Abstract
Müllerian Inhibiting Substance (MIS, Anti-Müllerian hormone) is a gonadal hormone that contributes to the subtle sex-biases in the nervous system. Mature neurons of both sexes also produce MIS, suggesting that MIS may be a paracrine regulator of adult neural networks. We report here that murine hypoglossal motor neurons produce MIS and its receptors, MISRII and bone morphogenetic protein receptor 1A (BMPR1A, ALK3), but differentially transport them, with only MIS being detectable in axons. The production of MIS and its receptors were rapidly down regulated after axonal damage, which is a characteristic of genes involved in mature neuronal function. MIS is a survival factor for embryonic spinal motor neurons, but the rate of cell loss after hypoglossal nerve avulsion was normal in Mis−/− mice and was not attenuated by intraventricular administration of MIS. These observations suggest that MIS may be involved in anterograde rather than autocrine or retrograde regulation of neurons.
Keywords: Axonal transport, Avulsion, MISRII, ALK3
Introduction
The development, maintenance and function of neurons are controlled by multiple regulators, some of which serve different functions as the neuron matures (Glebova and Ginty, 2005). Müllerian inhibiting substance (MIS) is a newly discovered regulator of neurons (Lebeurrier et al., 2008; Wang et al., 2005, 2009) whose function in the adult appears to be distinct from its role during development.
MIS (synonym, Anti-Müllerian hormone) is part of the classical pathway for male differentiation. The Sertoli cells of the testes are the only embryonic source of MIS (Teixeira et al., 2001; Wang et al., 2009), with its initial action being to trigger the degeneration of the uterine precursor (MacLaughlin and Donahoe, 2004). MIS continues to be present in blood throughout male development (Lee et al., 1996), with the brain being one of its targets (Wang et al., 2005, 2009). MIS promotes the survival of embryonic spinal motor neurons (Wang et al., 2005), which leads to a subtle male bias in the number of spinal motor neurons and in the exploratory behaviour of mice in an open-field test (Wang et al., 2009). It is thus one of the factors that contribute to the subtle sex-biases in the nervous system.
Mature neurons in both sexes express high levels of MIS receptors (Lebeurrier, et al. 2008; Wang et al., 2005, 2009), suggesting that MIS may regulate some aspect of the adult brain. The nature of this putative function is unknown, but is likely to be divergent from its role in the development of the brain, as the levels of MIS in the blood of men and women are similar, and low compared to those of prepubertal boys (de Vet et al., 2002; Lee et al., 1996). Furthermore, mature spinal motor neurons in both sexes begin to produce MIS (Wang et al., 2005). This late onset of neural production of MIS may indicate that it is linked to a property of neurons that only emerges once the neural networks in the brain are both functional and are comparatively stable.
As neurons mature, their dependency on external survival factors lessens, with the production of autocrine survival factors being one of the suspected causes of this change. MIS is a potential candidate for such a role, given that its neuronal expression is limited to mature neurons (Wang et al., 2005; Wang et al., 2009), and given that the neurons which produce it also express its unique type II receptor (MISRII), and type I co-receptors (BMPR1A, BMPR1B; synonym ALK3, ALK2) (Wang et al., 2005; Wang et al., 2009). In this paper, we have tested this hypothesis by determining the effect of an avulsion injury on the production of MIS and its receptors in hypoglossal neurons, and by comparing the survival of avulsed motor neurons in Mis−/− mice and mice treated with exogenous MIS.
Materials and methods
Animals
The Mis+/+ and Mis−/− mice (Behringer et al., 1994; Wang et al., 2009) were littermates produced from Mis+/− parents. The Mis+/− colony was maintained and housed as previously described (McLennan and Taylor-Jeffs, 2004; Wang et al., 2009). The University of Otago Animal Ethics Committee approved all procedures.
Hypoglossal avulsion
Seventy- to 90-day-old mice were anesthetized as previously described (Wang et al., 2007), their ventral neck surface shaved and a 1 cm midline incision made. The left hypoglossal nerve was exposed, cleared of connective tissue and pulled using constant traction until the separation from the brainstem (avulsion) occurred, removing approximately 1 cm of nerve. The wound was closed with sutures.
