Abstract
A major reason for dental resin composite restoration replacement is related to secondary caries promoted by acid production from bacteria including Streptococcus mutans (S. mutans). We hypothesized that S. mutans has esterase activities that degrade dental resin composites and adhesives. Standardized specimens of resin composite (Z250), total-etch (Scotchbond Multipurpose, SB), and self-etch (Easybond, EB) adhesives were incubated with S. mutans UA159 or uninoculated culture medium (control) for up to 30 days. Quantification of the BisGMA-derived biodegradation by-product, bishydroxy-propoxy-phenyl-propane (BisHPPP), was performed by high-performance liquid chromatography. Surface analysis of the specimens was performed by scanning electron microscopy (SEM). S. mutans was shown to have esterase activities in levels comparable with those found in human saliva. A trend of increasing BisHPPP release throughout the incubation period was observed for all materials and was more elevated in the presence of bacteria vs. control medium for EB and Z250, but not for SB (p < .05). SEM confirmed the increased degradation of all materials with S. mutans UA159 vs. control. S. mutans has esterase activities at levels that degrade resin composites and adhesives; degree of degradation was dependent on the material’s chemical formulation. This finding suggests that the resin-dentin interface could be compromised by oral bacteria that contribute to the progression of secondary caries.
Keywords: Streptococcus mutans, esterases, dental bonding, hydrolysis, biodegradation, chromatography
Introduction
Dental resin composites are currently the most popular restorative materials in dentistry (Krifka et al., 2013). Nearly 70% of resin composite restorations are replacements for failed restorations (Murray et al., 2002). Recurrent or secondary caries is the primary reason given for composite restorative replacement, followed by fracture (Ferracane, 2011).
Resin composite restorations require the application of resin adhesives to bond efficiently to the tooth structure (dentin and enamel). Currently, there are two main adhesive systems, total-etch (etch-and-rinse) and self-etch. In the total-etch adhesive systems, acid-etching and priming/bonding of the dentin are separate steps, whereas self-etch adhesive systems combine etching and priming/bonding in one step (Liu et al., 2011). For self-etch adhesive systems to etch and prime simultaneously, they have been designed to contain hydrophilic and acidic resin monomers (Moszner et al., 2005; Van Landuyt et al., 2007).
Human saliva contains esterase activities, cholesterol esterase-like (CE-like) and pseudocholinesterase, that degrade bis-phenyl glycidyl dimethacrylate (BisGMA)-containing resin composites and adhesives (Jaffer et al., 2002), yielding the degradation product bishydroxy-propoxy-phenyl-propane (BisHPPP) (Shokati et al., 2010). This process compromises the resin-dentin interface, allowing for cariogenic bacterial ingression along the interface (Kermanshahi et al., 2010).
Dental caries is the result of acid production from bacterial carbohydrate metabolism that leads to the demineralization of tooth structures. S. mutans is regarded as the chief etiological agent responsible for dental caries (Kidd and Beighton, 1996). In addition, streptococcus species have been shown to contain esterases (Zhu et al., 2009). While there have been studies investigating the impact of composite degradation products on bacterial growth and virulence gene expression (Khalichi et al., 2009; Singh et al., 2009), the potential effect of bacterial degradative activity on resin composites and adhesives has yet to be explored. Therefore, we hypothesized that, in addition to acid production, cariogenic bacteria contain esterase activities that degrade dental resin composites and adhesives.
Materials & Methods
Bacterial Esterase Activity Assay
S. mutans strains UA159, JH1005, LT11, NG8, UA140, BM71, and GS5 were subcultured on Todd-Hewitt agar plates supplemented with 0.3% yeast extract (THYE) (Khalichi et al., 2004). Colonies of S. mutans from THYE plates were cultivated overnight in THYE broth (37°C, 5% CO2) and then diluted 1:10 and allowed to grow to mid-log growth phase, washed, and re-suspended in phosphate buffer (pH = 7.0). We determined esterase activities, CE-like and PCE-like, by incubating 1 mL of the bacterial cell suspension in 0.5 mL of either p-nitrophenylbutyrate (p-NPB), o-nitrophenylbutyrate (o-NPB), p-nitrophenylacetate (p-NPA), or butyrylthiocholine iodide (BTC) substrates (Sigma, St. Louis, MO, USA), as described previously (Finer and Santerre, 2004a).
