Abstract
Background
To date, there are no studies reported in the literature on the possible use of bovine collagen, oxidized regenerated cellulose, or synthetic hyaluronic acid medications in the oral cavity. The aim of this paper is to report the use of bovine collagen, oxidized regenerated cellulose, and synthetic hyaluronic acid medications to improve wound healing in the oral cavity by stimulating granulomatous tissue.
Methods
From 2007 to 2011, 80 patients (median age 67 years) suffering from oral mucosal lesions participated in this double-blind study. The patients were divided into two groups, each consisting of 40 patients. One group received conventional medications, while the other group of patients were treated with the advanced medications.
Results
Advanced medications allowed re-epithelialization of the wound margin in 2–20 days, whereas patients receiving conventional medication showed a median healing duration of 45 days.
Conclusion
The results of this study demonstrate that treating oral mucosal wounds with advanced medication has an advantage with regard to wound healing time, allowing patients to have a rapid, functional, and esthetic recovery.
Keywords: bioengineering, oral cavity, mucosal recovery
Introduction
Wound healing is a physiologic process based on the homeostasis of reparative mechanisms which allow for formation of new tissue and destruction essential for removing damaged tissue.1–3 The oral mucosa is frequently exposed to a number of sources of stress, including mastication, speech, breathing, and bacterial invasion of the oropharynx. These factors delay oral wound healing and increase the risk of infection. Impaired saliva turnover and chronic mechanical and bacterial insults make the management of oral wounds particularly complex.
Application of bovine collagen, oxidized regenerated cellulose, and synthetic hyaluronic acid medications accelerate wound healing in the oral cavity in patients with traumatic, oncologic, and inflammatory pathologies, such as bisphosphonate-related osteonecrosis of the jaw. Promogran™ (Johnson & Johnson, New Brunswick, NJ, USA) consists of a sterile, freeze-dried matrix composed of collagen and oxidized regenerated cellulose pressed into a sheet approximately 3 mm thick and cut into hexagonal pieces.
In the presence of wound exudate, the matrix absorbs the liquid and forms a soft, conformable, and biodegradable gel. This gel physically binds and inactivates matrix metalloproteases, and has a detrimental effect on wound healing when present in excessive quantities. Additionally, the gel binds naturally occurring growth factors within the wound and protects them from degradation by proteases, releasing them back into the wound in an active form as the matrix is slowly broken down.
Promogran is indicated for the management of all types of chronic wounds that are free of necrotic tissue and visible signs of infection. These include leg ulcers, both venous and arterial in origin, pressure sores, and ulcers occurring on the feet of diabetic patients. The matrix, which also has hemostatic properties, can be used in conjunction with compression therapy. Promogran is delivered in a transparent waterproof peel pouch, sealed with a laminated cover and sterilized by gamma irradiation. It is available in two sizes, ie, 28 cm2 and 123 cm2.
Hyalomatrix™ (Fidia Advanced Biopolymers, Abano Terme, Italy) is a bilayered, sterile, flexible, and conformable wound dressing that acts as an advanced wound care device. It is comprised of a nonwoven pad made entirely of HYAFF, a benzyl ester of hyaluronic acid, and a semipermeable silicone membrane which controls water vapor loss, providing a flexible covering for the wound surface and adding increased tear strength to the device. As Hyalomatrix is applied to the wound bed, the HYAFF wound-contact layer provides a three-dimensional scaffold for cellular invasion and capillary growth. When integration of the HYAFF-based material into the newly formed dermal matrix has progressed, well vascularized granulation tissue forms. This provides for wound closure via spontaneous re-epithelialization and acts as a suitable dermal layer for skin grafting.
The effects of these medications are well documented, with several studies in chronic diabetic ulcers, corneal transplantation, tympanic membrane perforation, and joint surgery, as well as in burns patients having been reported.4–7 The major effects appear to be related to improved wound healing of injured tissue.6 To our knowledge, no studies concerning possible use of these medications in the oral cavity have been reported in the literature. The purpose of this paper is to report the use of the aforementioned materials to improve wound healing in the oral cavity.
