Abstract
The clinical translation of stem-cell-based dental pulp regeneration will require the use of injectable scaffolds. Here, we tested the hypothesis that stem cells from exfoliated deciduous teeth (SHED) can generate a functional dental pulp when injected into full-length root canals. SHED survived and began to express putative markers of odontoblastic differentiation after 7 days when mixed with Puramatrix™ (peptide hydrogel), or after 14 days when mixed with recombinant human Collagen (rhCollagen) type I, and injected into the root canals of human premolars in vitro. Roots of human premolars injected with scaffolds (Puramatrix™ or rhCollagen) containing SHED were implanted subcutaneously into immunodeficient mice (CB-17 SCID). We observed pulp-like tissues with odontoblasts capable of generating new tubular dentin throughout the root canals. Notably, the pulp tissue engineered with SHED injected with either Puramatrix™ or rhCollagen type I presented similar cellularity and vascularization when compared with control human dental pulps. Analysis of these data, collectively, demonstrates that SHED injected into full-length human root canals differentiate into functional odontoblasts, and suggests that such a strategy might facilitate the completion of root formation in necrotic immature permanent teeth.
Keywords: tissue scaffolds, biocompatible materials, stem cells, neovascularization, dentinogenesis, odontoblasts
Introduction
Forceful displacements of teeth, such as intrusion and avulsion, can result in rupture of the apical blood vessel network and dental pulp necrosis. Pulp necrosis interrupts dentinogenesis, leading to incomplete root formation (i.e., narrow dentin walls, large pulp chamber) and teeth prone to fracture upon secondary trauma (Cvek, 1992). The routine endodontic treatment for these cases allows for infection control, but does not facilitate the completion of root formation and protection against external root resorption. Tissue-engineering-based approaches have been considered an attractive strategy for dental pulp regeneration (Nakashima and Reddi, 2003; Nör, 2006; Rosa et al., 2011). The development of a clinically relevant strategy for dental pulp tissue engineering will require the characterization of injectable scaffolds that maintain stem cell viability during transplantation and enable a functional dental pulp to be generated.
Although mesenchymal stem cells can be found in many oral tissues (e.g., bone, periodontal ligament, gingivae), dental pulps from exfoliating primary teeth are an attractive source because they constitute a relatively “disposable” post-natal tissue from which stem cells can be readily isolated. Stem cells from human exfoliated deciduous teeth (SHED) are highly proliferative and capable of generating a dental pulp-like tissue and to differentiate into odontoblasts (Miura et al., 2003; Cordeiro et al., 2008; Casagrande et al., 2010; Chadipiralla et al., 2010) and endothelial cells (Sakai et al., 2010; Bento et al., 2013). Proof-of-principle experiments have used fairly rigid scaffolds, as for example poly(L-lactic acid (PLLA), cast within tooth slices and transplanted into mice (Cordeiro et al., 2008; Sakai et al., 2011). Although such a strategy provides valuable mechanistic information on the potential of dental pulp stem cells in pulp regeneration, it presents shortcomings: (A) The use of tooth slices does not take into full account the three-dimensional geometry of root canals. It is known that cytokine transport, cell signaling, and tissue development are influenced by the geometry of the environment where the cells are placed (Engler et al., 2006; Gelain et al., 2007). (B) While oxygen/nutrient diffusion might be sufficient for the maintenance of cell survival and metabolism in tooth slices, the quick generation of functional microvessel networks is required to maintain cell viability in full-length root canals. (C) The clinical translation of rigid scaffolds is hindered by the inherent difficulties of casting a scaffold that penetrates and adapts to the dentin walls throughout the entire root canal. For such reasons, the development of injectable scaffolds that can be used in full root canals is a key step toward clinically relevant pulp regeneration.