Preparation of Composite and Adhesive Resin Specimens
Cylindrical specimens (diameter, 4 mm; height, 4 mm) of resin composite resin (Z250), a total-etch (Scotchbond Multipurpose, SB), and a self-etch adhesive (Easybond, EB) (3M, London, ON, Canada) were prepared with TeflonTM molds and MylarTM strips, photopolymerized for 10 sec from each side (Sapphire plus, DenMat, Santa Maria, CA, USA), and post-cured in a vacuum oven (60°C, 48 hrs) (Jaffer et al., 2002). All instrumentation was autoclaved or disinfected in a 70% ethanol/water solution. Specimen preparation was performed in a biosafety cabinet.
Degree of Vinyl Group Conversion at the Surface
Degree of vinyl group conversion for the different specimens was measured as described previously (Finer and Santerre, 2007) with a Spectrum Bx FT-IR system (Perkin Elmer, Waltham, MA, USA) at the Analest Laboratory, University of Toronto. Analysis of the data was carried out with the software Spectrum version 5.0.1. Briefly, the absorbance ratios of the peak areas for the carbon double-bond (C=C methacrylate saturation) (1,636 cm-1) and the aromatic ring in BisGMA (1,608 cm-1) for uncured and cured materials were measured. Degree of conversion (DC) was calculated according to the equation:
X-ray Photoelectron Spectroscopy
Elemental composition analysis of all pre-incubated specimens was performed by x-ray photoelectron spectroscopy (XPS) at a 90° take-off angle relative to the sample surface, as described previously (Finer and Santerre, 2007). We analyzed the specimens in both low- and high-resolution modes to determine percentage atomic composition and functional group data for the C1s region, respectively (Thermo Scientific K-Alpha XPS system, East Grinstead, UK) at Surface Interface Ontario, University of Toronto.
Contact Angle Measurements
Advancing water contact angle measurements were obtained by means of a goniometer (NRL C.A. goniometer, Ramé-Hart, Inc., Mountain Lakes, NJ, USA). Briefly, a microsyringe was used to place a 20-μL droplet of distilled/deionized water on the materials’ surfaces (N = 3). For each droplet, the contact angle on either side was measured, and the average ± standard deviation was reported as a single measurement (Battiston et al., 2012).
Biodegradation Experiments
Cured specimens (N = 3 from each material, incubation condition, and period) were incubated in sterile vials containing either 2 mL of brain-heart infusion (BHI) (Becton, Dickinson and Co., Sparks, MD, USA) (control group) or a 1:10 dilution in BHI of overnight S. mutans UA159 grown in BHI (test group). Incubation solutions were collected every 48 hrs from each group and replaced with fresh solutions. Incubation solutions were accumulated, pooled, and analyzed for isolation and quantification of BisHPPP degradation product at 2, 4, 7, 14, and 30 days by high-performance liquid chromatography (HPLC) as previously reported (Shokati et al., 2010). The purity of the bacterial culture was assessed by gram stain at each media replacement (Fucio et al., 2008).
Scanning Electron Microscopy (SEM)
Surface morphology observations for pre- and post-incubation specimens (N = 3 from each material, incubation condition, and period) were performed by scanning electron microscopy (S2500, Hitachi, Mito City, Japan) at an operating voltage of 10 kV as described previously (Shokati et al., 2010). Prior to observation, specimens were sonicated to remove bacterial cells from the surface for direct observation of the materials’ surfaces. Specimens from each group were analyzed after critical-point-drying (CPD 7501, Fisons Instruments VG Microtech, Uckfield, East Sussex, UK) and coated with platinum (SC515 SEM coating system, Polaron Equipment Ltd, Hertfordshire, UK).
