Abstract
Aims
The contribution of blood flow to angiogenesis is incompletely understood. We examined the effect of blood flow on Notch signalling in the vasculature of zebrafish embryos, and whether blood flow regulates angiogenesis in zebrafish with constitutively up-regulated hypoxic signalling.
Methods and results
Developing zebrafish (Danio rerio) embryos survive via diffusion in the absence of circulation induced by knockdown of cardiac troponin T2 or chemical cardiac cessation. The absence of blood flow increased vascular Notch signalling in 48 h post-fertilization old embryos via up-regulation of the Notch ligand dll4. Despite this, patterning of the intersegmental vessels is not affected by absent blood flow. We therefore examined homozygous vhl mutant zebrafish that have constitutively up-regulated hypoxic signalling. These display excessive and aberrant angiogenesis from 72 h post-fertilization, with significantly increased endothelial number, vessel diameter, and length. The absence of blood flow abolished these effects, though normal vessel patterning was preserved.
Conclusion
We show that blood flow suppresses vascular Notch signalling via down-regulation of dll4. We have also shown that blood flow is required for angiogenesis in response to hypoxic signalling but is not required for normal vessel patterning. These data indicate important differences in hypoxia-driven vs. developmental angiogenesis.
Keywords: Zebrafish, Angiogenesis, Blood flow, Notch, Angio-/arteriogenesis
1. Introduction
Angiogenesis is essential for normal embryonic development, yet is central to postnatal human diseases such as retinopathies and cancer. During embryonic development, early vascular formation must precede onset of cardiac contraction, and hence vasculogenesis (formation of the axial vessels by migration of mesodermal angioblasts) occurs prior to, and independent of, blood flow. However, the contribution of blood flow to subsequent patterning of the vasculature by angiogenesis is less clear.
The zebrafish is an outstanding model of vertebrate angiogenesis due to optical clarity and a range of transgenic lines allowing in vivo visualization of cellular behaviour. Strikingly, the embryo is not dependent upon a circulation for oxygenation, as it obtains sufficient via diffusion from the incubating medium until several days old.1 Thus, the zebrafish embryo heart can be stopped without inducing hypoxia, allowing the discrimination of the effects of blood flow without the confounding influence of ischaemia.2 This makes it well suited to examining the effect of blood flow on vascular development.
We previously showed that during zebrafish development, formation of the paired intersegmental vessels (ISVs) running between the somites from the aorta occurs in the absence of blood flow.3 This suggests that initiation of ISV formation is blood flow independent, although a recent study suggests blood flow contributes to the anastomosis of ISVs to form the dorsal longitudinal anastomotic vessel (DLAV).4 In contrast with ISV formation, development of other vessels in the zebrafish does require blood flow, such as the accessory fifth aortic arch (AA5x).5 We previously showed blood flow negatively regulates endothelial expression of the chemokine receptor CXCR4a.3 CXCR4a is required for hindbrain vascularization (but not ISV formation)6 by stabilizing angiogenesis.7 It is unknown why development of the AA5x and hindbrain vasculature, but not ISVs, requires blood flow.
In contrast with these studies that have shown blood flow is either dispensable or necessary for vessel formation, other reports have shown a more inhibitory effect of blood flow on vascular formation. Shear stress has been shown to inhibit sprouting angiogenesis either in vitro8 or in vivo.9 Clearly, much remains to be understood how the physical forces exerted by blood flow integrate with molecular mechanisms to influence angiogenesis.
Notch signalling refers to a range of cell–cell interactions where Notch ligands, such as the delta-like ligands (dll1-4) and jaggeds (jag1&2) interact with Notch receptors on neighbouring cells to induce alterations in gene expression and cellular phenotype.10 These effects are predominantly mediated via the transcription factor CSL. Notch signalling plays a central role in vascular development and angiogenesis.10 Although Notch signalling is required for vasculogenesis and axial vessel formation,11 during later developmental angiogenesis Notch signalling plays an inhibitory role. For example, during ISV formation, endothelial tip cells express the Notch ligand dll4, which induces Notch signalling in adjacent endothelial stalk cells. Loss of function of dll4 leads to excessive and aberrant angiogenesis12 including of the ISVs in zebrafish.13
To identify mechanisms whereby blood flow might regulate angiogenesis, we examined whether blood flow influences Notch signalling in the developing zebrafish vasculature. We find that in the absence of blood flow the Notch ligand dll4 is up-regulated in the zebrafish vasculature, leading to increased Notch signalling. However, as previously demonstrated, the absence of blood flow does not affect ISV patterning in wild-type embryos, suggesting the up-regulation of Notch signalling is insufficient to perturb normal developmental angiogenesis of the ISV. We therefore examined ISV formation in zebrafish with constitutively up-regulated hypoxic signalling due to homozygous mutation in the von Hippel Lindau protein gene vhl.14 We find these embryos display abnormal and excessive ISV angiogenesis that is completely abolished in the absence of blood flow, whereas normal ISV patterning is preserved. Thus, our data reinforce the suggestion that the contribution of blood flow to angiogenesis is highly context dependent, and that blood flow is required in the clinically important context of angiogenesis driven by hypoxic signalling.
2. Methods
2.1. Fish strains
Studies were performed under Home Office licence 40/3434 and conformed to Directive 2010/63/EU of the European Parliament. The vhlhu2117 mutant was previously described.14 This was crossed with the following transgenic lines: Tg(fli:eGFP),15 kindly supplied by Brant Weinstein, Tg(flk1:EGFP-nls)16 kindly supplied by Markus Affolter, and Tg(kdrl:HRAS-mCherry)s91617 kindly supplied by Arndt Siekmann. The Tg(CSL-venus)qmc61transgenic line was generated by co-author Gering's group.
2.2. Morpholino knockdown
Morpholino antisense oligonucleotide (Gene Tools, Oregon USA) were diluted in phenol red (Sigma UK) 0.5% solution and injected into the yolk of a 1–4 cell embryo at a dose of 4.2 ng. Morpholino sequences were: tnnt2 (ATG/start) blocking morpholino 5′CATGTTTGCTCTGACTTGACACGCA3′ as published,18 Control morpholino 5′CCTCTTACCTCAGTTACAATTTATA 3′ (Gene Tools stock control), and dll4 (ATG/start) blocking morpholino: 5′-GAGAAAGGTGAGCCAAGCTGCCATG-3′ as published.19
2.3. Drug treatments
The myosin ATPase inhibitor 2,3-butanedione 2-monoxime (BDM) (Sigma UK) was used to block cardiac contraction in the developing embryo as previously published.20 This was dissolved at [15 mM] in E3 embryo media and added after established embryonic circulation at 36 h post-fertilization (hpf) until imaging at 3 dpf.
