Abstract
Aims
Signalling via cGMP-dependent protein kinase I (cGKI) is the major pathway in vascular smooth muscle (SM), by which endothelial NO regulates vascular tone. Recent evidence suggests that canonical transient receptor potential (Trpc) channels are targets of cGKI in SM and mediate the relaxant effects of cGMP signalling. We tested this concept by investigating the role of cGMP/cGKI signalling on vascular tone and peripheral resistance using Trpc6−/−, Trpc3−/−, Trpc3−/−/6−/−, Trpc1−/−/3−/−/6−/−, and SM-specific cGKI−/− (sm-cGKI−/−) mice.
Methods and results
α-Adrenergic stimulation induced similar contractions in L-NG-nitroarginine methyl ester (l-NAME)-treated aorta and comparably increased peripheral pressure in hind limbs from all mouse lines investigated. After α-adrenergic stimulation, 8-Br-cGMP diminished similarly aortic tone and peripheral pressure in control, Trpc6−/−, Trpc3−/−, Trpc3−/−/6−/−, and Trpc1−/−/3−/−/6−/− mice but not in sm-cGKI−/− mice. In untreated aorta, α-adrenergic stimulation induced similar contractions in the aorta from control and Trpc3−/− mice but larger contractions in sm-cGKI−/−, Trpc6−/−, Trpc3−/−/6−/−, and Trpc1−/−/3−/−/6−/− mice, indicating a functional link between cGKI and Trpc6 channels. Trpc3 channels were detected by immunocytochemistry in both isolated aortic smooth muscle cells (SMCs) and aortic endothelial cells (ECs), whereas Trpc6 channels were detected only in ECs. Phenylephrine-stimulated Ca2+ levels were similar in SMCs from control (Ctr) and Trpc6−/− mice. Carbachol-stimulated Ca2+ levels were reduced in ECs from Trpc6−/− mice. Stimulated Ca2+ levels were lowered by 8-Br-cGMP in Ctr but not in Trpc6−/− ECs.
Conclusions
The results suggest that cGKI and Trpc1,3,6 channels are not functionally coupled in vascular SM. Deletion of Trpc6 channels impaired endothelial cGKI signalling and vasodilator tone in the aorta.
Keywords: Phenylephrine, Smooth muscle, Relaxation, Nitric oxide, Endothelium
1. Introduction
The dilator action of endothelium-derived NO contributes to the control of basal and stimulated regional blood flow.1 The cellular effects of NO are mainly mediated by a rise in intracellular cGMP levels in the target cells.2,3 The major target for cGMP in vascular smooth muscle (SM) is the cGMP-dependent protein kinase I (cGKI).4 cGKI relaxes SM through interaction with the myosin-interacting subunit of myosin phosphatase 1,5 the regulator of G-protein signalling 2,6 and the IP3R-associated cGMP kinase substrate (IRAG7). Recent studies added new potential targets by showing that cGKI inhibits the activity of the canonical transient receptor potential (Trpc) channels in SM.8–11
Trpc channels (Trpc1–7) are a subfamily of the TRP channel family that is expressed in many cells including vascular SM and vascular endothelium cells.9,12 Trpc channels have been invoked to be essential for the Bayliss effect.13 Evidence for the control of Trpc channels by cGKI, especially of Trpc1, 3, and 6 channels, comes from in vitro and in vivo experiments. For instance, inhibition of Trpc1/Trpc3 by cGKI has been shown to contribute to NO-mediated vasorelaxation in freshly isolated smooth muscle cells (SMCs) from the rat carotid artery.8 In line with this finding, Trpc3 channels were directly phosphorylated by cGKI at position T11 and S263 when expressed in HEK293 cells.10 Further, the NO-cGMP-cGKI pathway negatively regulated Trpc6 channels in cultured vascular smooth muscle cells (VSMCs).11 In addition, a functional link between TRP channels and cGKI signalling has been proposed to explain the effects of atrial natriuretic peptides on trans-vascular fluid exchange.14
In the present study, we tested the concept of a functional coupling between Trpc3/6 channels and cGMP/cGKI in SM in vivo using the respective knockout mice. To rule out an effect of the reported overexpression of Trpc3 channels in Trpc6−/− mice,15 we also used Trpc3/Trpc6-double knockout mice and, as a further control, Trpc1/Trpc3/Trpc6-triple knockout mice. We measured vascular contraction in aortic rings and pressure in the hind limb system. The hind limb system represents a more confident model for vessels involved in blood pressure regulation than the aorta. Surprisingly, we found no functional coupling between cGMP/cGKI and Trpc3/6 channels in murine vascular SM. We further investigated the expression of Trpc channels in vascular SMC and EC and measured α-adrenergic- or muscarinic-induced Ca2+ levels in these cells. We present evidence for the existence of a functional coupling between cGMP/cGKI signalling and Trpc6 channels in ECs.