The nanofiber hydrogel Puramatrix™ is a self-assembling peptide that has shown promising results in neural and cardiac tissue regeneration (Davis et al., 2005; Gelain et al., 2007; Thonhoff et al., 2008). We have recently shown that this hydrogel maintained the viability and supported the odontoblastic differentiation of dental pulp stem cells (DPSC) in vitro (Cavalcanti et al., 2013). Collagen-based scaffolds are also attractive for dental pulp tissue engineering, considering that collagen is a major component of human dental pulps. Notably, collagen has been shown to support DPSC survival and growth (Gebhardt et al., 2009). The development of human recombinant collagen (rhCollagen) matrices is attractive from a translational standpoint because they are potentially safer than animal collagen and because they enable a crosstalk to occur between cells and extracellular matrix from the same species. Indeed, rhCollagen type I scaffold has shown promising results when used for bone and corneal regeneration (Yang et al., 2004; Merrett et al., 2008). Here, we tested the suitability of injectable scaffolds (i.e., Puramatrix™, rhCollagen type I) for dental pulp tissue engineering using SHED transplanted into full-length root canals of human premolars.
Materials & Methods
SHED Survival and Proliferation in Three-dimensional Injectable Scaffolds in vitro
Pooled SHED cells obtained from Dr. Songtao Shi (Miura et al., 2003) were re-suspended (105 cell/mL, passages 3-8) in 20 µL of 0.2% Puramatrix™ (BD Biosciences, Bedford, MA, USA) or rhCollagen type I (human recombinant BD™ Fibrogen Collagen Type I; BD Biosciences) and cultured in triplicate wells of 96-well plates. Cells were fixed in 3.7% formaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 10 min at room temperature, washed in PBS, and then stained for Actin-F (Alexa Fluor 488 phalloidin; Invitrogen, Grand Island, NY, USA) and ProLong Gold anti-fade reagent with DAPI (Invitrogen). Cells were counted by means of a grid under a fluorescent microscope in 3 independent experiments. After 1 or 7 days, confocal microscopy (LSM 510 META; Zeiss, Thornwood, NY, USA) was used to obtain images in Z–stack for FITC (λ = 488 nm) and DAPI (λ = 350 nm). Images and projections were reconstructed with IMARIS 7.0 (Bitplane, St. Paul, MN, USA). Here, and throughout this work, statistical analyses were performed with either the t test or one-way analysis of variance (ANOVA), followed by post hoc tests with SigmaStat 2.0 software (SPSS, Chicago, IL, USA).
Odontoblastic Differentiation in vitro
The crowns of immature human premolars extracted for orthodontic reasons in 12- to 14-year-old patients were sectioned, and the dental pulps were carefully removed without touching the predentin. The cervical end of each root was cut to standardize the length to 11 mm. The apical openings ranged from 1 to 3 mm. Teeth were cleaned and disinfected with ethylic alcohol and thoroughly washed with PBS (Invitrogen). Roots were kept in a vertical position by stabilizing them with inverted Transwell inserts (BD Falcon) in such a way that only the apical third of the root was immersed in cell culture medium. SHED were re-suspended in 50 µL of Puramatrix™ or rhCollagen type I and injected into the roots of human premolars (n = 24 teeth/experimental condition). After 7 to 28 days, SHED were removed from the root canals, and RNA purification (TRIzol Reagent; Invitrogen), amplification (SuperScript III Platinum Kit; Invitrogen), and RT-PCR for DMP-I, DSPP, and MEPE were performed, as shown previously (Casagrande et al., 2010). RNA obtained from human third molars was used as control. Negative controls were SHED grown in regular cell culture flasks, or SHED mixed with injectable scaffolds in the absence of root structure.
Dental Pulp Tissue Engineering in vivo
SHED stably transduced with GFP were re-suspended in either scaffold (6 x 105 cells/mL), injected into the roots of human premolars (n = 6), and transplanted subcutaneously into the dorsum of 5- to 7-week-old male immunodeficient mice (CB-17 SCID; Charles River, Wilmington, MA, USA), as shown (Appendix Fig. 1). Both sides of the roots were left opened in these proof-of-principle experiments. Negative controls were scaffolds devoid of cells and injected into roots (n = 4). Ten days after transplantation, and every 5 days thereafter, intraperitoneal injections of 41.6 nmol/g of body weight of tetracycline hydrochloride (Sigma-Aldrich) were administered, as described (Sakai et al., 2010). Mice were euthanized 35 days after transplantation, and the roots were retrieved, fixed in 10% buffered formalin, and demineralized in Decalcifier II (Surgipath, Richmond, IL, USA). Untreated immature human premolars were used as positive controls for tissue morphology. Apoptosis was detected by TUNEL with the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany). New dentin deposition was assessed by confocal microscopy, as shown previously (Sakai et al., 2010). Experiments performed here were approved by the appropriate institutional review boards and followed the ARRIVE guidelines.