2.4. Reverse transcription–quantitative polymerase chain reaction
RNA was extracted from the dissected trunk and tail segments of groups of 30 pooled 48 hpf embryos. RNA was isolated immediately using Trizol (Sigma, UK) and then chloroform extracted and subsequently precipitated. cDNA was synthesized from the purified RNA using the Verso cDNA reverse transcription kit (Thermo Scientific, UK). Reverse transcription–quantitative polymerase chain reaction (RT–qPCR) was performed using iQ SYBR green supermix with a My iQ cycler (BioRad USA). Primers: β-actin forward (5′-GCAGAAGGAGATCACATCCCTGGC-3′), B-actin reverse (5′-CATTGCCGTCACCTTCACCGTTC-3′), cxcr4a forward (5′-TTGTGCTCACTCTGCCATTC-3′), cxcr4a reverse (5′-ACCGGTCCAAACTGATGAAG-3′), dll4 forward (5′- GCTTGGCTCACCTTTCTCAT-3′), dll4 reverse (5′-CGGAAGAAAGTCCTGCAGTC-3′), Nrarpa forward (5′-AGCTGCTTCGGACTCGTTAC-3′), Nrarpa reverse (5′-CGAGGTAGCTGATGCAGAGA-3′), Notch 3 forward (5′- CGGCCTGGTTATATTGGTTC-3′), Notch 3 reverse (5′-TCTAAAGCCTCGCTGACACA-3′), her12 forward (5′-GCTGAGGAAGCCGATAGTTG-3′), her12 reverse (5′-GCGAGAGGAAGTGGACAGAC-3′), ephrin B2 forward (5′-ACCACGTTGTCACTCAGCAC-3′) ephrin B2 reverse (5′-AGATGTTTGCTGGGCTCTGT-3′), flt4 forward (5′-TCTCGTTAGTGCCGTATCCA-3′), flt4 reverse (5′- GATGATGTGTGCTGGCTGTT-3′), kdr/flt1 forward (5′-CGCGCAACAGGTCACTATT-3′), kdr/flt1 reverse (5′-GTGAGGAGGATGTCGAGGAG-3′), vegfcc forward (5′- AAGAAGCTGGATGAGGAGACG-3′), vegfcc reverse (5′-GAGGTTGACTCCTCGGACAC-3′), vegfab forward (5′-CAGTGTGAGCCTTGCTGTTC-3′), and vegfab reverse (5′- CCATAGGCCTCCTGTCATTT-3′).
2.5. RT–qPCR for miR-30b and miR-30c
qPCR of zebrafish mature microRNA (miR) was performed according to the manufacturer's instructions using TaqMan MicroRNA Assays. Briefly, total RNA from groups of 30 pooled 48 hpf zebrafish embryos was purified using Trizol. cDNA was prepared using the TaqMan microRNA Reverse Transcription kit (Applied Biosystems) and qPCR performed using TaqMan microRNA Assays for miR-30b and miR-30c (Applied Biosystems). The primers used for RT–qPCR were supplied with each assay. Each is a stem-loop oligonucleotide containing the sequence of the mature miR (miR-30b:UGUAAACAUCCUACACUCAGCU; miR-30c: UGUAAACAUCCUACACUCUCAG).
qPCR was performed in triplicate using a 7900HT Fast Real-Time PCR System (Applied Biosystems). miR-30b and miR-30c expression levels were normalized to mean expression of the control group at 48 hpf.
2.6. Whole mount RNA in situ hybridization
Experiments were performed as previously described.3 Expression was detected with RNA probes labelled with digoxigenin (Roche) and resolved using BM Purple (Roche). dll4 antisense probe was synthesized from delta-like 4 cDNA fragment (kind gift from Roger Patient, Oxford, UK).
2.7. Live imaging and microscopy
Embryos were lightly anaesthetized using MS-222 (Tricaine) added to the incubating medium and immobilized in 0.5% low-melting point agarose and mounted in a chamber slide for visualization. Confocal microscopy was performed using an IX81 inverted motorized microscope (Olympus), Ultraview VOX confocal spinning disc imaging system (Perkin Elmer, Waltham, MA, USA). Images were acquired using Velocity software v5.3.2 (Perkin Elmer). Z stack images were acquired in 2 μm slices using sequential laser scanning of the region of interest. All analyses and quantification of vessel parameters were performed using ImageJ (v1.44 public domain software http://rsbweb.nih.gov/ij/). Stacks are presented as the maximum intensity Z projections. To quantify aortic Notch signalling in CSL:venus transgenics, we quantified fluorescence intensity in a two somite length of the wall of the aorta just above the anus. To quantify the vessel length in Fli1:GFP or kdrl:HRAS-mCherry transgenics we quantified the entire length of endothelium present in a region spanning two somites (containing three ISVs, and the dorsal longitudinal anastamotic vessel and any aberrant ISV sprouts). To quantify endothelial cell nuclei in flk1:GFPnls transgenics we counted the total nuclei in two somites, including the aorta, ISVs, and dorsal longitudinal anastamotic vessel. Stacks were analysed blinded and group sizes are as specified.
2.8. Statistics
Statistical comparisons were by Student's t-test for two group comparisons or ANOVA for more than two groups, and were performed using Graphpad Prism software. Group sizes are indicated in the results section. A P < 0.05 was considered statistically significant. Quantitative data are presented as mean ± SEM.
3. Results
3.1. Absence of blood flow leads to up-regulation of endothelial notch signalling
Morpholino antisense knockdown of cardiac-specific troponin T2 (tnnt2) has been shown to prevent cardiac contraction during development,18 although embryos survive for several days with otherwise normal development.3
To examine whether blood flow alters Notch signalling, we induced morpholino antisense knockdown of tnnt2 in a transgenic zebrafish line Tg(CSL:venus)qmc61. This expresses the YFP derivative Venus driven by concatemerized CSL-binding sites (the DNA-binding site of the CSL transcription factor required for most Notch signalling). In these embryos, Venus is expressed at sites of Notch signalling, notably the neural tube and less strongly in the developing vasculature, and is a useful reporter for Notch signalling. tnnt2 knockdown prevented cardiac contraction in these embryos at all time points studied up to 5 d post-fertilization (dpf); no erythrocyte movement could be detected in any blood vessel in tnnt2 morphants, whereas in control morphants brisk blood flow was observed in all vessels.