Materials and methods
Eighty patients (median age 67 years) were enrolled in this study from 2007 to 2011 (Table 1). All patients suffered from oral mucosal lesions of traumatic, oncologic, or inflammatory etiology. The patients were divided into two groups, each consisting of 40 patients. Each group consisted of 26 females and 14 males. Twenty-two patients had undergone surgery for oral cancer (six superior maxillary cancers, six mucosal cancers, and ten mandibular cancers), with eight affected by bisphosphonate-related osteonecrosis of the jaw and ten by post-traumatic injury. The two patient groups had wounds of the same size and type. The average wound size was approximately 2 cm.
Table 1.
Patients enrolled in the study
| Conventional medication group | Advanced medication group | |
|---|---|---|
| Patients | 26 female | 26 female |
| 14 male | 14 male | |
| Healing time | 45 days | 2–20 days |
The first group of patients was treated with conventional medications (iodoform gauze, hydrogen peroxide, and iodopovidone). The second group of patients was treated with advanced medications, ie, Promogran (containing oxidized regenerated collagen) and Hyalomatrix (containing hyaluronic acid). The collagen medication was changed twice a day in the second group of patients, while hyaluronic acid was changed after 2 weeks. Medications were applied depending of the type and position of the wound using stitches when required. We did not use specific criteria for assessing wound re-epithelization and instead based this on direct observation of wound healing. Patients were followed daily for the first 20 days and weekly thereafter.
Results
Both conventional and advanced medications showed benefits, but the main difference between the two related to healing time. Advanced medications allowed wound healing within 2–20 days, whereas patients in the conventional medication group took at least 45 days to reach complete re-epithelialization of the oral mucosa. Only patients from the second group presenting with sloping or declivities lesions had a healing time close to the control group. All patients had good post-treatment results without any complications. The difference in healing time between the groups was statistically significant.
Discussion
The healing process includes three phases, ie, an initial inflammatory phase required to eliminate damaged tissue, the reconstructive phase, characterized by formation of granulation tissue, and tissue modeling.8 Proteases participate in each phase, and have been shown to be the principal protagonists in the balance between synthesis and degradation.9,10 Excessive protease activity can be caused by positive feedback in the expression of protease genes, increased activation of extracellular latent protease, or by reduction of the activity of protease endogen inhibitors; this manifests as an increase in extracellular proteolysis, with greater imbalance in tissue destruction.11–13 Collagen and oxidized regenerated cellulose, components of advanced medications, are an optimum substrate for proteases, which are bonded and cannot interact further with the tissue.1
Additionally, this bovine extract can act as a mechanical support and stimulate migration of fibroblasts and the metabolic activity of granulomatous tissue.12–14 The oral mucosa is hard to cleanse because of continuous mechanical, bacterial, and viral stimuli. Such factors predispose these patients to mucosal alterations, with loss of integrity and possible bone or osteosynthesis material exposure.
Within the field of dermal replacement products, derivatives of hyaluronic acid have a place of particular importance.15 Hyaluronic acid is a linear polymer of glucuronic acid N-acetylglucosamine disaccharide, a main glycosaminoglycan ubiquitously distributed in the extracellular space and involved in the process of wound repair, modulating the release of cytokines and other mediators.6,7,16 First isolated in 1934 from bovine vitreous humor, it was subsequently collected from other sources, such as soft connective tissues, synovial fluid, umbilical cords, and rooster combs. Hyaluronic acid is recognized by specific cell receptors such as CD44, and regulates the adhesion, growth, differentiation, locomotion, and activation of specific cell types, thereby modulating inflammation, angiogenesis, and healing processes.6,17 Both wound size and vascularization showed good improvement in response to treatment with hyaluronic acid.6 This may be partly explained by the effect of the degradation products of hyaluronic acid on endothelial cell proliferation, angiogenesis, and collagen deposition and organization.6,15,17 Its structural role is attributable to its hygroscopic properties which allow for hydration and modulation of the cellular microenvironment. For these reasons, it is easy to understand the important role of hyaluronic acid in tissue repair processes, and how it contributes to the orientation of the fibrous component of the extracellular matrix.