Axonal transport
The transport of MIS and MISRII in motor axons was examined by ligating either the hypoglossal or sciatic nerve at two sites, as previously described (Jiang et al., 2000a; Russell et al., 2000; Wang et al., 2007). Proteins being transported down the axon accumulate at the proximal ligation, whereas retrogradely transported proteins accumulate at the distal ligation. Individual nerves were either sectioned transversely or longitudinally, to enable Schwann cell and axonal processes to be unambiguously distinguished (Jiang et al., 2000a; Russell et al., 2000; Wang et al., 2007). The transported proteins were detected by immunohistochemistry, using anti-MIS and anti-MISRII antibodies, with an anti-neurofilament antibody (Sigma) used as an axonal marker and as a positive control for axonal transport.
Intraventricular administration of MIS
An anaesthetized male mouse was placed in a stereotaxic apparatus and a hole was drilled through the skull using a dental drill at a position 0.22 mm posterior and 1 mm lateral to bregma. A 2.5 mm, 30 G cannula was inserted, secured to the skull with superglue and used to administer 0.8, 8 or 800 ng/day of MIS or vehicle into the left lateral ventricle over 15 days (see also legend of Fig. 1). The cannula was connected to a micro-osmotic pump (ALZET model 1002; 0.23± 0.02 µl/h), and the pump was inserted under the skin between the scapulae. The MIS was full length140 kDa rhMIS from Chinese hamster ovary cells (Ragin et al., 1992), with its potency verified as previously described (Wang et al., 2005). MIS is stable in osmotic pumps (Parry et al., 1992).
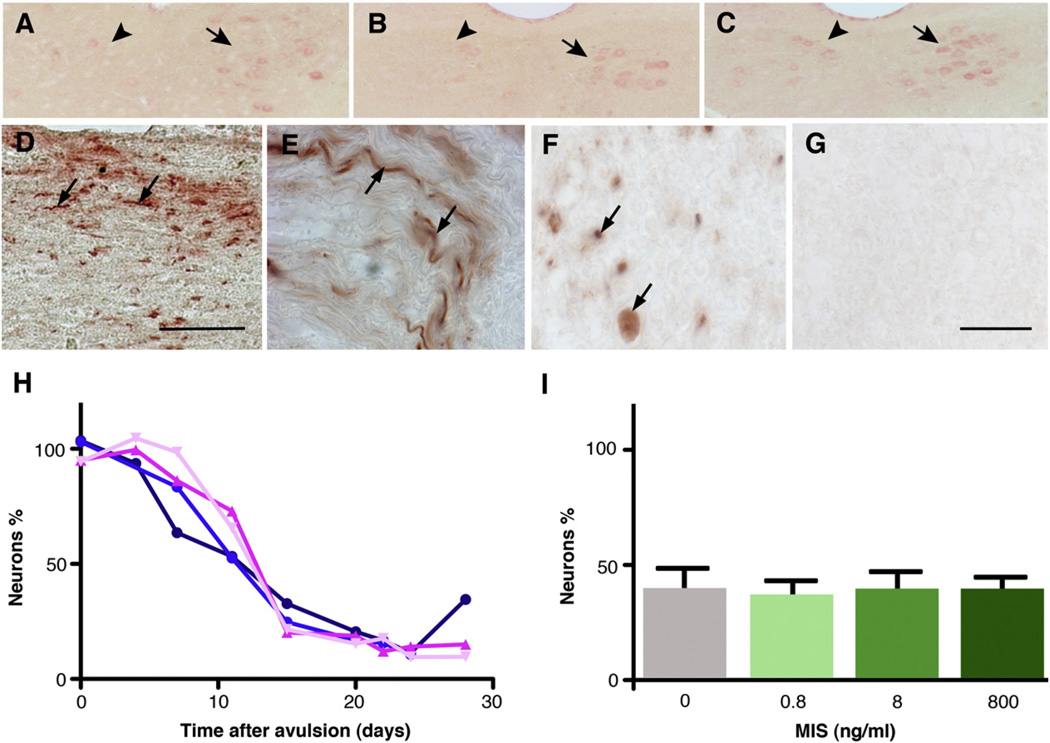
Fig. 1.