Statistical Analysis
One-way analyses of variance (ANOVA) and Tukey’s multiple comparison tests were performed to determine differences in esterase activity profiles, advancing water contact angle, degree of conversion, or BisHPPP release. A t test was used to determine differences between BisHPPP release for the two incubation conditions (S. mutans + BHI or BHI alone) at the same time-point (p < .05).
Results
Bacterial Esterase Activity Assay
All strains of S. mutans had activity toward the nitrophenyl esters (Fig. 1). All strains had preference toward the p-NPA and p-NPB vs. o-NPB (p < .05), with no significant difference between the para-isomers. All S. mutans strains showed no activity toward BTC substrate. The highest activity with p-NPB was observed with S. mutans UA159, at 2.07 ± 0.15 units/mg dry cell weight.
Figure 1.
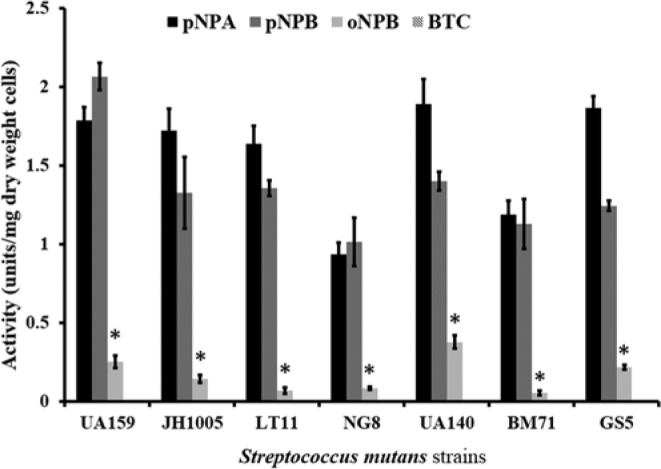
Activity profile for S. mutans UA159, JH1005, LT11, NG8, UA140, BM71, and GS5 with p-nitrophenylbutyrate (p-NPB), o-nitrophenylbutyrate (o-NPB), p-nitrophenylacetate (p-NPA), and butyrylthiocholine iodide (BTC). Data are plotted with standard error of the the mean (N = 3). *represents statistically significant differences (p < .05).
Material Characterization
The degree of vinyl group conversion ranged from 66.1% ± 4.5% to 74.9% ± 5.8% with no significant differences among the materials (Table).
Table.
Surface Properties and Composition (based on MSDS from the manufacturer, 3M) of Resin Composite (Z250), Total-etch (SB), and Self-etch (EB) Adhesives
| Z250 | SB | EB | ||
|---|---|---|---|---|
| Contact angle | 89 ± 4.1a | 75 ± 5.0b | 55.4 ± 4.0c | |
| Degree of conversion | 71.7 ± 2.2a | 66.1 ± 4.5a | 74.9 ± 5.8a | |
| Elements (atomic %) | C1s | 66.4 ± 3.6 | 72.2 ± 3.0 | 71.4 ± 2.3 |
| O1s | 24.8 ± 1.8 | 24.8 ± 2.1 | 25.0 ± 1.2 | |
| Si2p | 3.6 ± 1.4 | 1.7 ± 0.7 | 1.5 ± 0.9 | |
| Zr3d | 0.4 ± 0.2 | 0.1 ± 0.1 | 0.05 ± 0.04 | |
| Chemical Bonds (atomic %) | C-CC-O | 66.7 ± 1.522.4 ± 1.4 | 66.9 ± 2.221.8 ± 2.7 | 66.4 ± 1.323.7 ±1.6 |
| C=O | 9.4 ± 0.5 | 10.0 ± 3.1 | 9.8 ± 0.2 | |
| Z250 | SB | EB | ||
| Composition | Bisphenol A diglycidyl ether dimethacrylate (BisGMA) | 5-10 | 60-70 | 15-25 |
| 2-Hydroxyethyl methacrylate (HEMA) | — | 30-40 | 15-25 | |
| Diurethane dimethacrylate (UDMA) | 5-10 | — | — | |
| Bisphenol A polyethylene glycol diether dimethacrylate (BisEMA) | 5-10 | — | — | |
| Triethylene glycol dimethacrylate (TEGDMA) | 1-5 | — | — | |
| Silane-treated ceramic | 75-85 | — | 8-12 | |
| Water | 1-2 | — | 10-15 | |
| Ethanol | — | — | 10-15 | |
| Phosphoric acid-6-methacryloxy-hexylesters | — | — | 10-15 | |
| 1,6-Hexanediol dimethacrylate | — | — | 5-10 | |
| Copolymer of acrylic and itaconic acid | — | — | 1-5 |
Data are plotted with standard deviation of the mean. One-way analysis of variance (ANOVA) and Tukey’s post hoc tests were used to test for significant differences in contact angle or degree of conversion between and among the materials. Values with the same letter denote statistically non-significant differences between and among materials for the same assay (p > .05).
