
- AP
alkaline phosphatase
- APC
allophycocyanin
- BCR
B cell receptor
- DLN
draining lymph node
- FITC
fluorescein isothiocyanate
- GC
germinal center
- IRF4
Interferon response factor 4
- MFI
mean channel fluorescence
- NDLN
non-draining lymph node
- O.D.
optical densities
- PC
plasma cell
- SAV
streptavidin
- Tet-DP
tetramer-double positive
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, Cosio FG, Gandhi MJ, Kremers W, Stegall MD. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 10:582–9. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith RN, Colvin RB. Chronic alloantibody mediated rejection. Semin Immunol. 2012;24:115–21. doi: 10.1016/j.smim.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–17. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 4.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–99. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 5.Everly MJ. Donor-specific anti-HLA antibody monitoring and removal in solid organ transplant recipients. Clin Transpl. 2011:319–25. [PubMed] [Google Scholar]
- 6.Stegall MD, Gloor JM. Deciphering antibody-mediated rejection: new insights into mechanisms and treatment. Curr Opin Organ Transplant. 2010;15:8–10. doi: 10.1097/MOT.0b013e3283342712. [DOI] [PubMed] [Google Scholar]
- 7.Walsh RC, Alloway RR, Girnita AL, Woodle ES. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int. 2012;81:1067–74. doi: 10.1038/ki.2011.502. [DOI] [PubMed] [Google Scholar]
- 8.Snanoudj R, Candon S, Legendre C. Targeting B cells in sensitized kidney transplant patients: state of the art and future perspectives. Curr Opin Organ Transplant. 2010 doi: 10.1097/MOT.0b013e3283402cf4. [DOI] [PubMed] [Google Scholar]
- 9.Mulder A, Eijsink C, Kardol MJ, Franke-van Dijk ME, van der Burg SH, Kester M, Doxiadis, Claas FH. Identification, isolation, and culture of HLA-A2-specific B lymphocytes using MHC class I tetramers. J Immunol. 2003;171:6599–603. doi: 10.4049/jimmunol.171.12.6599. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Ma L, Yin D, Shen J, Chong AS. Long-term control of alloreactive B cell responses by the suppression of T cell help. J Immunol. 2008;180:6077–84. doi: 10.4049/jimmunol.180.9.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons RF, Vivek K, Rostami SY, Zekavat G, Ziaie SM, Luo Y, Koeberlein B, Redfield RR, Cancro MP, Naji A, Noorchashm H. Acquisition of humoral transplantation tolerance upon de novo emergence of B lymphocytes. J Immunol. 2011;186:614–20. doi: 10.4049/jimmunol.1002873. [DOI] [PubMed] [Google Scholar]
- 12.Pape KA, Kouskoff V, Nemazee D, Tang HL, Cyster JG, Tze LE, Hippen KL, Behrens TW, Jenkins MK. Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. J Exp Med. 2003;197:1677–87. doi: 10.1084/jem.20012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–7. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park MS, Terasaki PI, Barbetti A, Han H, Cecka JM. Significance of the HLA molecular structure to transplantation. Clin Transpl. 1988:301–28. [PubMed] [Google Scholar]
- 15.Kaneku H. Impact of donor-specific HLA antibodies in transplantation, a review of the literature published in the last three years. Clin Transpl. 2010:283–306. [PubMed] [Google Scholar]
- 16.Altman JD, Davis MM. MHC-peptide tetramers to visualize antigen-specific T cells. Curr Protoc Immunol Chapter. 2003;17 doi: 10.1002/0471142735.im1703s53. Unit 17 3. [DOI] [PubMed] [Google Scholar]
- 17.Nepom GT. MHC class II tetramers. J Immunol. 2012;188:2477–82. doi: 10.4049/jimmunol.1102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eijsink C, Kester MG, Franke ME, Franken KL, Heemskerk MH, Claas FH, Mulder A. Rapid assessment of the antigenic integrity of tetrameric HLA complexes by human monoclonal HLA antibodies. J Immunol Methods. 2006;315:153–61. doi: 10.1016/j.jim.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Zachary AA, Kopchaliiska D, Montgomery RA, Melancon JK, Leffell MS. HLA-specific B cells: II. Application to transplantation. Transplantation. 2007;83:989–94. doi: 10.1097/01.tp.0000259019.68244.d7. [DOI] [PubMed] [Google Scholar]