2. Methods
2.1. Mice
All procedures relating to animal care and treatment conformed to the institutional and governmental guidelines (Directive 2010/63/EU of the European Parliament) and were approved by local authorities (Regierung von Oberbayern). The following mouse lines were generated and used for the experiments: wild-type (WT) mice, mice lacking cGKI selectively in SM (sm-cGKI−/− mice16), mice lacking Trpc3 channels (Trpc3−/− mice17), mice lacking Trpc6 channels (Trpc6−/− mice15), mice lacking both Trpc3 and Trpc6 channels (Trpc3−/−/6−/− mice), and mice lacking Trpc1,18 Trpc3, and Trpc6 channels (Trpc1−/−/3−/−/6−/− mice). Control (Ctr) mice were heterozygous with respect to the respective knockout (see Supplementary material online, Figure S1A). Since none of the Ctr mice shows any differences in the effects investigated to those observed in WT mice (see Supplementary material online, Figure S1A), data obtained in the Ctr mouse lines were pooled with those obtained in WT mice. Mice of either sex were used for the experiments at the age of 3–6 months and sacrificed by decapitation.
2.2. Tension recordings
Segments of the thoracic aorta were taken from mice and placed in buffer solution at 37°C. Connective tissue and adhering fat were removed. Aortic rings were mounted into organ baths (Myograph 601, www.dmt.dk). Tension was recorded isometrically at 37°C. Rings were pre-stretched by 3 mN/mm. Contraction was measured after establishment of steady-state conditions with respect to the baseline.
2.3. Hind limb perfusion
After sacrifice, the aorta abdominalis was prepared and a 1 mm catheter was introduced, advanced to the bifurcation of the iliac arteries, and tied with a 6/0 silk suture. The inferior caval vein was slit open longitudinally to prevent venous congestion. Pressure was measured at 37°C using the universal perfusion set-up UP-100 (www.hugo-sachs.de). The hind limbs were perfused with buffer solution at a constant flow rate of 5–8 mL/min to give a resting pressure of ∼40–70 mmHg. The resting pressure was not different between the heterozygous and the respective knockout mice (see Supplementary material online, Figure S1B). Substances were applied by changing the buffer solution to a buffer containing the substances at the concentrations indicated. Responses were measured as changes with respect to the resting pressure.
2.4. Immunocytochemistry
VSMCs and ECs were isolated as described.19 The following primary antibodies were used: anti-cGKI (1:100 dilution20), anti-CD31 (2.5 µg/mL, www.bdbiosciences.com), anti-α-actin (0.5 µg/mL (rabbit, www.abcam.com) or 1:100 dilution (mouse, www.millipore.com)), anti-Trpc3, and anti-Trpc6 (1:200 dilution). Primary antibody/antigen complexes were detected with secondary antibodies conjugated to fluorescent dyes (1:300 dilutions for Alexa Fluor 488 and Alexa Fluor 594, www.lifetechnologies.com; 1:500 dilutions for Cy2 and Cy3, www.dianova.com). The fluorescence signals were visualized by a confocal laser scanning microscope using fixed adjustments for each condition (LSM510, www.zeiss.de).