XPS analyses showed that the initial surface elemental composition was similar for all materials with virtually pure resin on the surface (Table). High-resolution spectra of the C1s peak (Table) indicated that the C1s binding energies at 285.0, 286.5, and 289 eV corresponded, respectively, to the C-C, C-O, and C=O bonds. All materials had similar chemical group function, with a higher amount of C-C bonds, followed by C-O and C=O (p < .05). [The presence of C=O is assigned primarily to the ester bond of the resin and identified that ester groups were present in similar levels within the surface region of all materials and therefore potentially available for hydrolysis.]
Surface wettability of the materials was analyzed by advancing water contact angle measurements (Table). The most hydrophilic material was EB, having the lowest contact angle (55.4 ± 4.0), followed by SB and then Z250 (p < .05).
Biodegradation
A trend of increasing BisHPPP release with time throughout the incubation period was observed for all 3 materials (Fig. 2). The amount of BisHPPP released was elevated in the presence of bacteria vs. control for EB and Z250 but not for SB (p < .05) after 30 days and/or 14 days of incubation. The amount of BisHPPP released from EB after 30 days of incubation with S. mutans UA159 (143.15 ± 3.28 μg/cm2) was 39 and 82 times higher than that released from Z250 (3.71 ± 0.24 μg/cm2) and SB (1.74 ± 0.31 μg/cm2), respectively (p < .05). In the specimens incubated with bacteria, the total amount of BisHPPP released throughout the incubation period was significantly higher in EB (375.4 ± 3.6 μg/cm2) when compared with that in Z250 (13.1 ± 0.7 μg/cm2) and SB (6.1 ± 0.3 μg/cm2) (p < .05).
Figure 2.
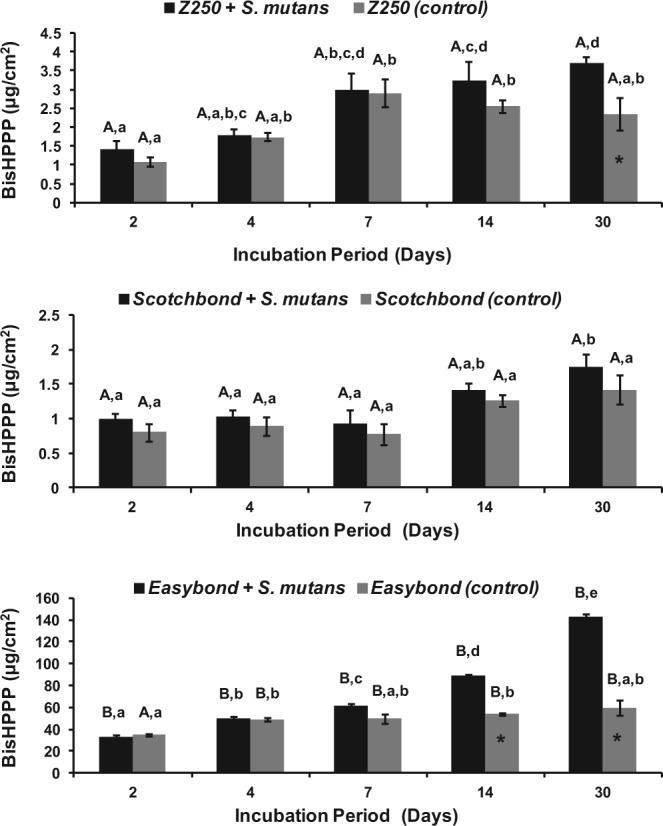
Cumulative amounts of BisHPPP production after incubation of a resin composite (Z250) (top), total-etch adhesive (SB) (middle), and self-etch adhesive (EB) (bottom) in BHI with S. mutans UA159 (black) and with BHI alone (gray). Data are plotted with standard error of the mean. *represents significant differences between the two incubation conditions for each material at the same time-point (p < .05). Values with the same lower case letter denote statistically non-significant differences within each material’s group (p > .05). Values with the same capital letters indicate non-significant differences among materials (p > .05).