Hyaluronic acid benefits epithelial regeneration and has free radical scavenging properties.15 Hyaluronic acid medications are physically coupled with a transparent and flexible film of medical-grade synthetic elastomer which acts as a semipermeable barrier against external contaminants.15 When the product is applied to the wound bed, the hyaluronic acid wound-contact layer provides a three-dimensional scaffold which enables the product to be colonized by fibroblasts and onto which extracellular matrix components are laid down, resulting in an ordered reconstruction of the dermal tissue.15 Disappearance of the materials is mediated by a macrophage response.15
Hyaluronic acid may also have a moderating effect in inflammation, via free radical scavenging and antioxidant effects, and prevent tissue degradation by enzymes from the immediate cellular environment and other structural components of the extracellular matrix.7 Hyaluronic acid binds with fibrin to form a temporary wound matrix.16 This matrix provides a scaffold for contact guidance and cell migration, analogous to its morphogenic role.
Advanced medications allowed us to improve oral wound healing (Figure 1). Further, we achieved a better esthetic and functional result in reconstructive procedures where Bichat’s fat pad was used (Figure 2). In cases where infection was present, advanced medications in association with argentic nitrate were used14 (Figure 3). In sloping oral lesions, the results were due to accumulation of saliva, which degraded components of the medication. It seems that the protease content of saliva also reduces the effect of the medication by degrading its components (Figures 4 and 5).
Figure 1.
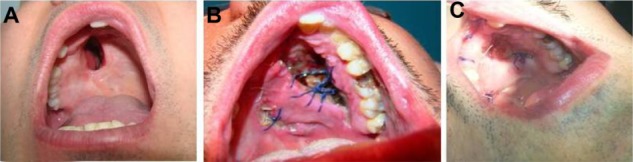
Post-traumatic palatal defect. (A) Outcome of massive trauma with a large residual palatal defect. (B) Reconstruction with palatal flap. Note a mucosal growth after advanced medication on follow-up at 7 days. (C) Total obliteration of the defect.
Figure 2.
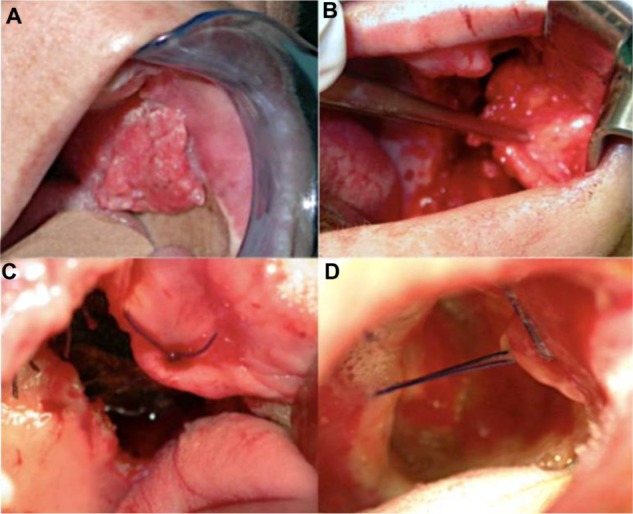
Right oral mucosal cancer. (A) Tumoral lesion involving the hard palate and right oral mucous. (B) Surgical resection and reconstruction with Bichat’s fat pad. (C) Post-surgical defect outcomes. (D) Follow-up at 7 days. Total mucosal growth.
Figure 3.
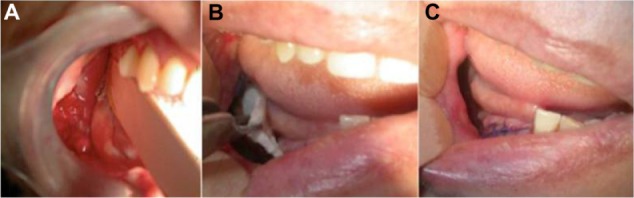
Bisphosphonate-related osteonecrosis of the jaw. (A) Right mandibular osteonecrosis with bone exposure. (B) Curettage and application of advanced medication. (C) Follow-up at 7 days.
Figure 4.
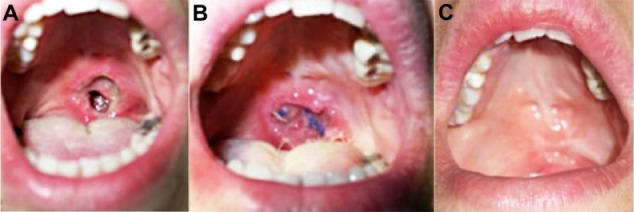
Iatrogenic soft palate lesion. (A) Iatrogenic soft palate defect. (B) Reconstruction with local mucosal flap and application of advanced medication. (C) Total recovery of the palatal defect.
