Abstract
Bone morphogenetic proteins (BMPs) are well-studied regulators of cartilage and bone development that have been Food and Drug Administration (FDA)-approved for the promotion of bone formation in certain procedures. BMPs are seeing more use in oral and maxillofacial surgeries because of recent FDA approval of InFUSE® for sinus augmentation and localized alveolar ridge augmentation. However, the utility of BMPs in medical and dental applications is limited by the delivery method. Currently, BMPs are delivered to the surgical site by the implantation of bulky collagen sponges. Here we evaluate the potential of detonation nanodiamonds (NDs) as a delivery vehicle for BMP-2 and basic fibroblast growth factor (bFGF). Nanodiamonds are biocompatible, 4- to 5-nm carbon nanoparticles that have previously been used to deliver a wide variety of molecules, including proteins and peptides. We find that both BMP-2 and bFGF are readily loaded onto NDs by physisorption, forming a stable colloidal solution, and are triggered to release in slightly acidic conditions. Simultaneous delivery of BMP-2 and bFGF by ND induces differentiation and proliferation in osteoblast progenitor cells. Overall, we find that NDs provide an effective injectable alternative for the delivery of BMP-2 and bFGF to promote bone formation.
Keywords: nanomedicine, drug delivery, biomaterials, bioengineering, nanoparticle, carbon
Introduction
Bone morphogenetic proteins (BMPs) are well-studied regulators of cartilage and bone development (Wozney et al., 1988; Celeste et al., 1990; Gautschi et al., 2007). Of the BMPs, rhBMP-2 and rhBMP-7 have been granted Food and Drug Administration approval and are used for a variety of on- and off-label procedures involving the promotion of bone formation (Govender et al., 2002; Ong et al., 2010). BMPs are also now finding usage in oral and maxillofacial procedures thanks to FDA approval of InFUSE® for use in sinus and localized alveolar ridge augmentation. Although in vitro studies indicate that BMP-2 is effective as a solution, the in vivo effects are dependent on the local concentration (Uludag et al., 1999). Because of this, it is widely accepted that BMP-2 delivery requires a carrier to prevent rapid clearance by diffusion. The current clinical standard is a collagen sponge soaked in a solution of BMP-2, which is then implanted at the desired location. Although this approach might be acceptable for larger, open procedures, the space limitations in oral and maxillofacial surgeries make it less ideal.
Several groups have evaluated different delivery methods for BMPs, including implant coatings and alternative organic and inorganic matrices (Allegrini et al., 2004; Schliephake et al., 2005). Nanoparticle suspensions present an interesting option as a delivery vehicle for BMP-2, since a liquid would be easy to use as an injection or rinse, while the particles would limit the rate of diffusion away from the active site (Zhang et al., 2009). Nanodiamonds (NDs) are produced at scale and are biocompatible (Chow et al., 2011; Zhang et al., 2012; Moore et al., 2013) 4- to 5-nm carbon nanoparticles that have previously been used to deliver a wide variety of cargos, including proteins varying in size from peptides to antibodies (Yeap et al., 2008; Shimkunas et al., 2009; Liu and Sun, 2010; Wei et al., 2010; Smith et al., 2011; Zhang et al., 2011). The related ultrananocrystalline diamond films, synthesized by chemical vapor deposition, have been shown to promote bone cell adhesion and proliferation (Yang et al., 2009) in addition to mineralization through the delivery of BMP-2 (Steinmuller-Nethl et al., 2006).
In this work, we evaluate the potential of ND suspensions as a protein delivery vehicle for the promotion of bone formation. Although BMP-2 and -7 are effective at promoting bone formation on their own, there are numerous proteins involved in the signaling cascade that may augment the response to BMPs. One such protein is fibroblast growth factor (FGF) (Kubota et al., 2002; Nakamura et al., 2005). FGF is known to promote myoblast proliferation and inhibit differentiation (Moore et al., 1991; Templeton and Hauschka, 1992); however, at low doses, FGF-basic (bFGF) has been found to enhance BMP-2 activity in vivo (Fujimura et al., 2002). Here we demonstrate that NDs are capable of simultaneously delivering various doses of 2 proteins, BMP-2 and bFGF, for the promotion of bone cell differentiation and proliferation in vitro.