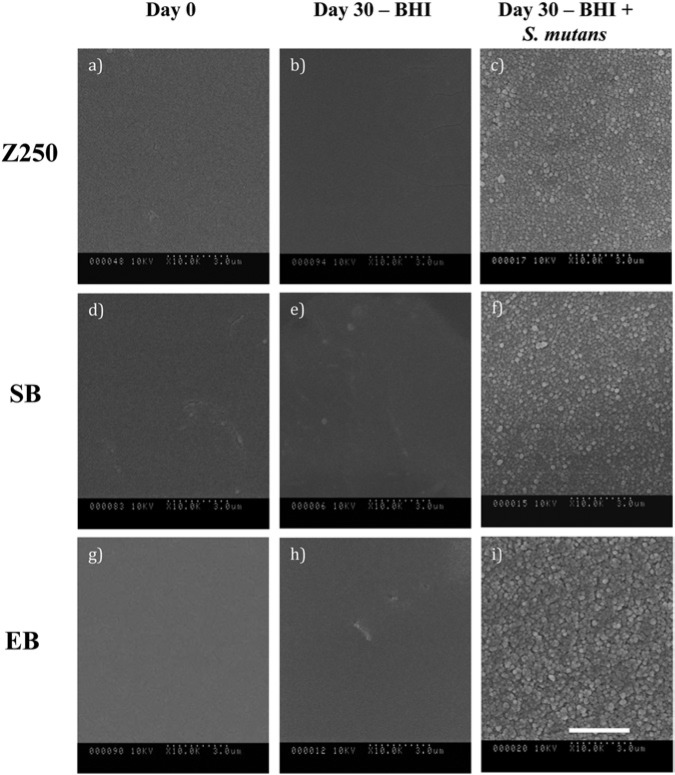
SEM micrographs (Fig. 3) demonstrate that the surfaces of the specimens incubated with S. mutans UA159 for 30 days appear rougher than those of BHI-incubated and non-incubated specimens.
Figure 3.
Scanning electron micrographs of Z250 (a-c), SB (d-f), and EB (g-i) at day 0 (a, d, g), and following 30 days of incubation with BHI (b, e, h) and with S. mutans UA159 (c, f, i) (104 x original magnification). Scale bar applies to all Figs. and represents 3 µm. Note the rougher surfaces of bacteria-incubated specimens, with rougher surfaces for EB vs. SB and Z250.
Discussion
The results of this study support the hypothesis that cariogenic bacteria (S. mutans) contain esterase activities at levels capable of hydrolytic-mediated degradation of cured dental resin composites and adhesives. This represents a significant finding for the field and identifies a clear vulnerability of current restorative materials to one of the most prominent bacteria in oral pathology.