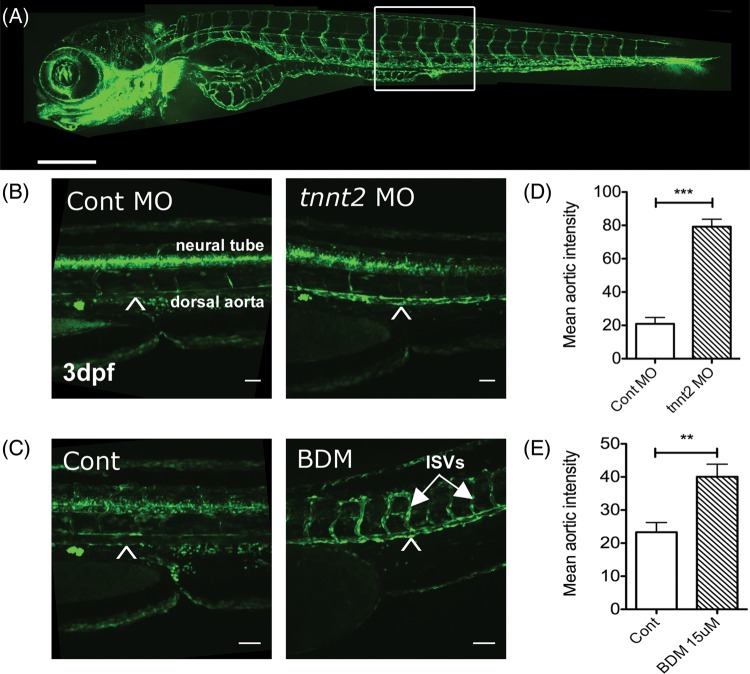
When we examined venus expression in the vasculature in these transgenic embryos at 72 h post-fertilization (hpf) we observed a statistically significant up-regulation of Notch signalling in the aorta of tnnt2 morphants. Figure 1A shows the vascular anatomy of a zebrafish embryo for orientation; the area of trunk examined in subsequent studies is indicated by a box. Figure 1B shows the trunk region of a representative control and tnnt2 morphant Tg(CSL:venus)qmc61 transgenic. Reporter expression can be seen in the aorta (arrowhead) and neural tube running dorsally to the aorta in both embryos, but the intensity of aortic expression is greater in the tnnt2 morphant. We quantified the reporter expression in the aorta in 72 hpf control and tnnt2 morphants (nine per group) and found a statistically significant increase in aortic fluorescence induced by tnnt2 knockdown (P < 0.001), shown in Figure 1D. We repeated this experiment in 48 hpf embryos and found a similar up-regulation of reporter expression (Supplementary material online, Figure S1). These observations suggested that blood flow may suppress CSL-mediated Notch signalling in the vasculature at these developmental stages.
Figure 1.
The absence of blood flow increases endothelial Notch signalling. (A) Vascular anatomy of a Fli1:eGFP transgenic zebrafish (vascular reporter line) for orientation. Scale bar = 500 μm. A boxed region of trunk indicates site of (B) and (C). (B) 3 dpf Tg(CSL-venus)qmc61 transgenic embryos with blood flow (control MO) or no blood flow (tnnt2 MO). Scale bar = 100 µm. Arrowhead indicates dorsal aorta. (C) The mean aortic fluorescence in 3 dpf Tg(CSL-venus)qmc61 embryos control and tnnt2 morphants. Scale bar = 100 µm. (D) 3 dpf Tg(CSL-venus)qmc61 transgenic embryos with blood flow (vehicle treated) or no blood flow (15 mM BDM treatment) from 36 hpf. ISVs, intersegmental vessels. Scale bar = 100 µm. (E) The mean aortic fluorescence in 3 dpf Tg(CSL-venus)qmc61 embryos in vehicle- and BDM-treated groups.
tnnt2 knockdown prevents circulation from ever commencing and could have effects other than preventing blood flow. We therefore sought to reproduce these findings using another method and examine whether absent blood flow increases Notch signalling in embryos that have previously experienced blood flow. Incubation of zebrafish embryos in the myosin ATPase inhibitor 2,3-butanedione 2-monoxime (BDM) has been shown to induce reversible cessation of cardiac contraction.20 We therefore used BDM to stop cardiac contraction in Tg(CSL:venus)qmc61 zebrafish embryos from 36 hpf (onset of circulation was at 26 hpf) until 72 hpf. Although a brisk normal circulation was established in all embryos at 36hpf, BDM treatment halted cardiac contraction within 15 min, causing complete absence of circulation. As with tnnt2 knockdown, cessation of cardiac output by BDM led to a significant up-regulation of Venus expression in the aorta (Figure 1C). When we quantified this effect, we found that cessation of blood flow led to a statistically significant increase in aortic Notch signalling (n = 8/group, P < 0.01) shown in Figure 1E). Taken together, these experiments suggest that the absence of blood flow leads to up-regulation of Notch signalling in the vasculature by some mechanism.
3.2. Absence of blood flow leads to up-regulation of endothelial Notch signalling by up-regulation of dll4
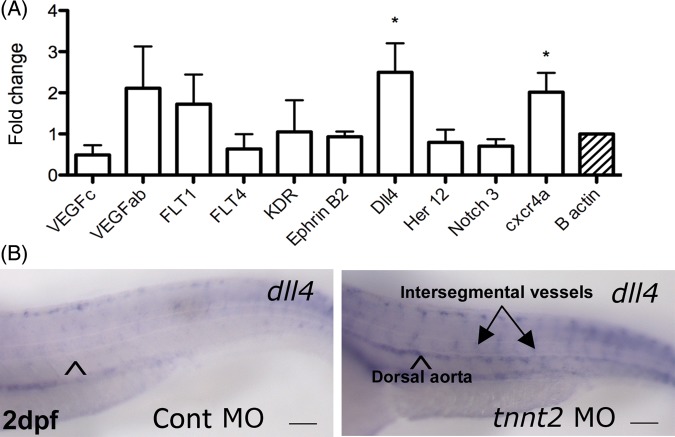
To determine how blood flow might regulate Notch signalling, we extracted RNA from the trunk of 48 hpf tnnt2 and control morphants (3 replicate experiments of 30 embryos per group) and performed quantitative rt–PCR for a range of Notch ligands and receptors, VEGF ligands and receptors, and CXCR4a (Figure 2A). As we previously described, CXCR4a was significantly up-regulated by the absence of blood flow (P < 0.05).3 The Notch ligand dll4 was also significantly up-regulated in tnnt2 morphants (P < 0.05).