Materials & Methods
Complete materials and methods can be found in the online Appendix.
Protein Loading
To maintain sterility, all syntheses and dilutions were performed in a class II biosafety cabinet. Proteins were mixed at the desired ratio and diluted in PBS to generate a 0.5 to 0.75X PBS solution after the addition of NDs in water. Autoclaved NDs at 200 to 500 µg/mL in water were added to the protein solution, mixed gently, and allowed to incubate for 15 min at 22°C. The suspension was centrifuged for 15 min at 16,200 x g at 4°C, and the supernatant was removed and re-suspended in water (for sizing) or cell culture media (for cell assays) with approximately 30 sec of bath sonication. Supernatants were either used immediately or stored at -20°C for future assays.
Cell Assays
Cells were plated at either 20,000 (for experiments with bFGF) or 30,000 cells/well in 24-well plates in DMEM containing 10% FBS. Cells were allowed to adhere overnight before treatment. Cells were treated with NDs or controls in DMEM containing 2.5% FBS for 72 hrs prior to assay. Cells underwent lysis with 200 µL per well of cell lysis solution (20 mM Tric-HCl, 1 mM EDTA, 150 mM NaCL, 1 mM MgCl2 hexahydrate, 1% NP-40, 5% glycerol at pH 7.9) for 10 min on an orbital shaker. Cell lysates were collected in microtubes and centrifuged for 5 min at 16,200 x g at 4°C. Three replicates of 20 µL of supernatant were transferred to a 96-well plate and combined with 200 µL of QuantiBlue solution (reconstituted according to the manufacturer’s protocol). Solution was incubated for 2 to 24 hrs at 37°C, and absorbance was read at 633 nm to determine alkaline phosphatase activity, which was then normalized to supernatant DNA content, as measured with a PicoGreen® kit. Three replicates of 5 µL of supernatant were added to 95 µL of kit buffer in a black-walled 96-well plate. A 100-µL quantity of PicoGreen® reagent was then added to each well, incubated for 5 min at room temperature and protected from light, and fluorescence was read at 480 nm excitation/520 nm emission. Results were compared with a standard curve generated from the kit. All experiments were performed in triplicate.
Statistics
All statistics were performed in GraphPad Prism. All analyses were done by 1- or 2-way analysis of variance (ANOVA). The Bonferroni correction was used to account for multiple comparisons.
Results
ND-BMP-2 and bFGF Cluster Synthesis
The first step toward using detonation nanodiamonds (NDs) to deliver BMP-2 and FGF is the optimization of a synthesis protocol. Previous studies have demonstrated that proteins can be rapidly loaded into and released from ND clusters through physisorption (Shimkunas et al., 2009; Smith et al., 2011). We elected to load recombinant human BMP-2 and FGF-basic using a similar method (Fig. 1). Incubation of NDs with the protein mixture in 0.5 to 0.75X PBS resulted in reproducible ND protein clusters. Varying the ratio of ND: BMP-2 (wt:wt) in solution demonstrated that there is a minimum amount of protein necessary to stabilize the colloidal solution (Fig. 2A). At ratios greater than 80:1, the average size and standard deviation increased, suggesting destabilization and agglomeration. Additionally, the particles synthesized with only bovine serum albumin (BSA) are slightly larger (p < .05 vs. 10:1 only) than those made with BMP-2, indicating that BMP-2 may have a role in cluster stabilization. Adding bFGF to the BMP-2 clusters did not affect the final cluster size (Fig. 2B). However, when FGF was added to the ND-BSA clusters (ND-FGF), we observed greater variation in size. In spite of the variability in the size of ND-FGF, we did not observe precipitation from solution over time. Because of the improved stability of the particles synthesized at 80:1 ND: BMP-2, this ratio was used for all subsequent syntheses. Because of the expense of recombinant proteins compared with NDs, which are a by-product of detonation, our goal was to obtain a stable colloidal solution using a minimum of protein. By minimizing the protein used during synthesis, we also theoretically minimized protein loss, which led to a lower cost for synthesis of the final product and reduced barriers to effective clinical translation.
Figure 1.