Results
To begin the evaluation of the suitability of Puramatrix™ and rhCollagen type I for dental pulp tissue engineering, we re-suspended SHED in the scaffolds and observed them microscopically (Fig. 1). While SHED appeared round at day 1, by day 7 the cells had become more elongated and formed clusters (Fig. 1A). By the end of the experimental period, we observed a 4-fold increase in cell numbers for both scaffolds (Fig. 1B). To evaluate the differentiation potential under these experimental conditions, we re-suspended SHED in Puramatrix™ or rhCollagen type I and injected them into human root canals (Fig. 2). For Puramatrix™, we began observing expression of MEPE after 7 days, and by day 21 all 3 putative markers of odontoblastic differentiation (DSPP, DMP-1, MEPE) were expressed (Fig. 2). Conversely, for rhCollagen type I we began observing expression of DSPP and MEPE on day 14, and only on day 28 were all markers clearly expressed. Notably, neither scaffold, by itself, was capable of inducing the odontoblastic differentiation of SHED, indicating that dentin-derived morphogenic factors are necessary.
Figure 1.
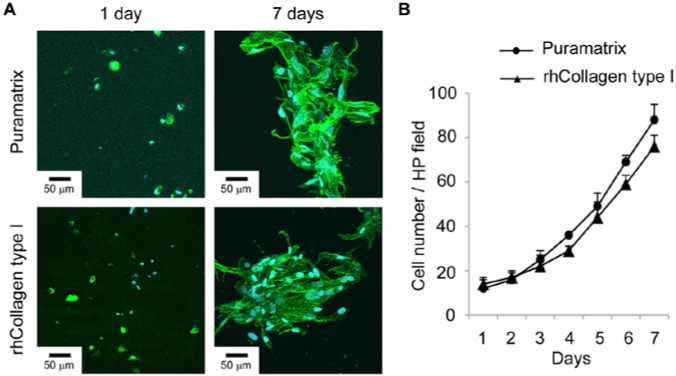
SHED morphology and proliferation in injectable scaffolds in vitro. (A) SHED are dispersed and present a round morphology when cultured in Puramatrix™ or rhCollagen type I for 1 day. Cells proliferate, elongate, and form clusters after 7 days. Green depicts Actin-F staining, and blue depicts DAPI staining (nuclei). (B) Graph depicting proliferation of SHED over time when mixed with Puramatrix™ or rhCollagen type I. Cell number throughout the experiment was not statistically different when cells were cultured in Puramatrix™ or rhCollagen type I, as determined by t test (p > .05).
Figure 2.
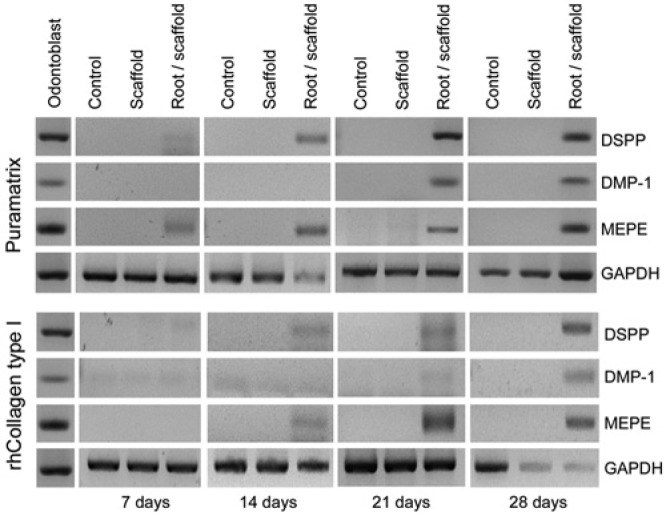
Expression of putative markers of odontoblastic differentiation by SHED in vitro. RT-PCR analysis of markers of odontoblastic differentiation (DMP-1, DSPP, MEPE) in SHED mixed with scaffolds (Puramatrix™, rhCollagen type I) and injected into human root canals. As controls, we evaluated SHED cultured in standard tissue culture plates (Control) or SHED cultured in the injectable scaffolds (Puramatrix™ or rhCollagen type I) but in the absence of tooth structure (Scaffold) for up to 28 days.