2.5. Ca2+ measurements
Intracellular [Ca2+] was monitored in freshly isolated aortic SMC and EC using Fura-2/AM at room temperature as described.21 [Ca2+]i was recorded as the fluorescence intensity (at 510 nm) at alternating 350 (F350) and 380 nm (F380) excitation wavelengths and their respective ratios (F350/F380) using a TILLvision device (www.till-photonics.de). Stimulation was performed by local application of phenylephrine (PE; 30 µM), carbachol (CCh; 30 µM), and 8-Br-cGMP (300 µM) via a syringe device.
2.6. RNA isolation
Total RNA isolation was performed on intact and endothelium-denuded aortas from 12 to 15 WT mice as described by the supplier (peqGOLD RNAPure, PEQLAB Biotechnologie, Erlangen, Germany).
2.7. PCR analysis
Total RNA (1 µg) was utilized as a template for semi-quantitative PCR to amplify primers against Trpc3, Trpc6, α-SMA (alpha smooth muscle actin), PECAM-1 (platelet endothelial cell adhesion molecule 1), and HPRT (hypoxanthine-guanine phosphoribosyltransferase). The analysis was performed using the OneStep RT-PCR Kit (QIAGEN, Hilden, Germany) according to manufacturer's instructions and using a peqSTAR thermocycler (PEQLAB Biotechnologie, Erlangen, Germany).
2.8. Substances
All salts and substances were used as pure as commercially available and purchased from Sigma (www.sigmaaldrich.com) unless otherwise indicated.
2.9. Statistical analysis
Results are presented as photos and original recordings or expressed as means ± SEM. Effects of substances were analysed in steady-state conditions. Statistical comparisons of data sets were performed by Student's t-test or by ANOVA followed by a Bonferroni post hoc test using Prism 5 (www.graphpad.com). Differences were considered significant at P < 0.05.
An expanded Method section is presented in the Supplementary material online.
3. Results
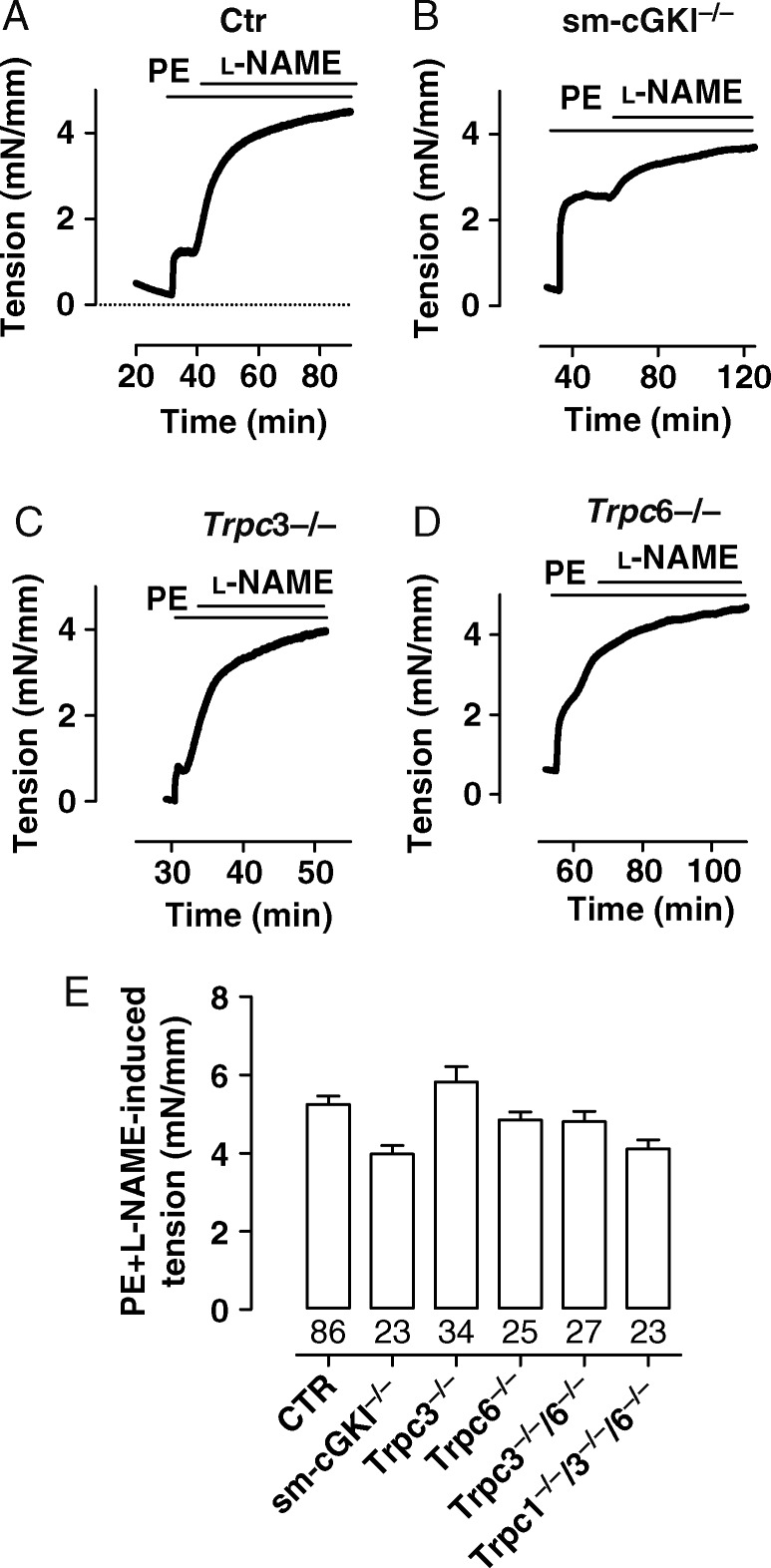