Human saliva has been shown to hydrolyze resin composites and adhesives (Shokati et al., 2010). Human salivary esterases were previously analyzed to have activity toward o- and p-nitrophenyl esters and BTC (Finer and Santerre, 2004a). In the current study, all tested strains of S. mutans had activity toward the nitrophenyl esters, but not BTC, in levels that were previously shown to degrade resin composites and adhesives (Lin et al., 2005; Shokati et al., 2010). Overall, the activity patterns of S. mutans suggest that these micro-organisms are contributors more to the para nitrophenyl acetate-like-dependent esterase activities of saliva and less to the more predominant para nitrophenyl butyrate-like-dependent esterases that are characteristic of human salivary esterase activity (Finer and Santerre, 2004a).
S. mutans UA159 was selected over the other strains for the subsequent biodegradation study because it had the highest esterase activity with respect to p-NPB, a characteristic activity previously shown to affect composites as well (Jaffer et al., 2002). BisHPPP, a BisGMA-derived biodegradation product, was analyzed since it is a good marker of true resin biodegradation due to the hydrophobic nature of its precursor, and therefore the results provide a good indication of the biodegradation potential of S. mutans UA159 in vivo (Finer and Santerre, 2007).
Many bacterial species express esterases; however, the overall function of S. mutans esterases and, more specifically, their importance in contributing to the biodegradation process of dental resin composite restorations are not well-understood. In other bacteria, esterases have been linked to virulence and pathogenesis (Lun and Bishai, 2007; Wall et al., 2007; Zhu et al., 2009). These studies highlight the importance of esterases and point toward the need for the study of the potential role of S. mutans esterases in aciduricity, virulence, and pathogenesis (i.e., dental caries).
The self-etch adhesive (EB) exhibited a higher release of BisHPPP relative to the total-etch adhesive (SB), and the resin composite (Z250), after incubation with bacteria or bacterial media. The differences ranged between 39 and 82 times higher than that released from Z250 and SB, respectively, after 30 days of incubation with bacteria. Because there was no difference in the degree of conversion (FTIR) and surface elemental composition of the specimens (XPS) among the different materials, and since the amount of BisHPPP production for each material was not correlated with the BisGMA content for each material, the difference in the amounts of released products could arise only from the materials’ inherent differences in chemical composition (Finer and Santerre, 2004b).
The incorporation of acidic monomers (phosphoric acid-6-methacryloxy-hexylesters, and copolymer of acrylic and itaconic acid) and water as a co-solvent in EB at high concentrations renders this material more hydrophilic compared with SB and Z250, as demonstrated by the advancing water contact angles. The increased hydrophilicity amplifies water sorption, which in turn leads to greater susceptibility of the ester bonds to hydrolysis (Ito et al., 2005; Hashimoto et al., 2008). Water sorption has also been shown to contribute to hydrolysis, plasticization of the polymer, and the lowering of mechanical properties (Ito et al., 2005; Hashimoto et al., 2008). In addition to esterase-mediated hydrolysis, hydrogen ions from acidic resin monomers and hydrogen ions produced by oral biofilms could catalyze the hydrolysis of the ester bonds present in the polymer matrix (Moszner et al., 2005; Borges et al., 2011; Silva et al., 2012). The above provides a possible contributing explanation for the reduced performance of self-etch vs. total-etch adhesive systems in clinical applications (Peumans et al., 2005; Van Meerbeek et al., 2010).
Advancing water contact angle values revealed that SB is more hydrophilic than Z250, which could be attributed in part to the inclusion of 30% to 40% by weight of HEMA in SB. A previous degradation study (Shokati et al., 2010) demonstrated greater degradation of SB vs. Z250 by human saliva. Yet, in the current study, SB showed slightly more stability and released less BisHPPP than Z250 in the presence of bacteria. Therefore, in addition to the material’s hydrophilicity, other factors affect the material’s relative biostability.