Figure 2.
The absence of blood flow up-regulates the expression of dll4 (A) Quantitative RT–PCR for Notch ligands and receptors, VEGF ligands and receptors, and cxcr4a performed on trunk RNA from 48 hpf control and tnnt2 morphants. (B) Whole mount in situ hybridization for dll4 in 48 hpf control and tnnt2 morphants. Arrowhead indicates dorsal aorta, ISVs in tnnt2 morphant arrowed. Scale bar = 100 μm.
To examine spatial localization of the dll4 expression, we performed whole mount in situ hybridization in 48 hpf control and tnnt2 morphants. This confirmed dll4 was up-regulated within the aorta and ISVs of tnnt2 morphants. Representative micrographs are shown in Figure 2B; 10 of 10 control morphants had the appearance shown, whereas 12 of 13 tnnt2 morphants had clear up-regulation of dll4 as depicted. It should be noted that our in situ protocol was optimised to show dll4 upregulation in the absence of flow; more prolonged staining revealed aortic and ISV expression of dll4 in embryos with blood flow, but this was substantially less than in embryos without flow).
The above data suggested that the up-regulation of Notch signalling induced by the absence of blood flow in Figure 1 could be mediated via dll4 up-regulation. To test this, we co-injected a previously published dll4 morpholino with or without the tnnt2 morpholino to knockdown dll4 in 3 dpf Tg(CSL:venus)qmc61 embryos with or without blood flow. In the presence of flow dll4 knockdown did not alter Venus expression in the aorta (n = 19) compared with control morphants (n = 18). This lack of effect of dll4 knockdown on reporter expression in embryos with blood flow may be due to insensitivity of the reporter, perdurance or consitutive CSL activity. However, as before, tnnt2 knockdown induced a significant increase in Notch signalling (n = 11, P < 0.001). However, in similar tnnt2 morphants, dll4 knockdown was sufficient to prevent the up-regulation of aortic Notch signalling detected by Venus expression induced by tnnt2 knockdown (n = 11). Figure 3A shows representative micrographs of the trunk vasculature in control and tnnt2 morphants with and without dll4 knockdown. Figure 3B shows the quantification of the aortic fluorescence from all the embryos examined. These data confirmed that the absence of blood flow leads to up-regulation of Notch signalling via up-regulation of dll4 expression.
Figure 3.
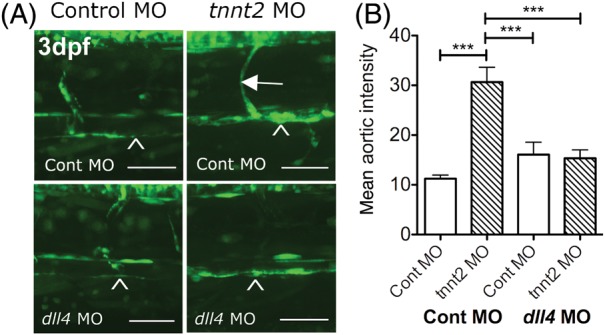
dll4 knockdown blocks the up-regulation of Notch signalling in the absence of blood flow (A) The effect of dll4 knockdown on reporter expression in 3 dpf Tg(CSL-venus)qmc61 embryos with and without blood flow due to tnnt2 knockdown. Arrowhead indicates dorsal aorta and arrow indicates an ISV. (B) The mean aortic fluorescence in 3 dpf control and dll4 morphant Tg(CSL-venus)qmc61 embryos with and without blood flow due to tnnt2 knockdown. Scale bar = 100 μm.
Our finding that dll4 was up-regulated in 48 hpf tnnt2 morphants was surprising, as previous studies reported down-regulation of dll4 in 36 hpf tnnt2 morphants (but not 24 hpf) by in situ hybridization and qPCR.21 We therefore extended our experiments to 72 hpf embryos, which continued to show up-regulation of dll4 in the vessels of tnnt2 morphants (Supplementary material online, Figure S2). This is consistent with the up-regulation of Notch signalling seen at the same developmental stage (Figure 1). It appears therefore that although Wang et al. showed down-regulation of dll4 in tnnt2 morphants at earlier stages of development, this is subsequently followed by up-regulation for at least the following 36 h.
Recent work has shown that the microRNA species miR-30b and miR-30c regulate dll4.22 We therefore questioned whether the effect of blood flow on dll4 levels is mediated via effects on the expression of this microRNA. We performed qPCR to quantify the expression of both miR-30b and miR-30c in 48 hpf control and tnnt2 morphant embryos. We found no significant difference in the expression of either miR (Supplementary material online, Figure S3), suggesting blood flow does not suppress dll4 expression via miR-30.
3.3. Absence of blood flow does not affect normal ISV patterning but prevents excessive angiogenesis in vhl mutants with constitutively activated hypoxic signalling
dll4 is expressed by endothelial tip cells during ISV formation, and reduction of dll4 expression leads to excessive and aberrant ISV angiogenesis.23 However, despite the absence of blood flow leading to up-regulation of dll4 expression and Notch signalling, we previously showed this is not associated with abnormal ISV formation in wild-type embryos,3 which do not experience hypoxia in the absence of blood flow.1 We therefore asked whether blood flow would be required for angiogenesis driven by up-regulation of hypoxic signalling. Low tissue oxygen levels lead to increased HIF1α signalling, which induces gene expression changes that are highly pro-angiogenic.24 However, incubation of zebrafish embryos in hypoxia leads to a reduction in cardiac output and blood flow, preventing direct testing of the interactions between low oxygen tension and blood flow during angiogenesis.
We therefore examined vascular development in zebrafish embryos that have constitutively activated HIF1α signalling due to mutation in the von Hippel Lindau (vhl) gene.14 These embryos have previously been shown to exhibit excessive angiogenesis due to up-regulation of VEGF signalling.25 These mutants therefore represent a powerful model of hypoxic-signalling driven angiogenesis. We crossed vhl mutants with existing transgenic lines that allowed visualization of endothelial cells, cytoplasm (Fli1:GFP15), nuclei (Flk1:GFPnls16), or endothelial membrane (kdrl:HRAS-mCherry17).