Trpc channels, especially the Trpc6 channel, have been proposed to play a central role in the control of vascular SM contraction.9,13,22 Consequently, we investigated the magnitude of vascular contraction in various mouse models including Ctr, Trpc3−/−, Trpc6−/−, Trpc3−/−/6−/−, Trpc1−/−/3−/−/6−/−, and sm-cGKI−/− mice. Contraction was induced by stimulation with the α-adrenergic agonist PE. Contraction was measured directly as an increase in tension using intact aortic rings and evaluated indirectly as an increase in pressure using the hind limb perfusion system. In aortic rings, the contraction by PE (3 µM) was assessed in the presence of the NO synthase inhibitor L-NG-nitroarginine methyl ester (l-NAME) (100 µM) to exclude the vasodilator tone provided by the NO generation in the vascular endothelium.3,23 In the presence of l-NAME, PE elicited contractions of similar magnitude in aortic rings from all mouse lines investigated (Figure 1A–E, see Supplementary material online, Figure S2). These findings suggest that, in the presence of an NOS inhibitor, α-adrenergic contraction of the murine aorta does not rely on the presence of Trpc1,3,6 channels.
Figure 1.
Magnitudes of PE-induced contractions in the murine aorta in the presence of l-NAME. (A–D) Original recordings of PE-induced contraction in aortic rings from a CTR (A), sm-cGKI−/− (B), Trpc3−/− (C), and Trpc6−/− (D) mouse. Bars indicate the presence of PE (3 µM) and l-NAME (100 µM), respectively. (E) Magnitudes of PE-induced contractions in aortic rings from the indicated mouse line in which NO synthases were inhibited by l-NAME. Columns represent mean ± SEM. Numbers represent the number of experiments. Data were analysed using ANOVA followed by the Bonferroni post hoc test, which indicated no statistically significant differences between Ctr and the respective mouse strain.