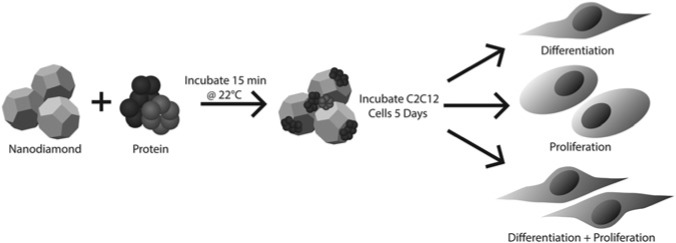
Schematic of ND cluster synthesis and cell culture experiments. Recombinant human BMP-2, recombinant human FGF-basic, and bovine serum albumin were loaded into ND clusters by incubation at room temperature in phosphate-buffered saline. The resulting clusters were then washed and added to C2C12 myoblasts in cell culture, where they induced differentiation into osteoblasts and/or proliferation, depending on the protein combination.
Figure 2.
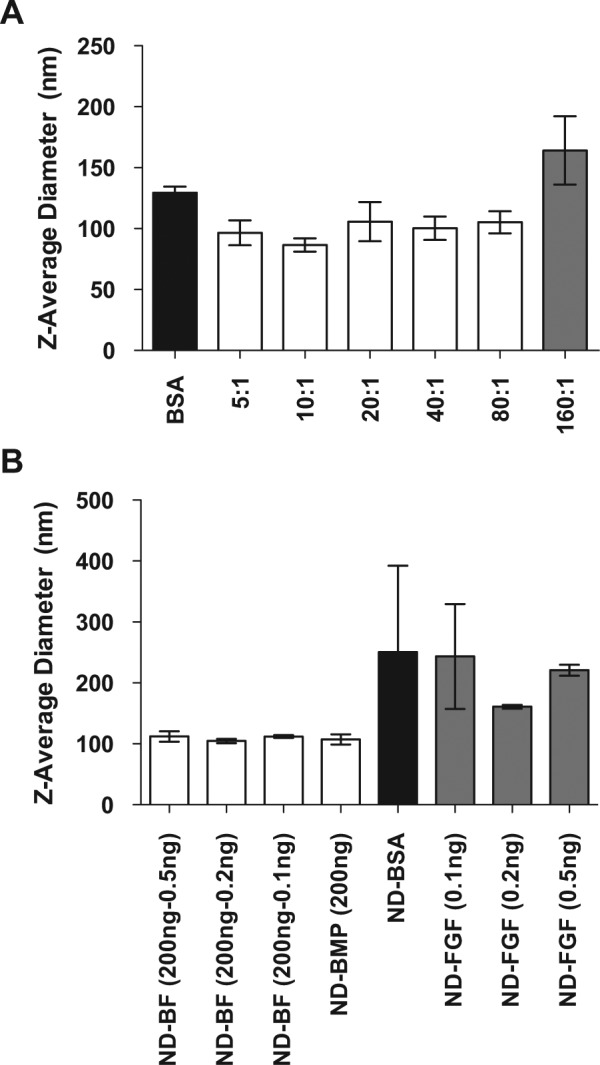
Nanodiamond-protein cluster size. Recombinant human BMP-2 and FGF-basic were loaded into ND clusters with phosphate-buffered saline and bovine serum albumin. (A) Varying the ratio of ND: BMP-2 (wt:wt) during the synthesis process resulted in differing final complex sizes. The maximum ratio providing stable ND-BMP clusters was 80:1. (B) The addition of FGF to the ND-BMP synthesized with 200 ng of BMP (ND-BF 200) did not alter the final cluster size. However, there was increased variability in size when FGF was added to NDs and BSA in the absence of BMP-2 (ND-FGF).