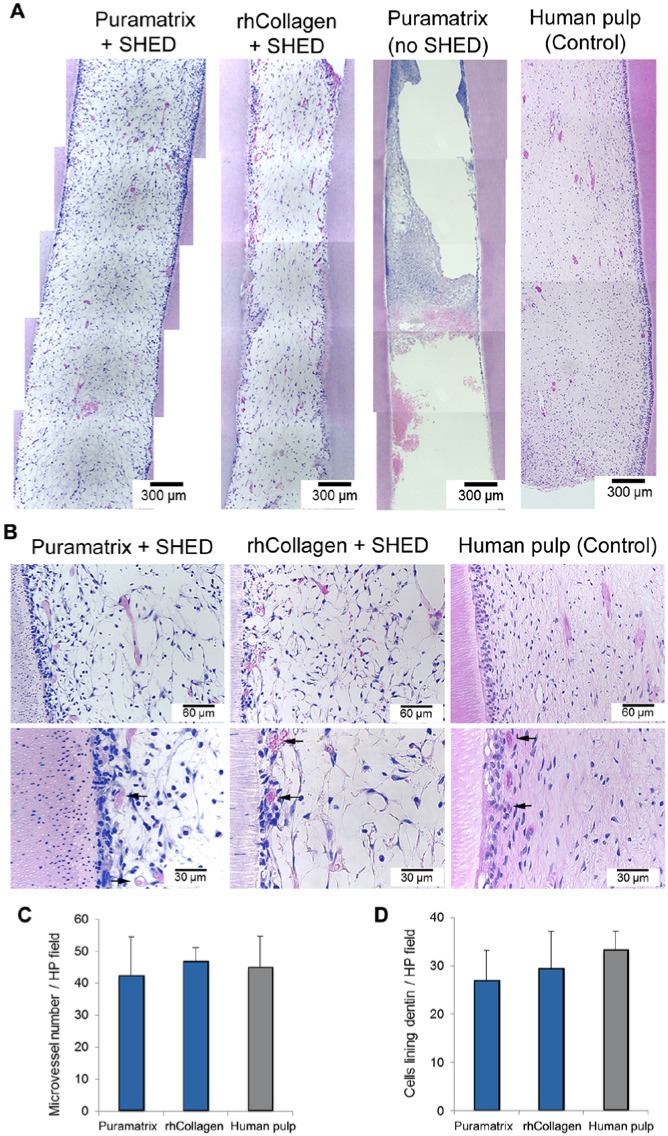
Next, we assessed the capacity of SHED injected into full-length root canals to generate functional dental pulps in vivo (Fig. 3). The tissue formed with SHED injected with either Puramatrix™ or rhCollagen type I occupied the full extension of the root canal (Fig. 3A). Histologically, it had the appearance of a connective tissue containing multiple blood vessels, many of them close to the predentin (Fig. 3B). However, the engineered pulps appeared to have less dense extracellular matrices when compared with those of the control pulps (Fig. 3B). When Puramatrix™ (Fig. 3A) or rhCollagen type I (data not show) was injected into the root canal without cells, the tissue formed was minimal and poorly organized. The quantification of microvessel density revealed that engineered pulps generated with both scaffolds had vessel density (p > .05) similar to that of the one observed in control human dental pulps (Fig. 3C). Likewise, the number of cells close to the predentin was similar (p > .05) in dental pulps engineered with either scaffold (Fig. 3D). The occurrence of apoptotic cells in the engineered dental pulps was consistent with that observed in control human pulps (Appendix Fig. 2). Using the tetracycline staining technique to mark new dentin formation in engineered pulps (Sakai et al., 2010), we observed that SHED transplanted in either Puramatrix™ or rhCollagen type I were capable of generating new dentin throughout the length of the root (Appendix Fig. 3).