- 20.Zachary AA, Kopchaliiska D, Montgomery RA, Leffell MS. HLA-specific B cells: I. A method for their detection, quantification, and isolation using HLA tetramers. Transplantation. 2007;83:982–8. doi: 10.1097/01.tp.0000259017.32857.99. [DOI] [PubMed] [Google Scholar]
- 21.Panoskaltsis-Mortari A, Taylor PA, Riddle MJ, Shlomchik MA, Blazar BR. In situ identification of allospecific B cells using pentamers. Blood. 2008;111:3904–5. doi: 10.1182/blood-2007-12-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwun J, Oh BC, Gibby AC, Ruhil R, Lu VT, Kim DW, Page EK, Bulut OP, Song MQ, Farris AB, Kirk AD, Knechtle SJ, Iwakoshi NN. Patterns of De Novo Allo B Cells and Antibody Formation in Chronic Cardiac Allograft Rejection After Alemtuzumab Treatment. Am J Transplant. 2012 doi: 10.1111/j.1600-6143.2012.04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–8. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 24.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–96. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 25.Kessler B, Michielin O, Blanchard CL, Apostolou I, Delarbre C, Gachelin G, Gregoire C, Malissen B, Cerottini JC, Wurm F, Karplus M, Luescher IF. T cell recognition of hapten. Anatomy of T cell receptor binding of a H-2kd-associated photoreactive peptide derivative. J Biol Chem. 1999;274:3622–31. doi: 10.1074/jbc.274.6.3622. [DOI] [PubMed] [Google Scholar]
- 26.Blaney JE, Jr., Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, Fu TM, Kawaoka Y, Tevethia SS. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol. 1998;72:9567–74. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin D, Dujovny N, Ma L, Varghese A, Shen J, Bishop DK, Chong AS. IFN-gamma production is specifically regulated by IL-10 in mice made tolerant with anti-CD40 ligand antibody and intact active bone. J Immunol. 2003;170:853–60. doi: 10.4049/jimmunol.170.2.853. [DOI] [PubMed] [Google Scholar]
- 28.Townsend SE, Goodnow CC, Cornall RJ. Single epitope multiple staining to detect ultralow frequency B cells. J Immunol Methods. 2001;249:137–46. doi: 10.1016/s0022-1759(00)00352-5. [DOI] [PubMed] [Google Scholar]
- 29.Naito Y, Takematsu H, Koyama S, Miyake S, Yamamoto H, Fujinawa R, Sugai M, Okuno Y, Tsujimoto G, Yamaji T, Hashimoto Y, Itohara S, Kawasaki T, Suzuki A, Kozutsumi Y. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. 2007;27:3008–22. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–92. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 31.Falini B, Fizzotti M, Pucciarini A, Bigerna B, Marafioti T, Gambacorta M, Pacini R, Alunni C, Natali-Tanci L, Ugolini B, Sebastiani C, Cattoretti G, Pileri S, Dalla-Favera R, Stein H. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95:2084–92. [PubMed] [Google Scholar]
- 32.Cattoretti G, Shaknovich R, Smith PM, Jack HM, Murty VV, Alobeid B. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol. 2006;177:6930–9. doi: 10.4049/jimmunol.177.10.6930. [DOI] [PubMed] [Google Scholar]
- 33.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–36. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Sciammas R, Li Y, Warmflash A, Song Y, Dinner AR, Singh H. An incoherent regulatory network architecture that orchestrates B cell diversification in response to antigen signaling. Mol Syst Biol. 2011;7:495. doi: 10.1038/msb.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R, Chong A, Klein U, Dinner A, Singh H, Sciammas R. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. doi: 10.1016/j.immuni.2013.04.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, Ohara O, Rajewsky K, Takemori T. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012;209:2079–97. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammerling GJ, Rusch E, Tada N, Kimura S, Hammerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982;79:4737–41. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemke H, Hammerling GJ. Clustering of antigenic determinants on H-2 molecules. J Immunol. 1982;128:2465–9. [PubMed] [Google Scholar]