MIS and its receptors are down regulated by avulsion (A–C). The levels of MIS (A), MISRII (B) and BMPR1A (C) protein were reduced in avulsed nuclei of male mice. The sections illustrate 3-day-post avulsion. day 0, 1 and 7 sections are illustrated in Fig. S3, together with measurements of the respective mRNA levels. The left avulsed hypoglossal nuclei are indicated with arrowheads, with arrows pointing to the right nucleus (arrows), which serves as an internal control. MIS protein was transported by motor neurons (D–G). Longitudinal sections (D–E) and cross sections (F and G) of ligated sciatic nerves (E–G) and hypoglossal nerves (D) from male mice were stained with anti-MIS (D–F) or control IgG (G) antibody. The MIS immunoreactivity was mainly observed at the proximal portion of the ligated nerves and trace level of MIS immunoreactivity at distal areas (see Fig. S4). The arrows point to the immunoreactivity of MIS in the axons. The scale bars=100 µm(D) and 25 µm(E–G). The rate and extent of neuronal loss after avulsion were not affected by MIS (H–I). The data is the ratio of the number of neurons in the avulsed nucleus, relative to the contralateral nucleus. (H) The time course of neuronal loss: Mis+/+ males (dark blue circles); Mis−/− males (light blue circles); Mis+/+ females (deep pink triangles) and Mis−/− females (light pink triangles). The four groups were not significantly different. (I) Infusion of rhMIS to the left ventricle of male mice did not alter the extent of neuronal loss after 15 days. The doses were designed to produce a concentration of MIS within the CSF of 5 ng/ml (0.8 ng/day), 50 ng/ml (8 ng/day) and 5 µg/ml (800 ng/day), based on a turnover rate of 160 µl/day (Johanson, et al., 2008).
Hypoglossal nuclei mRNA
Avulsed and non-avulsed motor neurons were collected by laser microdissection, and the mRNA levels were examined by qPCR as previously described (Wang et al., 2005) (see also Supplementary Methods).
Immunohistochemistry
Avulsed mice were killed by cardiac perfusion with 4% paraformaldehyde and their hypoglossal nuclei were sectioned in a cryostat at a thickness of 30 µm and floated in PBS. The sections were washed in 0.1 M glycine followed by PBS and then sequentially incubated with; either goat anti-MISRII, goat anti-MIS or goat anti-ALK3/BMPR1A (R & D Systems) at 4 °C for 48 h; a biotinylated donkey anti-Ig antibody (Sigma-Aldrich); methanol/H2O2 to inactivate endogenous peroxidises; streptavidin–biotinylated horseradish peroxidase complex (Amersham Biosciences), after which the immunoreactivity was visualized using 3-amino-9-ethylcarbamide (AEC; Sigma-Aldrich) as the chromogen. The anti-MISRII antibody precipitates a single band of appropriate size from the spinal cord (Wang et al., 2005), and the anti-MISRII and anti-MIS antibodies do not stain the brains from their respective null-mutant mice(Mis−/− and MISRII−/−) (Wang et al., 2005; Wang et al., 2009). Sections were also incubated with non-immune IgG (Sigma-Aldrich) as a further control for non-specific binding.
Motor neuron cell counts
The numbers of motor neurons in the ipsilateral and contralateral nuclei were estimated using the Fractionator (Gundersen et al., 1988), with the counting particle being the nucleolus (see also Supplementary Methods).
Results
The murine hypoglossal nucleus is not sexually-dimorphic
The number of spinal motor neurons has a subtle male-bias, which develops under the influence of testicular MIS (Wang et al., 2009). A similar bias was, however, not present in the hypoglossal nucleus, where the numbers of motor neurons were indistinguishable between male and female mice (Fig. S1). Similarly, the number of hypoglossal motor neurons in Mis−/− and Mis+/+ mice were not significantly different (Fig. S1). This makes the hypoglossal nucleus an attractive model in which to study the actions of MIS in the mature nervous system, as MIS has minimal or no effect on its development.