Figure 3.
Dental pulp tissue engineering with SHED injected into human root canals and transplanted into immunodeficient mice. (A) Low-magnification and (B) high-magnification images of tissues formed when SHED mixed with scaffolds (Puramatrix™, rhCollagen type I groups) were injected into full-length root canals of human premolars. A vascularized connective tissue occupied the full extension of the root canal. Cell densification and many blood vessels were observed along dentin walls. Scaffolds (Puramatrix™) injected into the root canals without cells were used as controls for SHED. Freshly extracted human premolars were used as tissue controls. Black arrows point to blood vessels close to the odontoblastic layer. (C) Graph depicting microvessel density and (D) cellular density of dental pulp tissues engineered with SHED injected into full human root canals. Microvessel density and cellular density were similar in both experimental conditions and the control group (human pulp), as determined by one-way ANOVA.
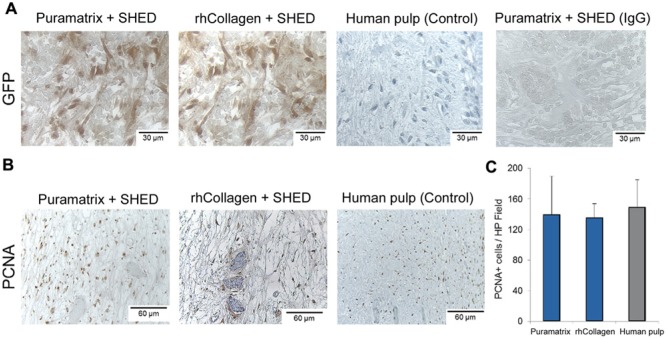
To evaluate the source of the cells in the engineered dental pulps, we transduced SHED with green fluorescence protein (GFP) prior to transplantation. Immunohistochemistry for GFP confirmed that tissue engineered with the root canals is primarily populated with SHED (Fig. 4A). Proliferative activity, as determined by positivity to proliferating cell nuclear activity (PCNA), was similar for both scaffolds (p > .05) and comparable with that in control human dental pulps (Figs. 4B, 4C).
Figure 4.
Characterization of the tissues engineered with SHED. SHED stably tranduced with green fluorescence protein (GFP) were mixed with scaffolds (Puramatrix™ or rhCollagen type I) and injected into full-length root canals of human premolars. (A) Immunohistochemistry for GFP (brown) confirmed that tissues were formed primarily with SHED transplanted into the root canal. Images were randomly captured from the center of engineered dental pulps or control human pulps. Immunohistochemistry with GFP of human dental pulps, and non-specific isotype-matched IgG, were used as controls for this experiment. (B) Immunohistochemistry with PCNA (brown) to evaluate cell proliferation in pulps engineered with Puramatrix™ or rhCollagen type I. Immunohistochemistry with PCNA of human dental pulps was used as control for this experiment. (C) Graph depicting the number of PCNA-positive cells in tissues formed with SHED injected into full-length human root canals. The number of PCNA-positive cells was similar in both experimental conditions and the control group (human pulp), as determined by one-way ANOVA.
Discussion
Studies in the tooth slice/scaffold model showed that SHED differentiate into functional odontoblasts capable of generating new tubular dentin (Sakai et al., 2010, 2011). These proof-of-principle studies were performed with 1- to 1.5-mm tooth slices in a cast scaffold (PLLA) and do not fully represent the challenges of regenerating a pulp tissue in complete roots. The larger dimension of the tissue that has to be engineered, compounded with the fact that vascularization can be obtained only through the apex, is a significant obstacle to the engineering of a pulp within a full root. Here, we used full roots from extracted human premolars and observed that the transplantation of SHED generated a pulp-like tissue throughout the extent of the root canal. Importantly, the engineered pulp was capable of generating new dentin. These results represent a critical step forward in the process of translating the concept of dental pulp tissue engineering to the clinic.