Protein Loading onto Nanodiamonds
Fourier transform infrared spectroscopy (FTIR) was used to confirm protein loading into ND clusters. The spectra of ND-BMP, ND-BMP+FGF (ND-BF), and ND-BSA showed peaks at 1,650 and 1,540 cm−1 that are not observed with plain NDs. These peaks are consistent with the presence of amide bonds found in proteins (Fig. 3A; see Appendix Table for complete peak list). More quantitative loading information can be achieved by analysis of the protein levels remaining in the supernatant after ND separation by centrifugation. Protein assay of the supernatants remaining after ND separation demonstrated the ability to load an average 73.1% of the protein added to the solution, or 45.6% (wt ND) (Appendix Fig. 1). However, the FDA-approved formulation of BMP-2, which we used, also contains a significant amount of BSA for stabilization (1 mg/mL). To assess the amount of BMP-2 in the supernatants, as opposed to total protein, we were able to use 2 methods: enzyme-linked immunosorbent assay (ELISA) and C2C12 cell alkaline phosphatase production. ELISA analysis demonstrated that, on average, 0.86% (wt) BMP-2, or 69% of the BMP-2 added, was loaded onto NDs (Fig. 3B). Loading did not vary across batch sizes (400 ng BMP-2 vs. 200 ng BMP) or when bFGF was added (0.5 ng added to 200 ng BMP-2). Similar results were obtained by incubation of C2C12 myoblasts with the supernatants. C2C12 myoblasts differentiated into alkaline-phosphatase-producing osteoblasts in the presence of BMP-2 (Katagiri et al., 1994). Alkaline phosphatase production from cells incubated in the particle synthesis supernatant indicated that an average of 0.89% wt BMP-2 loaded onto NDs (Appendix Fig. 2), which is consistent with the ELISA results.
Figure 3.
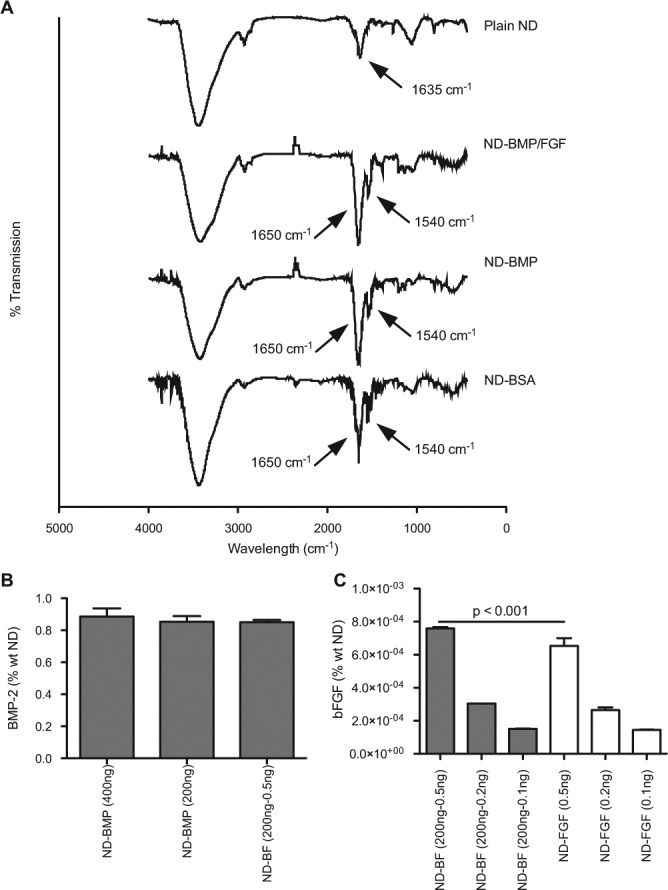
BMP-2 and bFGF loading. Assessment of BMP-2 and bFGF loading into ND clusters by FTIR and ELISA. (A) FTIR spectra of plain NDs, ND-BMP+FGF (200 ng BMP-2, 0.5 ng bFGF), ND-BMP (200 ng BMP-2), and ND-BSA (10 µg, equivalent to others). Additional peaks in ND-BMP, ND-BMP+FGF, and ND-BSA are consistent with the addition of amide functional groups. See Appendix Table for complete peak listing. (B) BMP-2 ELISA of supernatants demonstrated 0.86% wt BMP-2 loading. (C) bFGF ELISA of supernatants demonstrated loading ranging from 1.4 x 10−4 to 7.6 x 10−4% wt, depending on the initial protein input (listed in parentheses).