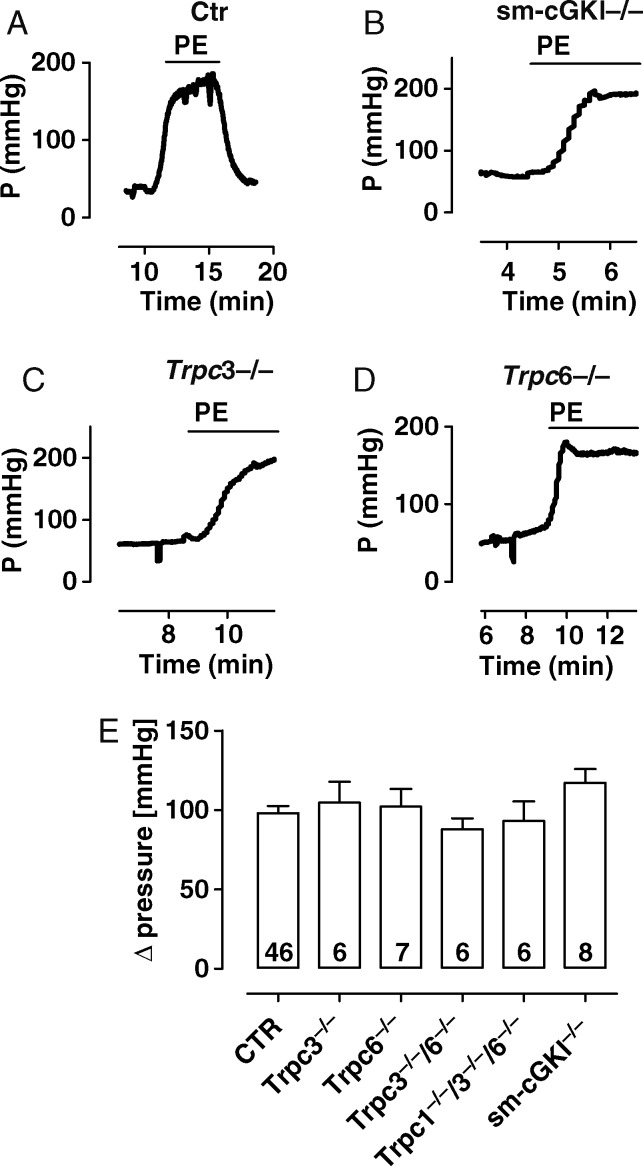
In agreement with the above experiments, PE (10 µM) induced similar increases in pressure in all mouse lines using the hind limb perfusion system (Figure 2A–E, see Supplementary material online, Figure S2). l-NAME (100 µM) had no major influence on this effect, indicating that the vessels of the hind limb system do not exhibit a significant relaxing tone due to spontaneous NO production, at least if they were stimulated by an α-adrenergic agonist (see Supplementary material online, Figure S3). These results indicate that, in the absence of an NO-dependent relaxing tone, Trpc1,3,6 channels are not involved in α-adrenergic-mediated contraction of vascular SM.
Figure 2.
Magnitudes of PE-induced pressure increase in the murine hind limb. (A–D) Original recordings of the increase in PE-induced pressure in the hind limb from a CTR (A), sm-cGKI−/− (B), Trpc3−/− (C), and Trpc6−/− (D) mouse. Bars indicate the presence of PE (10 µM). (E) Magnitudes of the increase in PE-induced pressure in the indicated mouse line. Columns represent means ± SEM. Numbers represent the number of experiments. Data were analysed using ANOVA followed by the Bonferroni post hoc test, which indicated no statistically significant differences between Ctr and the respective mouse strain.