- 40.Sherman LA, Chattopadhyay S, Biggs JA, Dick RF, 2nd, Bluestone JA. Alloantibodies can discriminate class I major histocompatibility complex molecules associated with various endogenous peptides. Proc Natl Acad Sci U S A. 1993;90:6949–51. doi: 10.1073/pnas.90.15.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulder A, Eijsink C, Kester MG, Franke ME, Kardol MJ, Heemskerk MH, van Kooten C, Verreck FA, Drijfhout JW, Koning F, Doxiadis, Claas FH. Impact of peptides on the recognition of HLA class I molecules by human HLA antibodies. J Immunol. 2005;175:5950–7. doi: 10.4049/jimmunol.175.9.5950. [DOI] [PubMed] [Google Scholar]
- 42.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB. Chronic antibody mediated rejection of renal allografts: pathological, serological and immunologic features in nonhuman primates. Am J Transplant. 2006;6:1790–8. doi: 10.1111/j.1600-6143.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 43.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–56. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 44.Wojciechowski D, Vincenti F. Belatacept for prevention of acute rejection in adult patients who have had a kidney transplant: an update. Biologics. 2012;6:385–93. doi: 10.2147/BTT.S23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker LS, Wiggett HE, Gaspal FM, Raykundalia CR, Goodall MD, Toellner KM, Lane PJ. Established T cell-driven germinal center B cell proliferation is independent of CD28 signaling but is tightly regulated through CTLA-4. J Immunol. 2003;170:91–8. doi: 10.4049/jimmunol.170.1.91. [DOI] [PubMed] [Google Scholar]
- 46.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–62. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 47.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–70. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, Mengel M, Matas A, Halloran PF. A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012;12:1168–79. doi: 10.1111/j.1600-6143.2011.03931.x. [DOI] [PubMed] [Google Scholar]
- 49.Morrow WR, Frazier EA, Mahle WT, Harville TO, Pye SE, Knecht KR, Howard EL, Smith RN, Saylors RL, Garcia X, Jaquiss RD, Woodle ES. Rapid reduction in donor-specific anti-human leukocyte antigen antibodies and reversal of antibody-mediated rejection with bortezomib in pediatric heart transplant patients. Transplantation. 2012;93:319–24. doi: 10.1097/TP.0b013e31823f7eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huber L, Lachmann N, Durr M, Matz M, Liefeldt L, Neumayer HH, Schonemann C, Budde K. Identification and therapeutic management of highly sensitized patients undergoing renal transplantation. Drugs. 2012;72:1335–54. doi: 10.2165/11631110-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1. Splenocytes prepared from naïve C57BL/6 mice or on day 14 after heart transplantation were stained with Kd-PE, Kd-FTIC and Kb-APC, or stained with Kd-PE, Kd FITC and Streptavidin(SAV)-APC. All data are the mean and SEM of 3-4 mice, and no significant differences in the percentage of Kb-APC- or SAV-APC-reactive Kd Tet-DP B cells are observed between the naïve and transplanted mice (p>0.05).
Supplemental Fig 2. Background staining with sera from naïve C57BL/6 mice on BALB/c targets. Sera from naïve mice or 7 days after BALB/c heart transplantation were incubated with BALB/c splenocytes, and then stained with anti-mouse IgG-FITC and anti-mouse IgM-PE. Control samples had no sera added. Data are presented as mean fluorescence intensity ± SEM (3 mice each group).
Supplemental Fig 3. IRF4 and CD138 staining of plasmablasts identifies a B220lo IRF4hi CD138+ plasmablast population. IRF4lo are activated B cells comprising the pre-memory/GC as well as the memory and GC subsets. CD138+B220hi B cells are likely to be activated B cells but not committed PCs.
Supplemental Fig 4. Detailed gating strategy for Fig 5 and Fig 6. After gating on lineage negative Dump−Kd Tet-DP population, ≥ 95% of the cells are B220+, so further B220 gating is not necessary. The Dump−Kd Tet-DP cells are then subdivided into IgDhi and IgDlo populations and their expression of GC (GL7+Fas+) or other markers (not shown) were assessed.