ELISA can also be used to assess bFGF loading based on the amount of protein remaining in the supernatant after particle synthesis. We were able to load between 1.4 x 10−4 and 7.6 x 10−4 % wt bFGF into ND clusters with and without BMP-2, depending on the initial amount of FGF used for synthesis (Fig. 3C). Particles synthesized from bFGF and BSA contained an average of 85% of the initial mass of bFGF added during synthesis. In contrast, we were able to load an average of 95% of the bFGF when the particles were made from bFGF and BMP-2. The additional bFGF loading we observed with ND-BF vs. ND-FGF may have been due to a charge interaction between the net-positive charge of FGF-basic (Moy et al., 1996) and amphiphilic BMP-2 (Scheufler et al., 1999).
ND-Protein Clusters Promote Osteoblast Differentiation and Proliferation
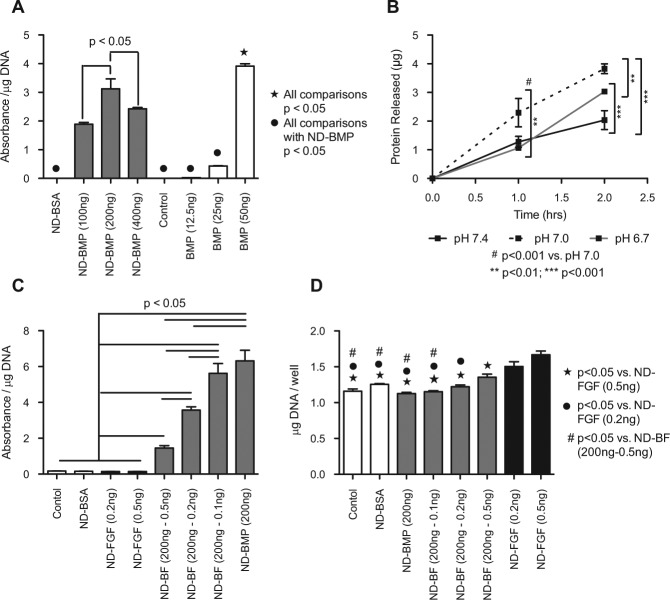
To examine if the BMP-2 delivered by NDs was still functional, we incubated C2C12 myoblasts with either ND-BMP or a known concentration of BMP-2. BMP-2 induced differentiation of the myoblasts into osteoblasts (Katagiri et al., 1994), which was detectable by alkaline phosphatase activity. We noted that ND-BMP induced osteoblast differentiation in the C2C12 cells at multiple doses (Fig. 4A). Alkaline phosphatase activity was highest at the middle dose of ND-BMP, which suggests that there may be an optimal dose. The normalized alkaline phosphatase activity in the ND-BMP wells corresponded to 37.8, 45.8, and 41.5 ng of free BMP-2 (low, medium, and high doses, respectively). Interestingly, when cells were washed after 24 hrs of incubation with ND-BMP or BMP-2 alone, this effect was not observed (Appendix Fig. 3). Additionally, the responses of the cells when exposed to ND-BMP for 24 hrs as opposed to 72 hrs were markedly reduced, indicating a delay in protein release from the ND clusters. Combined, these 2 findings indicate that BMP-2 release from ND clusters has a non-linear or delayed profile.
Figure 4.
NDs induced osteoblast differentiation and proliferation. BMP-2 and bFGF delivered by ND induced osteoblast differentiation and proliferation, respectively. (A) Day 5 alkaline phosphatase activity normalized to DNA content from C2C12 cells incubated with various concentrations of ND-BMP or BMP-2 alone (doses listed indicate initial synthesis conditions). (B) Total protein released from ND clusters demonstrates pH dependence and a near-linear profile during the first 2 hrs (Appendix Fig. 4). (C) Day 5 alkaline phosphatase activity normalized to DNA content from C2C12 cells incubated with various amounts of FGF as ND-BF or ND-FGF. FGF inhibited alkaline phosphatase production in a dose-dependent fashion. (D) Day 5 total DNA content from C2C12 cells incubated with various doses of FGF as ND-BF or ND-FGF. FGF induced proliferation of C2C12 cells in a dose-dependent fashion.