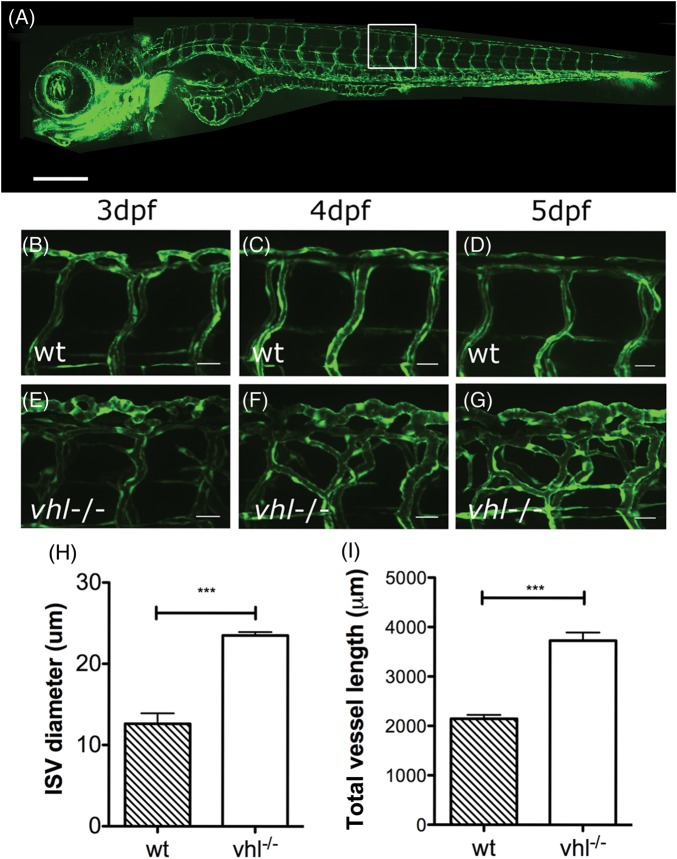
Figure 4B–D show higher power images of the ISVs in a representative wild-type embryo at 3, 4, and 5 dpf. By 3 dpf, the ISV pattern is largely established, with limited remodelling over the subsequent 2 days. Figure 4E–G show the same region in a vhl−/− mutant. As previously described,25 vhl−/− mutants exhibited aberrant ISV angiogenesis that became more apparent between 3 and 5 days post-fertilization (dpf). We quantified ISV diameter and compared with wild types (n = 5) this was significantly increased in vhl−/− mutants [n = 5, P < 0.001), Figure 4H]. Similarly, when we quantified ISV length, this was significantly greater in vhl−/− mutants (n = 5, P < 0.001) compared with wild types (n = 5).
Figure 4.
vhl−/− mutant zebrafish demonstrates excessive angiogenesis of trunk vessels during embryonic development. (A) Vascular anatomy of a Fli:eGFP wild-type embryo for orientation. Scale bar = 500 μm, regions of (B–G) boxed. (B–D) Mid-trunk intersegmental (ISV) and DLAV vessels from 3 to 5 dpf in a wild-type Fli:eGFP transgenic. (E–G) Mid-trunk vessels in a vhl−/− 3–5 dpf Fli:eGFP transgenic. Scale bars for B–G = 25 μm. H; ISV diameter of 3 dpf wt and vhl−/− at 3 dpf. I: total ISV length in 3 dpf wt and vhl−/− mutants.
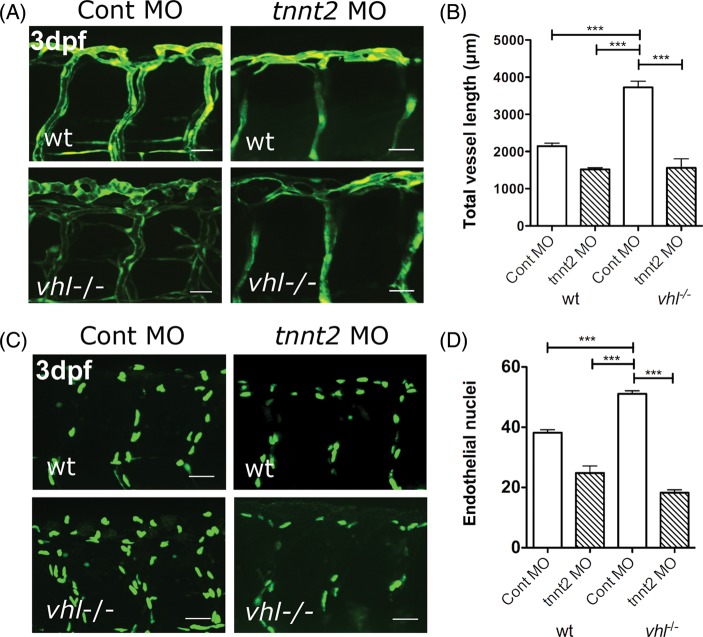
We next examined the effect of preventing cardiac contraction by tnnt2 knockdown on abnormal ISV formation in vhl−/− mutants. Representative 3 dpf Fli1:GFP wild-type and vhl−/− mutant transgenics with and without tnnt2 knockdown are shown in Figure 5A. Although as we previously described, tnnt2 knockdown did not affect ISV patterning in wild-type embryos (aside from a collapsed appearance due to lack of blood flow), the excessive ISV angiogenesis in homozygous vhl−/− mutants was completely abolished. When we quantified ISV length, we confirmed that tnnt2 knockdown did not significantly affect this in wild-type embryos (n = 5/group), but that tnnt2 knockdown reduced the increased total vessel length in vhl−/− mutants to wild-type levels (P < 0.001, n = 5–7/group) (Figure 5B).
Figure 5.
tnnt2 knockdown prevents excessive angiogenesis in vhl−/− zebrafish. (A) The mid-trunk vascular anatomy of 3 dpf Tg(fli:eGFP) wild type and vhl−/− with and without blood flow due to tnnt2 knockdown (site of imaging boxed in Figure 4A). (B) The total vessel length of 3 dpf wt control morphants, vhl−/− control morphants, and wt and vhl−/− tnnt2 morphants. (C) Endothelial nuclei in 3 dpf flk1:EGFP-nls wt and vhl−/− injected with tnnt2 or control morpholino. (D) The effect of tnnt2 knockdown on number of endothelial nuclei in a two-somite region of the mid trunk in 3 dpf wild type and vhl−/−.
