The role of proteins in defining the mechanical properties of cells, and how they are able to control their geometry and respond to varied environmental conditions, constitute an important frontier in biology. These traits are principally achieved by the cell’s cytoskeleton, a complex protein composite dominated by actin, microtubules, and vimentin intermediate filaments (1) (Fig. 1). However, the detailed mechanisms, by which cells control their stiffness continuously and reversibly as they adapt to changed environmental conditions, remain largely unknown.
Figure 1.
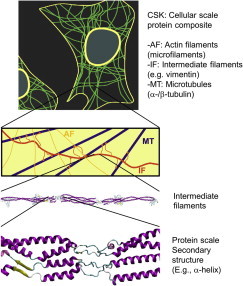
Structural makeup of cells and location of intermediate filament networks within the cytoskeletal network of actin filaments and microtubules. The figure depicts the complex range of scales from tens of micrometers at the cell level, to a few nanometers at the scale of individual protein molecules or assemblies thereof. The basic protein structure is dominated by coiled assembly of α-helices, with regions of unfolded or differently structured domains (e.g., β-sheets). The ability to knock out intermediate filaments from the composite structure provides a route to explore its role in defining certain mechanical functions at the cellular scale. MT, microtubules; IF, intermediate filaments; AF, actin filaments; and CSK, cytoskeleton. Images adapted and reprinted from Qin et al. (6).
Actin and microtubules are known to be important players in cell mechanics and have been widely studied. There is increasing evidence that proteins in the family of intermediate filaments (2–6), found abundantly in metazoan cells as well as bacteria, are also critical for important geometric and mechanical features of cells. Yet, despite their prominent presence in the cell’s cytoskeleton, they have so far escaped a detailed understanding, and their specific contributions to cellular behavior remain unclear. Early work indicated their significance in contributing to cell mechanical properties (7,8), and has raised important questions about their role in comparison with the other proteins found in the cytoskeleton. The name “intermediate” filament originates from the fact that their characteristic diameter of 10 nm is in between actin filaments (∼6–7 nm) and microtubules (∼25 nm) (2–6).
Intermediate filaments are α-helix-rich fibrous proteins that are highly sensitive to mechanical strain, undergo a series of molecular unfolding events as they are deformed, and can be stretched to more than several times their initial length (6,9). A salient feature of intermediate filaments is that they are extremely compliant at small deformation but stiffen severely at larger stretch. This concept of mechanical invisibility at small deformations forms the basis for the notion as a protecting element that enhances the mechanical integrity of cells against severe deformation (2–6). These mechanical traits are enabled by self-assembled hierarchical structures ranging from H-bonds, α-helical proteins, coiled helices, and larger-scale filaments that form a complex network at the cellular level (6,9). Intermediate filaments are not only found in the cytoskeleton but also in the nuclear envelope where lamin intermediate filaments provide structural support. One of their most prominent roles of lamin intermediate filaments in a pathological context is in rapid aging disease (progeria).
Now, new research by Weitz et al. (10) explains the mechanisms by which vimentin intermediate filaments control the stiffness of eukaryotic cells, identifying their role in affecting the stiffness of the cell cortex and the cell’s cytoplasm as well as the manner in which they contribute to intracellular mechanics. An elegant approach for studying the effect of a particular protein on the mechanical behavior of cells or tissues is to produce knockout cells that do not contain a specific type of protein. The availability of cells with and without a certain protein enables detailed testing for comparison. The Weitz group employed this technique, combining it with optical magnetic tools for the assessment of cortical stiffness and rheological methods for the assessment of intracellular stiffness and movements (10).
The cell cortex consists of a highly responsive and dynamic structural network that includes actin and myosin motor proteins and is relatively stiff. However, underneath the cortex, a much softer part exists which contains significant amounts of intermediate filament proteins. The new experiments (10) show that the stiffness of the internal part of the cytoplasm is strongly affected by either the presence or lack of vimentin intermediate filaments, leading to stiffness changes by almost a factor of two. However, presence or lack of vimentin intermediate filaments does not significantly affect the stiffness of the cortex. Another important finding is that the increased stiffness of the cytoplasm stabilizes intracellular components such as organelles within cells, as was shown by directly tracking submicron lipid vesicles. This work, for the first time to our knowledge, provides insights into the role of where and how intermediate filament proteins affect cellular properties. The data also directly shows that the intermediate filament network does more than just increase the mechanical integrity of cells at severe deformation.
These findings point to a more complex biological role for intermediate filaments, and presents a possibility for mechanism-based upscaling from the single molecule to the network scale (6,9). The refined view of the protein composite network could be used to expand existing models to larger scales, and may even pinpoint potential targets for drugs to treat some of the diseases with which the intermediate filaments are associated. Many questions about intermediate filaments remain, such as their role in mechanosensing, their interaction with actin and microtubules and other proteins, and their ability to self-organize under different physiological conditions. From a historical perspective, this work marks an important step in a long series of studies that date back to the late 1970s (2), when proteins of the intermediate family first became the focal point of attention. This body of work has shown the prominent role of this relatively little-known protein as a big player in cell mechanics.
From a broader viewpoint, this work supports the notion that the small-scale chemical features of biopolymers alone are not sufficient for understanding biological functions. Rather, the spatial distribution of biopolymer building blocks, and their dynamical interplay at different scales, emerge as key drivers in determining the properties of cells and tissues. Molecular-to-tissue upscaling efforts that integrate experiment and simulation can provide critical insight not only to physiological aspects but also into disease properties, and must include an appropriate description of the composite nature of the protein networks. The study of intermediate filaments can also stimulate the design of new biomaterials. For instance, intermediate filaments are found in hagfish slime (11), a remarkably tough gel (secreted at very short timescales in an aqueous environment) for providing mechanical protection against predators. This and other materials built from intermediate filament proteins could be a powerful platform for the design of new biomaterials.
Acknowledgments
We acknowledge support from the Office of Naval Research, Arlington, Virginia, under grant No. N000141010562.
References
- 1.Alberts B., Johnson A., Walter P. 5th Ed. Garland Science; New York: 2007. Molecular Biology of the Cell. [Google Scholar]
- 2.Franke W.W., Schmid E., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc. Natl. Acad. Sci. USA. 1978;75:5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann H., Bär H., Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann H., Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu. Rev. Biochem. 2004;73:749–789. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison C.J. Lamins: building blocks or regulators of gene expression? Nat. Rev. Mol. Cell Biol. 2002;3:848–858. doi: 10.1038/nrm950. [DOI] [PubMed] [Google Scholar]
- 6.Qin Z., Kreplak L., Buehler M.J. Hierarchical structure controls nanomechanical properties of vimentin intermediate filaments. PLoS ONE. 2009;4:e7294. doi: 10.1371/journal.pone.0007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janmey P.A., Euteneuer U., Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N., Stamenović D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am. J. Physiol. Cell Physiol. 2000;279:C188–C194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- 9.Qin Z., Buehler M.J. Flaw tolerance of nuclear intermediate filament lamina under extreme mechanical deformation. ACS Nano. 2011;5:3034–3042. doi: 10.1021/nn200107u. [DOI] [PubMed] [Google Scholar]
- 10.Guo M., Ehrlicher A.J., Weitz D.A. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys. J. 2013;105:1562–1568. doi: 10.1016/j.bpj.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreplak L., Fudge D. Biomechanical properties of intermediate filaments: from tissues to single filaments and back. Bioessays. 2007;29:26–35. doi: 10.1002/bies.20514. [DOI] [PubMed] [Google Scholar]