The clinical use of cell-based therapies will require the development of scaffolds that enable stem cells to be transplanted into root canals (Galler et al., 2011). These investigators developed customized self-assembling hydrogels that are highly suitable for dental pulp tissue engineering (Galler et al., 2012). Here, we began the evaluation of 2 commercially available injectable scaffolds, i.e., a self-assembling peptide hydrogel (Puramatrix™) and rhCollagen type I. We observed that both scaffolds sustained SHED cell survival and enabled these cells to express putative markers of odontoblastic differentiation when injected into root canals (e.g., DSPP, DMP-1). Notably, the scaffolds by themselves (without surrounding tooth structure) were not capable of inducing odontoblastic differentiation. These results confirmed previous observations that dentin-derived signaling molecules are necessary for odontoblastic differentiation of stem cells (Casagrande et al., 2010; AJ Smith et al., 2012). But the study reported here has a key difference when compared with the Casagrande study. Here, we injected cells into the full-length root and had culture medium covering only the apical third of the root, simulating the clinical condition where all influx of nutrients must come through the apex. In the Casagrande study, we had the cells in 1- to 1.5-mm-thick tooth slices, which facilitate ample diffusion of nutrients and oxygen to the cells. Interestingly, DSPP was among the first putative marker of odontoblastic differentiation to be expressed by SHED. The increased expression of DSPP in early stages suggests that SHED were quickly acquiring the capacity of secreting mineralizable dentin matrices (Suzuki et al., 2009). We also observed that it took longer for SHED cells to express all odontoblastic markers when they were seeded in recombinant human collagen than in Puramatrix™. While we do not know the reason for this difference in the in vitro experiments, we speculate that the higher viscosity of the rhCollagen scaffold may have contributed by: (A) delaying the migration of SHED toward the dentin; and/or (B) slowing the diffusion of dentin-derived morphogenic signals required for odontoblastic differentiation. It appears that the physical properties of the scaffold, such as viscosity, play an important role in dental pulp tissue regeneration.
The histology of the dental pulps engineered with either scaffold showed a connective tissue containing blood vessels and cell densification along the dentin walls. Although this cell layer was not as organized as the one found in the control human pulp, the number of cells lining the dentin was fairly similar. We observed that the numbers of proliferating cells (PCNA-positive) and apoptotic cells (TUNEL-positive) were similar for engineered and control pulps, indicative of tissue homeostasis in the engineered pulps. The overall tissue microvessel density was similar in engineered tissues and control human pulps. A high concentration of blood vessels close to the dentin walls was observed in the engineered pulps. This finding is in line with recent observations that dentin contains functional pro-angiogenic factors (Zhang et al., 2011) that support pulp regeneration. Finally, a noticeable difference between engineered tissues and controls was the higher density of extracellular matrix in the human pulps. This might be correlated with the more advanced maturation status of the control pulps when compared with the 30-day-old engineered tissues.
The engineered pulp tissues contained primarily cells that were transplanted, not invading murine cells, as demonstrated by the predominance of GFP-positive cells. Notably, we observed that SHED transplanted into full root canals are capable of generating new dentin at a rate of approximately 10 µm/day in both experimental conditions, as demonstrated by tetracycline staining. These findings give support to the concept that SHED might constitute a singular source of cells for pulp regeneration, capable of differentiating into odontoblasts, vascular endothelial cells, resident fibroblasts, and neural cells (Miura et al., 2003; Huang et al., 2009; Casagrande et al., 2010; Sakai et al., 2010, 2012) that can generate a functional dental pulp.
This study suggests that the reliance on host cells to regenerate dental pulps throughout an entire root canal may pose challenges, despite the fact that dentin contains chemotactic factors that induce cell migration (Fitzgerald et al., 1990; JG Smith et al., 2012). We observed the ingrowth of cells in scaffolds devoid of SHED injected into root canals, but the resulting tissue was incomplete and disorganized. The authors are aware that the experimental model used here has limitations inherent to the transplantation of roots into the subcutaneous tissue of mice, and therefore devoid of the apical stem cells that are important for pulp regeneration (Sonoyama et al., 2008). Nevertheless, these results suggest that chemotactic factors would likely have to be incorporated into scaffolds for active recruitment of cells from the periapical region in cell-free tissue-engineering approaches.