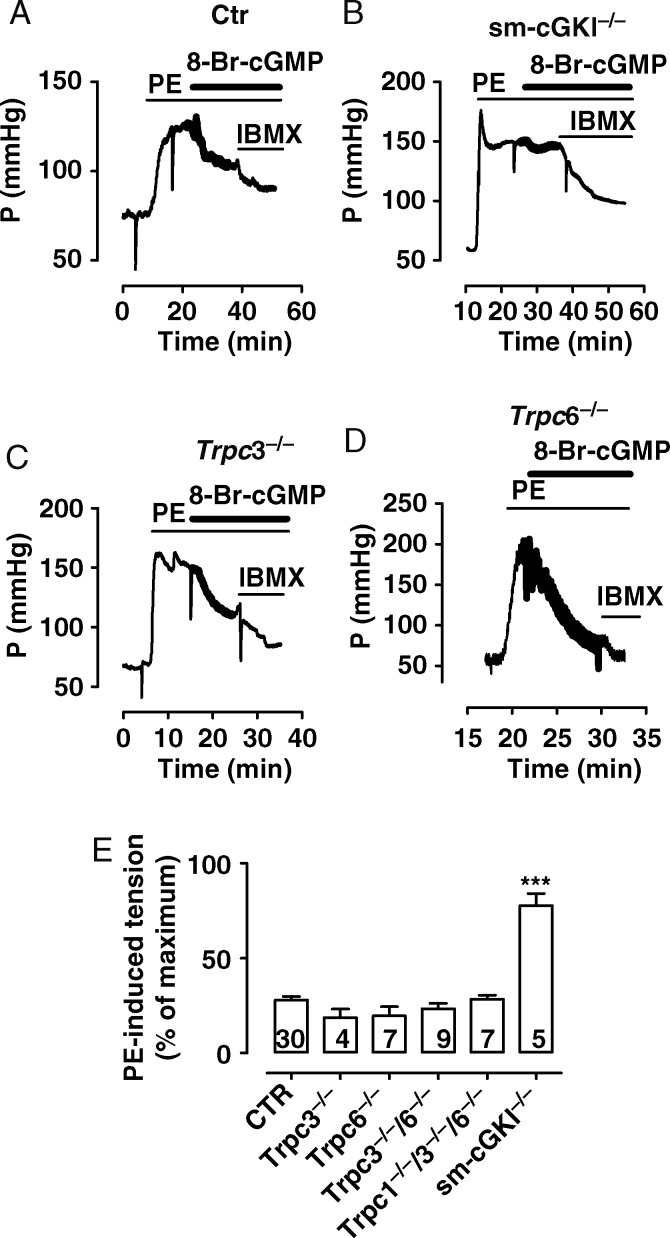
Recent evidence indicates that NO-mediated relaxation partially involves cGKI-dependent inhibition of Trpc channel activity.8 Therefore, we tested the significance of a functional coupling between cGMP/cGKI and the presence of Trpc channels, in particular the Trpc1, Trpc3, and Trpc6 channels, using aortic rings and hind limbs from Ctr, Trpc3−/−, Trpc6−/−, Trpc3−/−/6−/−, Trpc1−/−/3−/−/6−/−, and sm-cGKI−/− mice. Aortic rings were contracted by PE (3 µM) and l-NAME (100 µM) and then relaxed by the membrane-permeable analogue of cGMP, 8-Br-cGMP (300 µM). 8-Br-cGMP similarly reduced these contractions in aortic rings from all mouse lines investigated except rings from sm-cGKI−/− mice (Figure 3A–E, see Supplementary material online, Figure S2). Likewise, 8-Br-cGMP (300 µM) comparably reduced PE-induced pressure increases in hind limbs from all mouse lines investigated except hind limbs from sm-cGKI−/− mice (Figure 4A–E, see Supplementary material online, Figure S2). These results demonstrate (i) that cGKI is essential for the effect of 8-Br-cGMP in aortic rings and hind limb vessels, and (ii) that the effect of 8-Br-cGMP does not require Trpc1, Trpc3, or Trpc6 channels.
Figure 3.