The Flk1:GFPnls transgenic labels endothelial nuclei, allowing quantification of endothelial cell number. We therefore examined the effect of prevention of blood flow by tnnt2 knockdown on endothelial cell number in 3 dpf wild-type and vhl−/− mutant embryos. Representative 3 dpf Flk1:GFPnls wild-type and vhl−/− mutant transgenics with and without tnnt2 knockdown are shown in Figure 5C. When we quantified endothelial cell number, we found that compared with wild-type embryos with normal blood flow (n = 20), tnnt2 knockdown (n = 8) did not significantly affect endothelial cell number in a two-somite region of the mid trunk. As might be expected from the excessive vessel formation seen in Fli1:GFP vhl−/− mutants shown in Figure 4E–G, in the presence of blood flow vhl−/− mutants (n = 11), had a significantly greater number of endothelial cells (P < 0.001) compared with wild-types (Figure 5D). However, prevention of blood flow in vhl−/− mutants by tnnt2 knockdown (n = 11) reduced this to wild-type levels (P < 0.001), confirming that blood flow is required for the excessive angiogenesis in vhl−/− mutants.
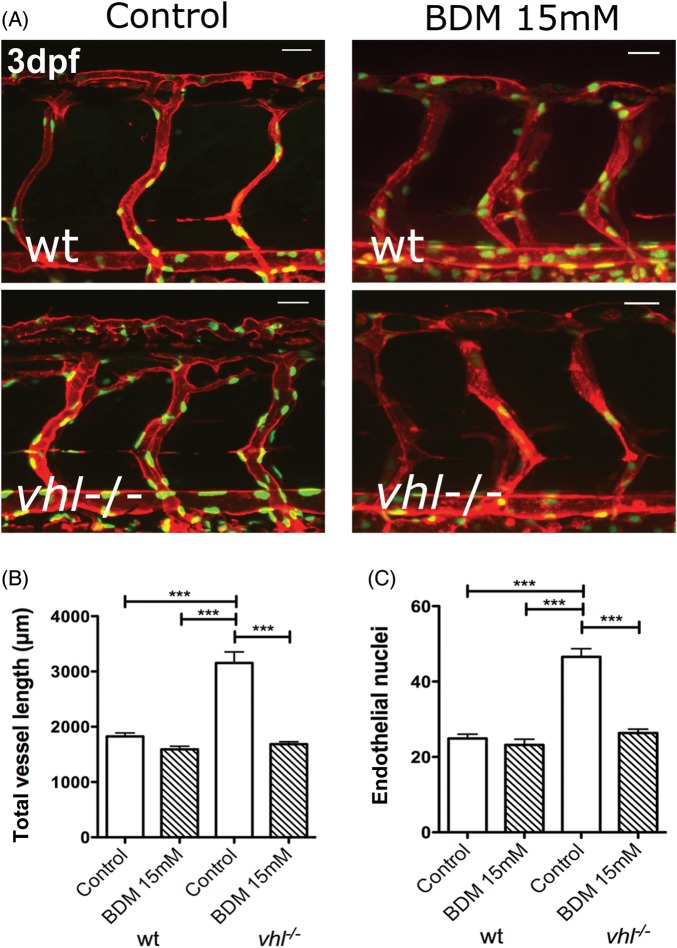
To confirm that the effect of tnnt2 knockdown on angiogenesis in vhl−/− mutants could be seen by preventing blood flow using an alternative method, we exposed wild-type and vhl−/− mutant embryos to BDM from 36 hpf until 72 hpf. We performed these experiments using a double transgenic (flk1:EGFP-nls labelling endothelial nuclei with GFP and kdrl:HRAS-mCherry labelling endothelial membrane with mCherry), allowing simultaneous quantification of vessel length and endothelial cell number. Figure 6A shows representative 3 dpf wild-type and vhl−/− double transgenics with and without BDM treatment. In control-treated vhl−/− mutants with blood flow, excessive endothelial cell number and ISV sprouting is apparent, whereas BDM treatment significantly reduced both vessel length (Figure 6B, P < 0.001) and endothelial cell number (Figure 6C, P < 0.001) to wild-type levels (n = 11/group). The data confirmed that the absence of blood flow prevented the excess ISV angiogenesis in vhl−/− mutants, though preserving normal ISV architecture and endothelial cell number.
Figure 6.
Pharmacological inhibition of blood flow blocks excessive angiogenesis in vhl−/− mutant zebrafish. (A) 3 dpf Tg(kdrl:memRFP; flk1:EGFP-nls) double transgenic wild type or vhl−/− mutants, treated with vehicle or [15 mM] BDM to stop blood flow from 36 hpf until 3 dpf (site of imaging boxed in A). (B) A total vessel length at 3 dpf of wt, vhl−/−, and BDM-treated groups. (C) The mean number of endothelial nuclei in a two-somite mid-trunk region in wt and vhl−/− treated with BDM or vehicle.
4. Discussion
Most studies of angiogenesis use in vitro models that seldom reproduce the exposure to blood flow. The contribution of blood flow to vascular development has been little studied, perhaps because angiogenesis clearly can proceed in the absence of blood flow in vitro and in several in vivo contexts, such as those that precede cardiac output.
However, recent studies have revealed that the contribution of blood flow to angiogenesis is highly complex and context-dependent. Dependent upon the model and vessel studied, published reports have shown blood flow to be required,5,7 dispensable,3 or inhibitory8,9 for vessel formation. This uncertainty led us to investigate potential mechanisms for integration of blood flow with angiogenic pathways. The zebrafish is an attractive model for this purpose due to its proven ability to oxygenate via diffusion even when cardiac contraction is entirely halted,1 at least in the first several days of development.
The Notch pathway is a complex and fundamental mechanism in embryonic development and disease. Although Notch signalling is required for vascular development,11 including lymphatic development,19 it can also play an inhibitory role in angiogenesis, by repressing tip cell formation.13,26 The regulation of this multifaceted contribution to vascular development is likely to be multi-layered. However, we find that the Notch ligand dll4 is clearly transcriptionally repressed by blood flow in the aorta and ISVs of 48 h post-fertilization zebrafish embryos. This provides a potential pathway for blood flow to integrate with other signals to modulate angiogenesis. Although Notch ligand/receptor interactions lead to signalling in both ligand and receptor bearing cells, this does not appear to affect angiogenesis in the ligand-bearing cell.27 This suggests that any modulation of angiogenesis induced by flow mediated alteration in dll4 expression is likely to be mediated by effects on Notch receptor-expressing cells, rather than in an autocrine fashion.