Hypoglossal motor neurons express MIS and its receptors
MISRII and BMPR1A mRNA were readily detectable by real-time PCR in laser micro-dissected hypoglossal nuclei. The MISRII mRNA was 64% more abundant than BMPR1A mRNA (Fig. S2A), which contrasts with spinal motor neurons where the abundance of MISRII is ten-fold greater than BMPR1A and the other type-I, TGFβ-superfamily receptors (Wang et al., 2005; Wang et al., 2007). Significant levels of MIS mRNA was also present in the hypoglossal nucleus, but with an abundance that was lower than for its receptors (Fig. S2A).
MISRII-lineage tracing and immunohistochemistry were used to determine which cell types in the hypoglossal nuclei expressed MIS and MISRII. Antibodies to MISRII, BMPR1A and MIS selectively stained the neurons of the hypoglossal nucleus, with no detectable stain being associated with other cell types (Fig. S3A–C). Similarly, all neurons in the MISRII-Cre+ve, LacZ+ve mice were lacZ positive, providing further confirmation that hypoglossal neurons express MISRII (Fig. S2B).
Axon damage down-regulates the expression of MIS and its receptors
The genes that control the repair and growth of axons are up regulated when a nerve is cut, whereas those associated with mature synaptic function are down regulated (Armstrong et al., 1991; Mesnard et al., 2010). Immature neurons do not express significant levels of MIS, suggesting that neuronal MIS may relate to mature function, rather than axonal growth. We therefore examined the effect of avulsion on the levels of MIS and its receptors. The levels of MIS, MISRII and BMPR1A mRNAs (Fig. S3D–F) and protein (Figs. 1A–C; S3A–C) fell rapidly, to below 50% of the starting values within 1 day of axonal damage. The levels did not decline further during the next 2 days, although immunohistochemical staining of neurons suggested that further down regulation occurred at longer time points. This could not be quantified by qPCR as extensive death of avulsed neurons occurs after the 3rd day (Fig. 1H).
MIS-deficiency does not exacerbate avulsion-induced death
The production of both MIS and its receptors by hypoglossal neurons raised the possibility that MIS is an autocrine survival factor for mature neurons. If so, then MIS-deficient neurons may die more rapidly after avulsion, due to the absence of this putative survival mechanism. This did not occur (Fig. 1H). There was a minimal loss of neurons during the few days post avulsion in all mice, after which a rapid loss of neurons occurred in Mis+/+ and Mis−/− mice, of both sexes. This loss was largely complete after 15 days in all mice, with a lesser decline over the next few days (Fig. 1H). A small number of neurons did not die after avulsion, and these may be interneurons located within the hypoglossal nucleus. There was no sex or MIS genotype bias to the number of neurons surviving.
The effect of endogenous MIS should diminish over time, due to the down regulation of MIS production (Fig. S3). We therefore perfused the brains of avulsed mice with exogenous rhMIS to determine whether elevated levels of MIS sustained the avulsed neurons. Exogenous MIS had no effect on the number of neurons that survived 15 days after avulsion (Fig. 1I). The MIS recovered from the pumps at the end of the experiment retained approximately 70% of the initial MIS activity, in a bioassay based on the survival of embryonic motor neurons in vitro.
MISRII is not anterogradely transported
The growth factor receptors are transported into the axon, which is the largest component of motor neurons (Jiang et al., 2000b). Consequently, an avulsion potentially limits the ability of a neuron to respond to survival factors, due to the loss of receptors. We therefore determined whether MIS and MISRII were transported into motor axons, using the double ligation technique. MIS accumulated at the proximal tie on the hypoglossal nerve, indicating that it is transported down motor axons (Fig. 1D–G, S4). The anti-MIS stain appeared to be axonal. It had a similar appearance and distribution to the anti-neurofilament stain observed in adjacent sections, and in cross-section, it was distinct from the hollow appearance of Schwann cells (see (Jiang et al., 2000a; Russell et al., 2000)).
In marked contrast to MIS, and other receptors studied by us (Jiang et al., 2000a; Russell et al., 2000; Wang et al., 2007), no MISRII could be detected within the ligated or non-ligated nerves.