Magnitudes of cGMP-mediated relaxation in pre-contracted murine aorta. (A–D) Original recordings of 8-Br-cGMP-mediated relaxation in PE-contracted aorta from a CTR (A), sm-cGKI−/− (B), Trpc3−/− (C), and Trpc6−/− (D) mouse. Bars indicate the presence of PE (3 µM), l-NAME (100 µM), 8-Br-cGMP (300 µM), IBMX (100 µM), and isradipine (1 µM), respectively. (E) Magnitudes of tension in the presence of 8-Br-cGMP (300 µM) in PE-contracted aortic rings from the indicated mouse line. Values are calculated as the % of maximal contraction with respect to the baseline in the presence of IBMX. Columns represent means ± SEM. Numbers represent the number of experiments. Data were analysed using ANOVA followed by the Bonferroni post hoc test. Asterisks indicate a significant difference from Ctr with ***P < 0.001.
Figure 4.
Magnitudes of decrease in cGMP-induced pressure in the murine hind limb. (A–D) Original recordings of increase in PE-induced pressure in the hind limb from a CTR (A), sm-cGKI−/− (B), Trpc3−/− (C), and Trpc6−/− (D) mouse. Bars indicate the presence of PE (10 µM), 8-Br-cGMP (300 µM), and IBMX (100 µM). (E) Magnitudes of pressure in the presence of 8-Br-cGMP (300 µM) in PE-treated hind limbs from the indicated mouse line. Values are calculated as the % of maximal pressure with respect to the baseline in the presence of IBMX. Columns represent means ± SEM. Numbers represent the number of experiments. Data were analysed using ANOVA followed by the Bonferroni post hoc test. Asterisks indicate a significant difference from Ctr with ***P < 0.001.
In parallel experiments, the effect of PE on contraction was assessed in intact aorta in which a relaxing tone exists partially due to the presence of spontaneous endothelial NO production.3,23 In the absence of l-NAME, PE (3 µM) induced similar contractions in aortic rings from Ctr and Trpc3−/− mice but a significantly larger contraction in rings from sm-cGKI mice (see Supplementary material online, Figure S4A–E). PE also induced significant larger contractions in rings from Trpc6−/− mice (see Supplementary material online, Figure S4D and E). The larger PE-induced contractions were likewise observed in rings from Trpc3−/−/6−/−, and Trpc1−/−/3−/−/6−/− mice (see Supplementary material online, Figures S2 and S4E) challenging the view that the larger contractility observed in the aorta from Trpc6−/− mice is due to an up-regulation of Trpc3 channels.15 Equal results were obtained if the effects of PE in intact aorta were expressed in relation to the contraction stimulated by both PE and l-NAME (paired conditions; see Supplementary material online, Figure S5) or to an analysis per mouse basis (see Supplementary material online, Figure S5). Together, these results indicate that there exists a relaxing component in intact aorta that depends on the presence of sm-cGKI and that may represent the NO-dependent relaxing tone reported previously.3 Further, the enhanced α-adrenergic-mediated contraction in intact aortic rings from Trpc6−/−, Trpc3−/−/6−/−, and Trpc1−/−/3−/−/6−/− mice suggests that Trpc6 channels are partially involved in the maintenance of the NO-mediated relaxing tone.
So far, our results indicate that cGKI and Trpc6 channels are necessary to observe a fully activated vasodilator tone in intact aortic rings but that they are not functionally coupled in SM. To further elucidate this issue, we performed semi-quantitative PCR analysis on intact aorta and endothelium-denuded aorta using primer pairs against Trpc3 and Trpc6 and, as a reference, against HPRT, α-SMA (acting as a marker for SM), and PECAM-1 (acting as a marker for the endothelium). Amplification products for α-SMA and PECAM-1 were detected in intact aorta, whereas only products for α-SMA were detected in endothelium-denuded aorta, confirming the absence of the endothelium in the latter preparation. Semi-quantitative RT–PCR analysis revealed signals for Trpc3 in intact and endothelium-denuded aorta, whereas signals for Trpc6 were found in intact but not in endothelium-denuded aorta (see Supplementary material online, Figure S6). This result indicates that Trpc3 is expressed in both aortic SM and endothelium, whereas Trpc6 is only expressed in aortic endothelium but not in aortic SM.