The previous work by Wang et al.21 found that the absence of blood flow led to a decrease in dll4 expression at 36 hpf. Our finding that at later time points the same manipulation leads to increased dll4 expression is supported by a number of lines of evidence. Wang et al. did not examine whether tnnt2 knockdown led to alteration in Notch signalling, whereas we found this is indeed up-regulated by the absence of blood flow. We also confirmed that a second method of halting circulation (incubation in the drug BDM) induced up-regulation of Notch signalling at the same time points used for tnnt2 knockdown studies. Our findings do not contradict Wang et al., since they studied earlier stages of development. It is possible that tnnt2 knockdown does reduce dll4 expression at 38 hpf, but this is followed by the up-regulation we demonstrate at later time points. The reason for such a biphasic response is unclear, but the data from our Notch reporter studies up to 72 hpf strongly suggest that the overall effect of absent blood flow is an increase, not a decrease, in vascular Notch signalling.
The exact mechanism whereby the physical forces of blood flow influence the expression of dll4 remains unclear. Although several transcription factors (notably klf2) have been demonstrated to mediate the transcription of hundreds of genes in response to shear stress, recent work has highlighted the potential for microRNAs to similarly influence gene expression in response to haemodynamic alteration.5 Nicoli et al. showed that blood flow induces klf2a which in turn up-regulates miR-126. However, recent work suggests miR-126 increases, rather than decreases dll4 expression,28 suggesting miR-126 is not the mechanism whereby blood flow suppresses dll4.
We examined one candidate, miR-30 which regulates dll4 expression in other circumstances,22 but found no evidence to suggest this particular miR is the link between blood flow and dll4. Although target prediction algorithms suggest miR-30 is the microRNA species most likely to target dll4, this does not exclude the possibility that other miRs regulate dll4 under conditions of altered haemodynamic forces. The up-regulation of hypoxic signalling in vhl mutants would be predicted to up-regulate so-called hypoxamirs29 such as miR-210,30 which are highly likely to underlie the angiogenic phenotype and these may also integrate blood flow with angiogenic signalling. Such a link would be interesting to explore in future work.
Despite our finding that blood flow regulates Notch signalling, this is insufficient in itself to alter ISV formation during normal angiogenic development, since ISVs form normally, with normal endothelial cell number, even in the complete absence of blood flow. We therefore sought to identify whether other forms of angiogenesis might be more sensitive to alterations in blood flow.
Up-regulation of hypoxic signalling induces a profoundly pro-angiogenic response, and unsurprisingly constitutive activation of this process by homozygous mutation of vhl induces excessive angiogenesis in the ISVs of developing zebrafish embryos. We show for the first time that angiogenesis promoted by this up-regulation of hypoxic signalling is prevented in the absence of blood flow. Although a recent study has suggested that a reduction in cardiac output may induce hypoxic signalling in zebrafish, this occurred after 7 dpf,31 significantly later than the 3–5 dpf embryos examined in our work. Even if reducing blood flow induced hypoxia, this would be predicted to increase, not decrease the angiogenic response.
We are unable to say for certain how cessation of blood flow exerts its effect on Notch signalling and aberrant angiogenesis in vhl mutants. Various stimuli will occur in response to either preventing or halting blood flow in the vasculature, as we achieved in our models. Even if oxygenation is maintained, it is possible that CO2, being a larger, less diffusible molecule may have accumulated in the tissue. Since teleosts have been shown to possess carbonic anhydrase,32 an accumulation of CO2 could lead to increased osmotically active HCO3− which themselves may induce alterations in physical forces exerted on the vasculature. However, we believe that the effects of blood flow on Notch signalling and angiogenesis seen in our study are more likely to be exerted via mechanotransduction of the physical forces exerted by blood flow, rather than biochemically.
vhl−/− mutants experience increased levels of cardiac output and blood flow,14 and this must be considered in interpreting our results. It is possible that increased levels of blood flow might suppress Notch signalling still further than in wild types, and this would be predicted to lead to increased angiogenesis. However, as VEGF inhibition reduces angiogenesis in vhl mutants, it seems likely the abnormal angiogenesis seen in these mutants is predominantly driven by hypoxic signalling, rather than by increased blood flow. Nevertheless, our data suggest that blood flow and hypoxic signalling integrate at some level to permit angiogenesis.
Our work has several limitations. Most notably, we have been unsuccessful in demonstrating whether the requirement for blood flow in hypoxic-signalling driven angiogenesis is mediated by suppression of Notch signalling. Notch inhibition in vhl mutants without blood flow, either by morpholino knockdown of dll4 or pharmacological inhibition with gamma secretase inhibitors leads to embryonic death, which has prevented us from testing the hypothesis that Notch inhibition might rescue the excessive angiogenesis in vhl mutants even in the absence of blood flow. Exposing embryos directly to hypoxia reduces blood flow. This prevented us from examining the interaction of blood flow with angiogenesis stimulated by low oxygen tension (true hypoxia) as opposed to angiogenesis driven by up-regulated hypoxic signalling in normoxic conditions as in the vhl mutant. Nevertheless, the twin observations that blood flow suppresses Notch signalling via the repression of dll4 expression, and that blood flow is required for angiogenesis driven by hypoxic signalling, both add to our understanding of how blood flow may contribute to angiogenesis, and underline that mechanisms of angiogenesis differ according to context.
Our data suggest that targeting mechanosensory pathways that sense blood flow might prevent angiogenesis in situations where this is driven by hypoxia. This could potentially open novel therapeutic strategies for conditions such as diabetic retinopathy where ischaemia stimulates the neoangiogenesis that leads to visual impairment.33 In addition, since Notch inhibition may sensitize some tumours to chemotherapy,34 an ability to down-regulate Notch by exposure to fluid forces or blood flow might have adjuvant benefits for cancer therapeutics. As VEGF drives much of the angiogenic reponse to hypoxia, a requirement for blood flow for angiogenesis to occur in response to VEGF could underlie the disappointing clinical results of VEGF treatment for coronary artery disease35 as it may not have induced neovascularization when delivered to poorly perfused tissues. Further study of the interactions between blood flow and angiogenesis seems likely to reveal further intricacies of the regulation of vascular formation in development and disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by BHF project grants (09/287/28051 and 12/12/29433) awarded to T.J.A.C. F.J.M.E. was supported by EC-FP7 HEALTH-F4-2010-242048. MRC Centre Grant (G0700091) awarded to Professor PW Ingham provided a clinical training fellowship to OJW. Funding for Open Access was provided by he research was funded by the British Heart Foundation.