Examination of protein released from ND-BMP clusters indicated that BMP-2 release was dependent on solution pH (Appendix Fig. 4). At all pHs, there appeared to be some burst release in the first 2 hrs, followed by a plateau over 24 hrs. However, at pH 5.0, there was 4.7-fold more protein released than at pH 7.4, and 2.5-fold more protein released than at pH 3.0. The pH effect was also observed during the first 2 hrs at more physiological pHs (Fig. 4B). During the first 2 hrs, we noted a nearly linear release profile, with the maximal amount of protein released at pH 7.0, with pH 6.7 catching up at 2 hrs. Combined, these results demonstrate that protein release from ND-BMP clusters is pH-dependent, with improved release in slightly acidic environments. Additionally, we observed that not all of the protein is released after 24 hrs, suggesting that some protein may bind irreversibly to the ND clusters.
FGF-basic has previously been shown to promote proliferation while inhibiting myoblast differentiation (Templeton and Hauschka, 1992). However, several other groups have demonstrated in vivo that low-dose FGF can augment the response to BMP-2 (Kubota et al., 2002; Nakamura et al., 2005). To examine the effect of simultaneous delivery of BMP-2 and FGF, we incubated the C2C12 cells with ND-FGF and ND-BF carrying various amounts of FGF-basic (Fig. 4C). We observed that ND-BF induced alkaline phosphatase activity, while ND-FGF did not. Consistent with other in vitro reports (Hughes-Fulford and Li, 2011), FGF appeared to inhibit the osteoblast differentiation induced by BMP-2 (Fig. 4C). However, FGF induced cellular proliferation, as measured by total DNA content, in a dose-dependent fashion (Fig. 4D). NDs and ND-BMP appeared to have little impact on overall DNA content; however, the addition of 0.5 ng bFGF per well induced a 1.44-fold increase in total DNA content. These results indicate that BMP-2 and bFGF are both active when delivered simultaneously by NDs.
Discussion
Here we have demonstrated that NDs are capable of simultaneously delivering 2 functional proteins: BMP-2 and bFGF. ND-BMP induced differentiation of C2C12 myoblasts into alkaline-phosphatase-producing osteoblasts (Fig. 4A), while the combination of BMP-2 and bFGF in ND clusters (ND-BF) induced proliferation of the precursor myoblasts in addition to differentiation (Figs. 4C, 4D). However, the response to BMP-2 and FGF in cultured cells is complex. In vitro, consistent with other reports (Hughes-Fulford and Li, 2011), we observed that bFGF induced proliferation while inhibiting myoblast differentiation (Fig. 4C). In contrast, multiple in vivo reports have demonstrated that the addition of FGF augments bone formation in response to BMP-2 (Kubota et al., 2002; Nakamura et al., 2005). Although the BMP-2 and bFGF pathways are competitive in cultured cells, both pathways are necessary for effective bone healing in vivo. bFGF (FGF-2) is critical for promoting the proliferation of osteoblast progenitors, while BMP-2 is necessary for stimulating osteoblast differentiation and mineral production (Hughes-Fulford and Li, 2011). Therefore, although combinatorial therapy does not induce augmented alkaline phosphatase activity in vitro, augmented bone formation is likely to occur in vivo, since we have demonstrated that both proteins are functional.
In addition to the ability to deliver 2 functional proteins in one cluster, ND-mediated delivery offers an advantage in terms of protein release profile. We observed that the response rate of the C2C12 myoblasts to ND-BMP was reduced compared with that of free BMP-2 (Fig. 4A). The most likely reason for this is delayed release of protein from the ND clusters (Fig. 4B, Appendix Fig. 4), inducing a delayed cellular response (Fig. 4A vs. Appendix Fig. 3). Previous reports on proteins or peptides loaded onto NDs have demonstrated that a stimulus, such as a pH change, is necessary for release of the protein from the ND after cluster formation (Shimkunas et al., 2009; Smith et al., 2011). In our case, the natural acidification of cell culture media over the course of culture was the likely trigger for protein release from ND clusters.