Discussion
Hypoglossal motor neurons from adult mice expressed both MIS and its receptors, whereas the glia and other cells associated with them did not (Fig. S2)(see also (Wang et al., 2005; Wang et al., 2009). This implicates MIS as a regulator of mature neurons. A priori, this could involve autocrine regulation, injury responses and anterograde or retrograde regulation of neuronal networks. As discussed below, several of these possibilities can be excluding, with the evidence pointing to MIS being involved in some aspect of anterograde regulation.
MIS differs from classical regulators
The framework for the understanding of neuronal regulators has been greatly influenced by the historic study of target-derived (retrograde) factors, such as nerve growth factor (NGF). In almost all respects, the neurobiology of MIS differs from NGF, and related molecules. Target-derived regulators are transported up the axon, whereas MIS was transported in the opposite direction, namely down the axon. Similarly, the receptors for NGF and other such factors are transported down the axon (Curtis et al., 1998), whereas MISRII was not (Fig. 1, S4). This suggests that motor neurons are unresponsive to MIS in their periphery. Furthermore, the differential transport of MIS and MISRII argues against autocrine (self) regulation, as this requires MIS and its receptors to collocate within a neuron.
MIS does not attenuate axonal injuries
MIS is a physiological survival factor for embryonic lumbar spinal motor neurons (Wang et al., 2005, 2009). Thus, the presence of MIS receptors in mature motor neurons raised the possibility that MIS is a survival factor at all stages of the life cycle. Classical survival factors provide defense against peripheral injuries, and their receptors are consequently upregulated when axons are severed (Armstrong et al., 1991; Mesnard et al., 2010). In complete contrast to this, MIS and MISRII were down-regulated after avulsion (Fig. 1, S3) and neither endogenous nor exogenous MIS influenced avulsion-induced loss of neurons (Fig. 1). Hence, MIS is not protective against a severe axonal injury.
Motor neurons are responsive to a wide range of survival factors, and some factors may only protect against specific insults. For example, endogenous interleukin-6 (IL6) regulates local immune responses in the brain after viral injury and reduces viral-induced death of motor neurons (Pavelko et al., 2003), but has little effect on axotomy-induced death (Galiano et al., 2001). Similarly, the survival effects of endogenous vascular endothelial growth factor appear to be part of a wider defense against hypoxia, which includes stimulation of capillary growth, as well as promotion of motor neuron survival (Lambrechts et al., 2003; Vande Velde and Cleveland, 2005). We therefore do not exclude MIS being part of the defense system of mature hypoglossal motor neurons, particularly as MIS protects striatal and cortical neurons against NMDA-induced excitotoxicity (Lebeurrier et al., 2008).
MIS as an anterograde regulator
Neuronal networks are stabilized and regulated by anterograde signals, which modulate neurotransmission and provide protection against anterograde toxins. The observations relating to MIS are broadly consistent with it having such a role. MIS is produced by multiple neurons (Wang et al., 2009), and in hypoglossal neurons it was transported down the axon, whereas its receptors are located in the cell bodies and dendritic trees (Fig. 1, S2,4). As noted above, MIS protects at least some neurons from excitotoxicity (Lebeurrier et al., 2008), which is the main anterograde toxin. Furthermore, genes involved in neurotransmission and dendritic function are down regulated by axon damage (Armstrong et al., 1991; Mesnard et al., 2010), which was the case for both MIS and MISRII (Fig. 1, S3). This evidence is indirect and not definitive, but nevertheless collectively provides a rationale for MIS to be studied as a putative anterograde regulator of neuronal networks, using electrophysiology and other means.