Figure 5.
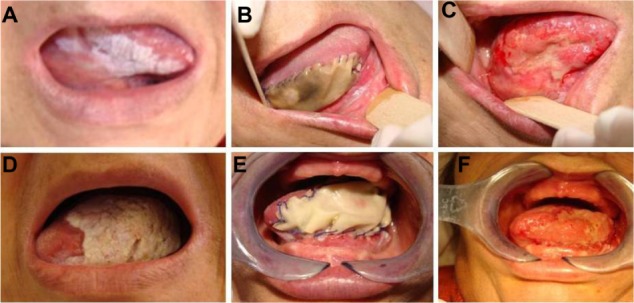
Massive bilateral tongue leucoplakia. (A) Massive left tongue leucoplakia, (B) lesion removal and application of a sheet of oxidized regenerated cellulose, and (C) full recovery. (D) Massive right tongue leucoplakia, (E) lesion removal and application of a sheet of oxidized regenerated cellulose, and (F) full recovery.
Advanced medications are recommended for use in clinical practice because they reduce both healing time and the risk of infection, ultimately leading to an improvement in the patient’s quality of life. We conclude that oral mucosal wounds can be treated with conventional and advanced medications. Advanced medications improve patient management by decreasing wound healing time, resulting in a prompt functional and esthetic recovery.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of Promogran, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10:16–25. doi: 10.1046/j.1524-475x.2002.10703.x. [DOI] [PubMed] [Google Scholar]
- 2.Guarnera G, Restuccia A. Promogran and complex surgical lesion: a case report. J Wound Care. 2004;13:237–239. doi: 10.12968/jowc.2004.13.6.26628. [DOI] [PubMed] [Google Scholar]
- 3.Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg. 1998;25:341–356. [PubMed] [Google Scholar]
- 4.Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care. 2002;11:335–341. doi: 10.12968/jowc.2002.11.9.26438. [DOI] [PubMed] [Google Scholar]
- 5.Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care. 2002;11:70–74. doi: 10.12968/jowc.2002.11.2.26675. [DOI] [PubMed] [Google Scholar]
- 6.Campoccia D, Doherty P, Radice M, Brun P, Abatangelo G, Williams DF. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials. 1998;19:2101–2127. doi: 10.1016/s0142-9612(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark RAF. Wound repair: overview and general considerations. In: Clark RAF, editor. The Molecular Biology of Wound Repair. 2nd ed. New York, NY: Plenum Press; 1996. [Google Scholar]
- 9.Ravanti L, Kähäri VM. Matrix metalloproteinases in wound repair. Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- 10.Barrick B, Campbell EJ, Owen CA. Leukocyte proteinases in wound healing: roles in physiologic and pathologic processes. Wound Repair Regen. 1999;7:410–422. doi: 10.1046/j.1524-475x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 11.Vaheri A, Stephens RW, Salonen EM, Pollanen J, Tapiovaara H. Plasminogen activation at the cell surface-matrix interface. Cell Differ Dev. 1990;32:255–262. doi: 10.1016/0922-3371(90)90038-x. [DOI] [PubMed] [Google Scholar]
- 12.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137:822–827. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 13.Motta G, Ratto GB, De Barbieri A, et al. Can heterologous collagen enhance the granulation tissue growth? An experimental study. Ital J Surg Sci. 1983;13:101–108. [PubMed] [Google Scholar]
- 14.Derbyshire A. The use of combination treatments and dressing for a traumatic wound. Br J Nurs. 2004;13:987–993. doi: 10.12968/bjon.2004.13.16.15976. [DOI] [PubMed] [Google Scholar]
- 15.Price RD, Das-Gupta V, Leigh IM, Navsaria HA. A comparison of tissue-engineered hyaluronic acid dermal matrices in a human wound model. Tissue Eng. 2006;12:2985–2995. doi: 10.1089/ten.2006.12.2985. [DOI] [PubMed] [Google Scholar]
- 16.Myers SR, Partha VN, Soranzo C, Price RD, Navsaria HA. Hyalomatrix: a temporary epidermal barrier, hyaluronan delivery, and neodermis induction system for keratinocyte stem cell therapy. Tissue Eng. 2007;13:2733–2741. doi: 10.1089/ten.2007.0109. [DOI] [PubMed] [Google Scholar]
- 17.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]