In addition, we observed that the total BMP-2 loaded onto the ND clusters did not correlate perfectly with the observed alkaline phosphatase response. The normalized alkaline phosphatase activity in the ND-BMP wells corresponded to 37.8, 45.8, and 41.5 ng of free BMP-2 (low, medium, and high doses, respectively). However, based on the ELISA of the supernatants, the cells were treated with 69.0, 138, or 276 ng of BMP-2 on nanodiamonds. This may have been due in part to the delay of protein release until the acidification of cell culture media occurred, but it may also have been due to incomplete protein release. The maximal protein release from ND-BMP at 24 hrs was 2.73 µg (Appendix Fig. 4, pH 5), which is less than 30% of the 9.14 µg loaded onto the ND clusters (Appendix Fig. 1). This suggests that only 30% of the BMP-2 would be released after 24 hrs in well-acidified media, which would occur only at the end of cell culture. Thirty percent of 138 ng is 41.2 ng, which correlates well with the alkaline phosphatase production data (45.8 ng, Fig. 4A). While perhaps not ideal for in vitro applications, the mouth naturally maintains an average pH of 6.78 (Aframian et al., 2006) and readily tolerates more acidic solutions like black coffee (pH 5) and orange juice (pH 3), making protein release in an acidified environment ideal for oral/dental applications.
In consideration of the translational potential of NDs as a delivery vehicle for BMP-2 and bFGF, the delayed release, response saturation, and nanometer-sized clusters are all advantageous. Delayed or triggered release may allow for more precise control over the location of protein delivery. The combination of delayed release and response saturation could allow for depot formation and sustained delivery. Furthermore, the 100-nm clusters will limit clearance by diffusion, keeping the proteins at the desired location for longer and enhancing activity. However, the greatest advantage of a protein delivery system of NDs is that it is a liquid suspension. This would be particularly advantageous in surgeries where space is limited, such as in oral and maxillofacial procedures. Instead of implanting a bulky collagen sponge, the clinician could administer a solution of ND-BF by injection or rinse. Furthermore, in the event of a non-union or implant failure, it might be possible to avoid a second surgery by injecting or rinsing with an ND-BF solution.
In summary, we have demonstrated that NDs are an effective delivery vehicle for BMP-2 and bFGF. ND-BF provides an attractive alternative to collagen sponges for the promotion of osteoblast progenitor cell differentiation and proliferation.
Supplementary Material
Acknowledgments
We thank Dr. C. Newcomb, S. Lee, and F. Tantakitti for their guidance in working with C2C12 cells and for protocol assistance. D.H. gratefully acknowledges support from the National Science Foundation CAREER Award (CMMI-0846323), Center for Scalable and Integrated NanoManufacturing (DMI-0327077), CMMI-0856492, DMR-1105060, V Foundation for Cancer Research Scholars Award, Wallace H. Coulter Foundation Translational Research Award, Society for Laboratory Automation and Screening Endowed Fellowship, Beckman Coulter, and European Commission funding program FP7-KBBE-2009-3.
Footnotes
D.H. and L.M. gratefully acknowledge support from National Cancer Institute grant U54CA151880. (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.) L.M. gratefully acknowledges support from the National Cancer Institute Grant 1F30CA174156 and the Northwestern University Malkin Scholarship. Additionally, this work was facilitated by core facilities provided by the Northwestern University Institute for Bionanotechnology and Medicine and the Northwestern University Atomic and Nanoscale Experimental Center.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Aframian DJ, Davidowitz T, Benoliel R. (2006). The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis 12:420-423. [DOI] [PubMed] [Google Scholar]
- Allegrini S, Jr, Yoshimoto M, Salles MB, Konig B., Jr (2004). Bone regeneration in rabbit sinus lifting associated with bovine BMP. J Biomed Mater Res B Appl Biomater 68:127-131. [DOI] [PubMed] [Google Scholar]
- Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, et al. (1990). Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA 87:9843-9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EK, Zhang X-Q, Chen M, Lam R, Robinson E, Huang H, et al. (2011). Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 3:73ra21. [DOI] [PubMed] [Google Scholar]
- Fujimura K, Bessho K, Okubo Y, Kusumoto K, Segami N, Iizuka T. (2002). The effect of fibroblast growth factor-2 on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in rat muscle. Arch Oral Biol 47:577-584. [DOI] [PubMed] [Google Scholar]