Supplementary Material
References
- 1.Pelster B, Burggren WW. Disruption of hemoglobin oxygen transport does not impact oxygen-dependent physiological processes in developing embryos of zebra fish (Danio rerio) Circ Res. 1996;79:358–362. doi: 10.1161/01.res.79.2.358. doi:10.1161/01.RES.79.2.358. [DOI] [PubMed] [Google Scholar]
- 2.Gray C, Packham IM, Wurmser F, Eastley NC, Hellewell PG, Ingham PW, et al. Ischemia is not required for arteriogenesis in zebrafish embryos. Arterioscler Thromb Vasc Biol. 2007;27:2135–2141. doi: 10.1161/ATVBAHA.107.143990. doi:10.1161/ATVBAHA.107.143990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packham IM, Gray C, Heath PR, Hellewell PG, Ingham PW, Crossman DC, et al. Microarray profiling reveals CXCR4a is downregulated by blood flow in vivo and mediates collateral formation in zebrafish embryos. Physiol Genomics. 2009;38:319–327. doi: 10.1152/physiolgenomics.00049.2009. doi:10.1152/physiolgenomics.00049.2009. [DOI] [PubMed] [Google Scholar]
- 4.Zygmunt T, Trzaska S, Edelstein L, Walls J, Rajamani S, Gale N, et al. ‘In parallel’ interconnectivity of the dorsal longitudinal anastomotic vessels requires both VEGF signaling and circulatory flow. J Cell Sci. 2012;125:5159–5167. doi: 10.1242/jcs.108555. doi:10.1242/jcs.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and VEGF signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. doi:10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita M, Cha YR, Pham VN, Sakurai A, Roman BL, Gutkind JS, et al. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development. 2011;138:1705–1715. doi: 10.1242/dev.058776. doi:10.1242/dev.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussmann J, Wolfe SA, Siekmann AF. Arterial–venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development. 2011;138:1717–1726. doi: 10.1242/dev.059881. doi:10.1242/dev.059881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci USA. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. doi:10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouinard-Pelletier G, Jahnsen ED, Jones EA. Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis. 2013;16:71–83. doi: 10.1007/s10456-012-9300-2. doi:10.1007/s10456-012-9300-2. [DOI] [PubMed] [Google Scholar]
- 10.Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. doi:10.1016/S1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 12.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the notch ligand delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. doi:10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 13.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. doi:10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 14.van Rooijen E, Voest EE, Logister I, Korving J, Schwerte T, Schulte-Merker S, et al. Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood. 2009;113:6449–6460. doi: 10.1182/blood-2008-07-167890. doi:10.1182/blood-2008-07-167890. [DOI] [PubMed] [Google Scholar]
- 15.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. doi:10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 16.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. doi:10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41:396–398. doi: 10.1038/ng.321. doi:10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- 18.Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DYR. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–110. doi: 10.1038/ng875. doi:10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- 19.Geudens I, Herpers R, Hermans K, Segura I, Ruiz de Almodovar C, Bussmann J, et al. Role of delta-like-4/notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol. 2010;30:1695–1702. doi: 10.1161/ATVBAHA.110.203034. doi:10.1161/ATVBAHA.110.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr Biol. 2002;12:492–497. doi: 10.1016/s0960-9822(02)00694-2. doi:10.1016/S0960-9822(02)00694-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Zhang PP, Wei YL, Gao Y, Patient R, Liu F. A blood flow-dependent klf2a-NO signaling cascade is required for stabilization of hematopoietic stem cell programming in zebrafish embryos. Blood. 2011;118:4102–4110. doi: 10.1182/blood-2011-05-353235. doi:10.1182/blood-2011-05-353235. [DOI] [PubMed] [Google Scholar]
- 22.Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, et al. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood. 2012;120:5063–5072. doi: 10.1182/blood-2012-04-423004. doi:10.1182/blood-2012-04-423004. [DOI] [PubMed] [Google Scholar]
- 23.Siekmann AF, Lawson ND. Notch signalling and the regulation of angiogenesis. Cell Adhes Migr. 2007;1:104–106. doi: 10.4161/cam.1.2.4488. doi:10.4161/cam.1.2.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. doi:10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 25.van Rooijen E, Voest EE, Logister I, Bussmann J, Korving J, van Eeden FJ, et al. Von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis Model Mech. 2010;3:343–353. doi: 10.1242/dmm.004036. doi:10.1242/dmm.004036. [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. doi:10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 27.Liebler SS, Feldner A, Adam MG, Korff T, Augustin HG, Fischer A. No evidence for a functional role of bi-directional notch signaling during angiogenesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0053074. e53074. doi: 10.1371/journal.pone.0053074. [Epub 2012 Dec 28]. doi:10.1371/journal.pone.0053074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate notch ligand delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med. 2013;31:484–492. doi: 10.3892/ijmm.2012.1200. [DOI] [PubMed] [Google Scholar]
- 29.Madanecki P, Kapoor N, Bebok Z, Ochocka R, Collawn JF, Bartoszewski R. Regulation of angiogenesis by hypoxia: the role of microRNA. Cell Mol Biol Lett. 2013;18:47–57. doi: 10.2478/s11658-012-0037-0. doi:10.2478/s11658-012-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19:215–223. doi: 10.1111/j.1549-8719.2011.00154.x. doi:10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopp R, Schwerte T, Egg M, Sandbichler AM, Egger B, Pelster B. Chronic reduction in cardiac output induces hypoxic signaling in larval zebrafish even at a time when convective oxygen transport is not required. Physiol Genom. 2010;42A:8–23. doi: 10.1152/physiolgenomics.00052.2010. doi:10.1152/physiolgenomics.00052.2010. [DOI] [PubMed] [Google Scholar]
- 32.Gilmour KM, Thomas K, Esbaugh AJ, Perry SF. Carbonic anhydrase expression and CO2 excretion during early development in zebrafish Danio rerio. J Exp Biol. 2009;212:3837–3845. doi: 10.1242/jeb.034116. doi:10.1242/jeb.034116. [DOI] [PubMed] [Google Scholar]
- 33.Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2012;12:CD007419. doi: 10.1002/14651858.CD007419.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs JJ, et al. Inhibition of the Wnt-beta-catenin and notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun. 2013;431:274–279. doi: 10.1016/j.bbrc.2012.12.118. doi:10.1016/j.bbrc.2012.12.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. doi:10.1161/01.CIR.0000061911.47710.8A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.