MIS may differentially regulate neurons
All neurons express MIS receptors, but this does not necessarily imply that MIS has the same action on all neurons. The GDNF receptors, Ret and GFRα1, are also ubiquitously expressed by spinal motor neurons, but only a specific sub-population of spinal motor neurons die when Ret or GFRα1 are conditionally inactivated in motor neurons (Gould et al., 2008). The hypoglossal nucleus did not exhibit a MIS dependent male bias (Fig. S1), which contrasts with lumbar spinal motor neurons (Wang et al., 2005; Wang et al., 2009). This suggests that MIS is not a physiological survival factor for embryonic hypoglossal motor neurons, and raises the possibility that MIS serves as a different function in hypoglossal and spinal motor neurons. Hypoglossal and spinal motor neurons have significant commonalities, but hypoglossal motor neurons are involved in respiration and mastication, whereas spinal motor neurons control movement and stance. Their pattern of use is therefore different and they can be differentially affected in some forms of motor neuron disease (Rowland, 2010).
One of the differences between spinal and hypoglossal neurons is their relative level of BMPR1A and MISRII. BMPR1A is also a receptor for the BMPs (Shi and Massague, 2003; Teixeira et al., 2001), and MIS and the BMPs are thus likely to have a common downstream action. Consistent with this, MIS and BMP6 have similar action on motor neurons in vitro (Wang et al., 2005, 2007). The comparative high levels of BMPR1A in hypoglossal neurons (cf (Wang et al., 2005) vs Fig. S2) may indicate that these neurons are more dependent on BMPs than MIS, whereas the spinal motor neurons may be the converse.
Conclusion
In conclusion, hypoglossal motor neurons produce MIS and MISRII, implicating MIS as a regulator of mature neurons. The current study provides evidence against neuronally-produced MIS having either an autocrine or classical retrograde action. The intracellular location of MIS and MISRII are consistent with MIS being a regulator of anterograde mechanisms, although further proof of this will require multiple studies, using a variety of techniques.
Supplementary Material
Acknowledgments
This work was supported by Otago Innovation Limited (ISM), NERF (Foundation for Science Research and Technology) (ISM), Maurice and Phyllis Paykel Trust and AFJ Mickle Scholarship (CLT) and Postdoctoral Fellowship from the Neurological Foundation of New Zealand (ANC). The authors thank N. Batchelor for production and care of the mice.
References
- Armstrong DM, Brady R, Hersh LB, Hayes RC, Wiley RG. Expression of choline acetyltransferase and nerve growth factor receptor witin hypoglossal motoneurons following nerve injury. J. Comp. Neurol. 1991;304:596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Curtis R, Tonra JR, Stark JL, Adryan KM, Park JS, Cliffer KD, Lindsay RM, DiStefano PS. Neuronal injury increases retrograde axonal transport of the neurotrophins to spinal sensory neurons and motor neurons via multiple receptor mechanisms. Mol. Cell. Neurosci. 1998;12:105–118. doi: 10.1006/mcne.1998.0704. [DOI] [PubMed] [Google Scholar]
- de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil. Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- Galiano M, Liu ZQ, Kalla R, Bohatschek M, Koppius A, Gschwendtner A, Xu S, Werner A, Kloss CU, Jones LL, Bluethmann H, Raivich G. Interleukin-6 (IL6) and cellular response to facial nerve injury: effects on lymphocyte recruitment, early microglial activation and axonal outgrowth in IL6-deficient mice. Eur. J. Neurosci. 2001;14:327–341. doi: 10.1046/j.0953-816x.2001.01647.x. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Ann. Rev. Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Gould TW, Yonemura S, Oppenheim RW, Ohmori S, Enomoto H. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J. Neurosci. 2008;28:2131–2146. doi: 10.1523/JNEUROSCI.5185-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol. Microbiol. Immunol. Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, McLennan IS, Koishi K, Hendry IA. Transforming growth factor-beta 2 is anterogradely and retrogradely transported in motoneurons and up-regulated after nerve injury. Neuroscience. 2000a;97:735–742. doi: 10.1016/s0306-4522(00)00084-1. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhang M, Koishi K, McLennan IS. TGF-beta 2 attenuates the injury-induced death of mature motoneurons. J. Neurosci. Res. 2000b;62:809–813. doi: 10.1002/1097-4547(20001215)62:6<809::AID-JNR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, III, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, Wyns S, Thijs V, Andersson J, van Marion I, Al-Chalabi A, Bornes S, Musson R, Hansen V, Beckman L, Adolfsson R, Pall HS, Prats H, Vermeire S, Rutgeerts P, Katayama S, Awata T, Leigh N, Lang-Lazdunski L, Dewerchin M, Shaw C, Moons L, Vlietinck R, Morrison KE, Robberecht W, Van Broeckhoven C, Collen D, Andersen PM, Carmeliet P. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat. Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Lebeurrier N, Launay S, Macrez R, Maubert E, Legros H, Leclerc A, Jamin SP, Picard JY, Marret S, Laudenbach V, Berger P, Sonderegger P, Ali C, di Clemente N, Vivien D. Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J. Cell Sci. 2008;121:3357–3365. doi: 10.1242/jcs.031872. [DOI] [PubMed] [Google Scholar]
- Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, Hasegawa Y, Noto RA, Schoenfeld D, MacLaughlin DT. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J. Clin. Endocrinol. Metab. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- MacLaughlin DT, Donahoe PK. Sex determination and differentiation. New Eng. J. Med. 2004;350:367–378. doi: 10.1056/NEJMra022784. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Taylor-Jeffs J. The use of sodium lamps to brightly illuminate mouse houses during their dark phases. Lab Anim. 2004;38:1–9. doi: 10.1258/0023677041958927. [DOI] [PubMed] [Google Scholar]
- Mesnard NA, Alexander TD, Sanders VM, Jones KJ. Use of laser microdissection in the investigation of facial motoneuron and neuropil molecular phenotypes after peripheral axotomy. Exp. Neurol. 2010;225:94–103. doi: 10.1016/j.expneurol.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RL, Chin TW, Epstein J, Hudson PL, Powell DM, Donahoe PK. Recombinant human Müllerian inhibiting substance inhibits human ocular melanoma cell lines in vitro and in vivo. Cancer Res. 1992;52:1182–1186. [PubMed] [Google Scholar]
- Pavelko KD, Howe CL, Drescher KM, Gamez JD, Johnson AJ, Wei T, Ransohoff RM, Rodriguez M. Interleukin-6 protects anterior horn neurons from lethal virus-induced injury. J. Neurosci. 2003;23:481–492. doi: 10.1523/JNEUROSCI.23-02-00481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin RC, Donahoe PK, Kenneally MK, Ahmad MF, MacLaughlin DT. Human Mullerian inhibiting substance: enhanced purification imparts biochemical stability and restores antiproliferative effects. Protein Expr. Purif. 1992;3:236–245. doi: 10.1016/1046-5928(92)90020-w. [DOI] [PubMed] [Google Scholar]
- Rowland LP. Progressive muscular atrophy and other lower motor neuron syndromes of adults. Muscle Nerve. 2010;41:161–165. doi: 10.1002/mus.21565. [DOI] [PubMed] [Google Scholar]
- Russell FD, Koishi K, Jiang Y, McLennan IS. Anterograde axonal transport of glial cell line-derived neurotrophic factor and its receptors in rat hypoglossal nerve. Neuroscience. 2000;97:575–580. doi: 10.1016/s0306-4522(00)00079-8. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Teixeira J, Maheswaran S, Donahoe PK. Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr. Rev. 2001;22:657–674. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- Vande Velde C, Cleveland DW. VEGF: multitasking in ALS. Nat. Neurosci. 2005;8:5–7. doi: 10.1038/nn0105-5. [DOI] [PubMed] [Google Scholar]
- Wang PY, Koishi K, McGeachie AB, Kimber M, Maclaughlin DT, Donahoe PK, McLennan IS. Mullerian inhibiting substance acts as a motor neuron survival factor in vitro. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16421–16425. doi: 10.1073/pnas.0508304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PY, Koishi K, McLennan IS. BMP6 is axonally transported by motoneurons and supports their survival in vitro. Mol. Cell. Neurosci. 2007;34:653–661. doi: 10.1016/j.mcn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wang PY, Protheroe A, Clarkson AN, Imhoff F, Koishi K, McLennan IS. Müllerian Inhibiting Substance contributes to sex-linked biases in the brain and behavior. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7203–7208. doi: 10.1073/pnas.0902253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.