- Gautschi OP, Frey SP, Zellweger R. (2007). Bone morphogenetic proteins in clinical applications. ANZ J Surg 77:626-631. [DOI] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. (2002). Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84-A:2123-2134. [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M, Li CF. (2011). The role of FGF-2 and BMP-2 in regulation of gene induction, cell proliferation and mineralization. J Orthop Surg Res 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. (1994). Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127(6 Pt 1):1755-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Iseki S, Kuroda S, Oida S, Iimura T, Duarte WR, et al. (2002). Synergistic effect of fibroblast growth factor-4 in ectopic bone formation induced by bone morphogenetic protein-2. Bone 31:465-471. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun K. (2010). Protein functionalized nanodiamond arrays. Nanoscale Res Lett 5:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JW, Dionne C, Jaye M, Swain JL. (1991). The mRNAs encoding acidic FGF, basic FGF and FGF receptor are coordinately downregulated during myogenic differentiation. Development 111:741-748. [DOI] [PubMed] [Google Scholar]
- Moore L, Chow EK, Osawa E, Bishop JM, Ho D. (2013). Diamond-lipid hybrids enhance chemotherapeutic tolerance and mediate tumor regression. Adv Mater 25:3532-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy FJ, Seddon AP, Böhlen P, Powers R. (1996). High-resolution solution structure of basic fibroblast growth factor determined by multidimensional heteronuclear magnetic resonance spectroscopy. Biochemistry 35:13552-13561. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, Wakitani S. (2005). Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone 36:399-407. [DOI] [PubMed] [Google Scholar]
- Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. (2010). Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine 35:1794-1800. [DOI] [PubMed] [Google Scholar]
- Scheufler C, Sebald W, Hulsmeyer M. (1999). Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J Mol Biol 287:103-115. [DOI] [PubMed] [Google Scholar]
- Schliephake H, Aref A, Scharnweber D, Bierbaum S, Roessler S, Sewing A. (2005). Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin Oral Implants Res 16:563-569. [DOI] [PubMed] [Google Scholar]
- Shimkunas RA, Robinson E, Lam R, Lu S, Xu X, Zhang X-Q, et al. (2009). Nanodiamond-insulin complexes as pH-dependent protein delivery vehicles. Biomaterials 30:5720-5728. [DOI] [PubMed] [Google Scholar]
- Smith AH, Robinson EM, Zhang XQ, Chow EK, Lin Y, Osawa E, et al. (2011). Triggered release of therapeutic antibodies from nanodiamond complexes. Nanoscale 3:2844-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmuller-Nethl D, Kloss FR, Najam-Ul-Haq M, Rainer M, Larsson K, Linsmeier C, et al. (2006). Strong binding of bioactive BMP-2 to nanocrystalline diamond by physisorption. Biomaterials 27:4547-4556. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Hauschka SD. (1992). FGF-mediated aspects of skeletal muscle growth and differentiation are controlled by a high affinity receptor, FGFR1. Dev Biol 154:169-181. [DOI] [PubMed] [Google Scholar]
- Uludag H, D’Augusta D, Palmer R, Timony G, Wozney J. (1999). Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res 46:193-202. [DOI] [PubMed] [Google Scholar]
- Wei L, Zhang W, Lu H, Yang P. (2010). Immobilization of enzyme on detonation nanodiamond for highly efficient proteolysis. Talanta 80:1298-1304. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. (1988). Novel regulators of bone formation: molecular clones and activities. Science 242:1528-1534. [DOI] [PubMed] [Google Scholar]
- Yang L, Sheldon BW, Webster TJ. (2009). Orthopedic nano diamond coatings: control of surface properties and their impact on osteoblast adhesion and proliferation. J Biomed Mater Res A 91:548-556. [DOI] [PubMed] [Google Scholar]
- Yeap WS, Tan YY, Loh KP. (2008). Using detonation nanodiamond for the specific capture of glycoproteins. Anal Chem 80:4659-4665. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Mochalin VN, Neitzel I, Knoke IY, Han J, Klug CA, et al. (2011). Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials 32:87-94. [DOI] [PubMed] [Google Scholar]
- Zhang S, Doschak MR, Uludag H. (2009). Pharmacokinetics and bone formation by BMP-2 entrapped in polyethylenimine-coated albumin nanoparticles. Biomaterials 30:5143-5155. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hu W, Li J, Tao L, Wei Y. (2012). A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol Res 1:62-68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.