The co-existence of both CE-like and PCE-like activities in in vitro systems was shown to result in a more efficient biodegradation of the resin matrix (Finer et al., 2004). The lack of activity for S. mutans toward the PCE-like substrate BTC, as compared with the broader activity of saliva which contains both CE-like and PCE activity (Finer and Santerre, 2004a), could explain the less efficient degradation of SB by bacteria, in part because SB contains water-soluble moieties, such as HEMA, that may show more susceptibility to PCE-like enzymes. In previous work, it was demonstrated that CE has greater specificity than PCE to hydrolyze phenol-containing monomers, such as BisGMA and BisEMA, while PCE showed greater affinity than CE toward short water-soluble monomers such as TEGDMA (Yourtee et al., 2001; Finer and Santerre, 2004a).
XPS data and SEM micrographs revealed that the surfaces of all pre-incubated specimens were composed mainly of resin, precluding any influence of the filler on the initial amount of resin surface available for degradation. Following 30-day incubation, SEM analyses demonstrated rougher surfaces for all materials incubated with bacteria vs. controls. This observation corroborates results from previous studies that demonstrated rougher surfaces for some restorative materials incubated with bacteria but did not undertake quantitative liquid chromatography measurements of the incubation media within the study to draw the association with specific esterase activity from the bacteria (Fucio et al., 2008; Gregson et al., 2012). The current study provides a possible contributing explanation for these observations, i.e., that esterases from the bacteria degrade the materials while releasing specific degradation by-products.
Kermanshahi et al. (2010) showed that exposure of dentin-composite restorations to salivary esterase-like activity resulted in the formation of micro-gaps that were infiltrated and colonized by biofilms of cariogenic bacteria such as S. mutans. When present within the confined space of the restoration-tooth marginal interface, S. mutans has the potential to contribute to the deterioration of the resin-dentin interface by producing both acids (Borges et al., 2011) and esterases, affecting the hybrid layer, tooth, and composite, ultimately compromising the integrity of the margins and reducing the longevity of the restoration. Although esterase-mediated degradation occurred in all materials used in this study, the extent of degradation was material-dependent, and material chemistry appeared to be a critical factor in determining a restoration’s biochemical stability. Manufacturers of dental resin composites and adhesives should test for the materials’ biochemical stability to develop more biostable materials.
Acknowledgments
The authors thank Dr. Dilani Senadheera, Martha Cordova, and Kirsten Krastel for their technical support.
Footnotes
This research was supported by the National Institute of Dental & Craniofacial Research (R01DE021385) and by the Canadian Institute of Health Research (MOP115113). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research and the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Battiston KG, Labow RS, Santerre JP. (2012). Protein binding mediation of biomaterial-dependent monocyte activation on a degradable polar hydrophobic ionic polyurethane. Biomaterials 33:8316-8328. [DOI] [PubMed] [Google Scholar]
- Borges MA, Matos IC, Mendes LC, Gomes AS, Miranda MS. (2011). Degradation of polymeric restorative materials subjected to a high caries challenge. Dent Mater 27:244-252. [DOI] [PubMed] [Google Scholar]
- Ferracane JL. (2011). Resin composite—state of the art. Dent Mater 27:29-38. [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. (2004a). Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res 83:22-26. [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. (2004b). The influence of resin chemistry on a dental composite’s biodegradation. J Biomed Mater Res A 69:233-246. [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. (2007). Influence of silanated filler content on the biodegradation of bisGMA/TEGDMA dental composite resins. J Biomed Mater Res A 81:75-84. [DOI] [PubMed] [Google Scholar]
- Finer Y, Jaffer F, Santerre JP. (2004). Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials 25:1787-1793. [DOI] [PubMed] [Google Scholar]