In conclusion, our results show that SHED survive and differentiate into odontoblasts when transplanted into full-length human root canals with injectable scaffolds. The pulp tissue generated under these experimental conditions contains functional odontoblasts capable of regenerating tubular dentin. The clinical translation of these laboratory findings will require the collaboration of pulp biologists, materials scientists, and clinicians to develop safe and effective approaches for pulp regeneration.
Supplementary Material
Acknowledgments
This work was done in partial fulfillment of the requirements for the Doctoral degree in Dental Materials of Vinicius Rosa. The authors thank Songtao Shi (USC) for providing us with the SHED cells used in this study and Zhihong Dong for technical support.
Footnotes
This work was funded by the NIH/NIDCR (grant R01-DE21410 to J.E.N.) and by CAPES (grant BEX441808-5 to V.R.)
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Bento LW, Zhang Z, Imai A, Nör F, Dong Z, Shi S, et al. (2013). Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res 92:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. (2010). Dentin-derived BMP-2 and odontoblastic differentiation of SHED. J Dent Res 89:603-608. [DOI] [PubMed] [Google Scholar]
- Cavalcanti BN, Zeitlin BD, Nör JE. (2013). A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater 29:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadipiralla K, Yochim JM, Bahuleyan B, Huang CY, Garcia-Godoy F, Murray PE, et al. (2010). Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res 340:323-333. [DOI] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962-969. [DOI] [PubMed] [Google Scholar]
- Cvek M. (1992). Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol 8:45-55. [DOI] [PubMed] [Google Scholar]
- Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, et al. (2005). Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 111:442-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126:677-689. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Chiego DJ, Jr, Heys DR. (1990). Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol 35:707-715. [DOI] [PubMed] [Google Scholar]
- Galler KM, D’Souza RN, Hartgerink JD, Schmalz G. (2011). Scaffolds for dental pulp tissue engineering. Adv Dent Res 23:333-339. [DOI] [PubMed] [Google Scholar]
- Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. (2012). A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A 18:176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt M, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. (2009). Cell survival within pulp and periodontal constructs. J Endod 35:63-66. [DOI] [PubMed] [Google Scholar]
- Gelain F, Horii A, Zhang S. (2007). Designer self-assembling peptide scaffolds for 3-d tissue cell cultures and regenerative medicine. Macromol Biosci 7:544-551. [DOI] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrett K, Fagerholm P, McLaughlin CR, Dravida S, Lagali N, Shinozaki N, et al. (2008). Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: performance of type I versus type III collagen. Invest Ophthalmol Vis Sci 49:3887-3894. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Reddi AH. (2003). The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol 21:1025-1032. [DOI] [PubMed] [Google Scholar]
- Nör JE. (2006). Tooth regeneration in operative dentistry. Oper Dent 31:633-642. [DOI] [PubMed] [Google Scholar]
- Rosa V, Botero TM, Nör JE. (2011). Regenerative endodontics in light of the stem cell paradigm. Int Dent J 61(Suppl 1):23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado M, Shi S, et al. (2010). SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89:791-796. [DOI] [PubMed] [Google Scholar]
- Sakai VT, Cordeiro MM, Dong Z, Zhang Z, Zeitlin BD, Nör JE. (2011). Tooth slice/scaffold model of dental pulp tissue engineering. Adv Dent Res 23:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. (2012). Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122:80-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR. (2012). Dentine as a bioactive extracellular matrix. Arch Oral Biol 57:109-121. [DOI] [PubMed] [Google Scholar]
- Smith JG, Smith AJ, Shelton RM, Cooper PR. (2012). Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp Cell Res 318:2397-2406. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. (2008). Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, et al. (2009). Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol 28:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonhoff JR, Lou DI, Jordan PM, Zhao X, Wu P. (2008). Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res 1187:42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hillas PJ, Baez JA, Nokelainen M, Balan J, Tang J, et al. (2004). The application of recombinant human collagen in tissue engineering. BioDrugs 18:103-119. [DOI] [PubMed] [Google Scholar]
- Zhang R, Cooper PR, Smith G, Nör JE, Smith AJ. (2011). Angiogenic activity of dentin matrix components. J Endod 37:26-30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.