- Fucio SB, Carvalho FG, Sobrinho LC, Sinhoreti MA, Puppin-Rontani RM. (2008). The influence of 30-day-old Streptococcus mutans biofilm on the surface of esthetic restorative materials - An in vitro study. J Dent 36:833-839. [DOI] [PubMed] [Google Scholar]
- Gregson KS, Shih H, Gregory RL. (2012). The impact of three strains of oral bacteria on the surface and mechanical properties of a dental resin material. Clin Oral Investig 16:1095-1103. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Fujita S, Kaga M, Yawaka Y. (2008). Effect of water on bonding of one-bottle self-etching adhesives. Dent Mater J 27:172-178. [DOI] [PubMed] [Google Scholar]
- Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. (2005). Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26:6449-6459. [DOI] [PubMed] [Google Scholar]
- Jaffer F, Finer Y, Santerre JP. (2002). Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials 23:1707-1719. [DOI] [PubMed] [Google Scholar]
- Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y. (2010). Biodegradation of resin-dentin interfaces increases bacterial microleakage. J Dent Res 89:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalichi P, Cvitkovitch DG, Santerre JP. (2004). Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials 25:5467-5472. [DOI] [PubMed] [Google Scholar]
- Kidd EA, Beighton D. (1996). Prediction of secondary caries around tooth-colored restorations: a clinical and microbiological study. J Dent Res 75:1942-1946. [DOI] [PubMed] [Google Scholar]
- Krifka S, Spagnuolo G, Schmalz G, Schweikl H. (2013). A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 34:4555-4563. [DOI] [PubMed] [Google Scholar]
- Lin BA, Jaffer F, Duff MD, Tang YW, Santerre JP. (2005). Identifying enzyme activities within human saliva which are relevant to dental resin composite biodegradation. Biomaterials 26:4259-4264. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. (2011). Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90:953-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun S, Bishai WR. (2007). Characterization of a novel cell wall-anchored protein with carboxylesterase activity required for virulence in Mycobacterium tuberculosis. J Biol Chem 282:18348-18356. [DOI] [PubMed] [Google Scholar]
- Moszner N, Salz U, Zimmermann J. (2005). Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater 21:895-910. [DOI] [PubMed] [Google Scholar]
- Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF. (2002). Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Crit Rev Oral Biol Med 13:509-520. [DOI] [PubMed] [Google Scholar]
- Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. (2005). Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater 21:864-881. [DOI] [PubMed] [Google Scholar]
- Shokati B, Tam LE, Santerre JP, Finer Y. (2010). Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface. J Biomed Mater Res B Appl Biomater 94:230-237. [DOI] [PubMed] [Google Scholar]
- Silva EM, Almeida GS, Poskus LT, Guimarães JG. (2012). Influence of organic acids present in the oral biofilm on the microtensile bond strength of adhesive systems to human dentin. J Biomed Mater Res B Appl Biomater 100:735-741. [DOI] [PubMed] [Google Scholar]
- Singh J, Khalichi P, Cvitkovitch DG, Santerre JP. (2009). Composite resin degradation products from BisGMA monomer modulate the expression of genes associated with biofilm formation and other virulence factors in Streptococcus mutans. J Biomed Mater Res A 88:551-560. [DOI] [PubMed] [Google Scholar]
- Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. (2007). Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 28:3757-3785. [DOI] [PubMed] [Google Scholar]
- Van Meerbeek B, Peumans M, Poitevin A, Mine A, Van Ende A, Neves A, et al. (2010). Relationship between bond-strength tests and clinical outcomes. Dent Mater 26:e100-e121. [DOI] [PubMed] [Google Scholar]
- Wall T, Bath M, Britton RA, Jonsson H, Versalovic J, Roos S. (2007). The early response to acid shock in Lactobacillus reuteri involves the ClpL chaperone and a putative cell wall-altering esterase. Appl Environ Microbiol 73:3924-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourtee DM, Smith RE, Russo KA, Burmaster S, Cannon JM, Eick JD, et al. (2001). The stability of methacrylate biomaterials when enzyme challenged: kinetic and systematic evaluations. J Biomed Mater Res 57:522-531. [DOI] [PubMed] [Google Scholar]
- Zhu H, Liu M, Sumby P, Lei B. (2009). The secreted esterase of Group A streptococcus is important for invasive skin infection and dissemination in mice. Infect Immun 77:5225-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]