To confirm this finding, we performed immunocytochemistry to study more directly the expression of Trpc3 and Trpc6 channels in isolated VSMCs and, for comparison, in vascular endothelium cells. For this purpose, ECs and SMCs were freshly isolated from the murine aorta. Cells were identified using anti-CD31 as a marker for ECs and anti-α-actin as a marker for SMCs. The markers showed a specific signal for the respective cell type (see Supplementary material online, Figure S7). Co-staining of ECs with anti-Trpc3 and anti-Trpc6 showed that both Trpc3 and Trpc6 channels were present in ECs (Figure 5A and B). Co-staining of SMCs with anti-cGKI, anti-Trpc3, and anti-Trpc6 revealed a signal for cGKI and Trpc3 channels in SMCs, but not for Trpc6 channels (Figure 5C, see Supplementary material online, Figure S8A–C). No signals of anti-cGKI, anti-Trpc3, and anti-Trpc6 were detected in cells from sm-cGKI−/−, Trpc3−/−, and Trpc6−/− mice, respectively, confirming the specificity of the used antibodies (Figure 5, see Supplementary material online, Figures S8 and S9). These findings indicate that Trpc3 channels are expressed in both freshly isolated vascular endothelial and SMCs, whereas Trpc6 channels are expressed in freshly isolated endothelial but not in freshly isolated aortic SMCs.
Figure 5.

Expression of Trpc3 and Trpc6 channels in endothelial cells (ECs) and smooth muscle cells (VSMCs) from the murine aorta. (A) Immunolabelling of isolated ECs from a Ctr (left) and a trpc3−/− (right) mouse with anti-CD31 and anti-Trpc3. (B) Immunolabelling of isolated ECs from a Ctr (left) and a trpc6−/− (right) mouse with anti-CD31 and anti-Trpc6. (C) Immunolabelling of isolated VSMCs from a Ctr (left) and a TrpC6−/− (right) mouse with anti-α-actin and anti-TrpC6.
From these findings, we speculated that Trpc6 channels are involved in regulating intracellular Ca2+ homoeostasis in ECs. Ca2+ modulates endothelial NO production by endothelial NO synthase.24 Consequently, we studied agonist-induced changes in intracellular [Ca2+] in aortic SMCs and ECs from Ctr, Trpc3−/−, and Trpc6−/− mice. In addition, we tested again the possibility that Trpc6 channels are functionally coupled to cGMP/cGKI signalling by measuring the effect of 8-Br-cGMP on agonist-induced changes in intracellular [Ca2+]. PE (30 µM) similarly increased intracellular Ca2+ levels in aortic SMCs from Ctr, Trpc3−/−, and Trpc6−/− mice (Figure 6A, see Supplementary material online, Figure S10). Application of 8-Br-cGMP (300 µM) equally reduced the PE-stimulated increases in intracellular Ca2+ levels in the SMCs from all three mouse lines investigated (Figure 6A, see Supplementary material online, Figure S10), indicating that Trpc3 and Trpc6 have no major impact on α-adrenergic-induced Ca2+ signals and their attenuation by cGKI signalling in SM. CCh (30 µM) increased intracellular Ca2+ levels in ECs from Ctr, Trpc3−/−, and Trpc6−/− mice but the Ca2+ levels in the cells from Trpc6−/− mice were significantly lower than those found in cells from Ctr and Trpc3−/− mice (Figure 6B, see Supplementary material online, Figure S10). After the activation of cGKI signalling by 8-Br-cGMP (300 µM), the CCh-induced Ca2+ levels were reduced in ECs from Ctr and Trpc3−/− mice but not in those cells from Trpc6−/− mice (Figure 6B, see Supplementary material online, Figure S10). These results suggest that CCh-induced Ca2+ signals in ECs depend partially on the presence of Trpc6 channels. The missing effect of 8-Br-cGMP on CCh-induced Ca2+ signals in ECs from Trpc6−/− mice points to a possible coupling of cGMP/cGKI signalling to Trpc6 channels in this cell type. To strengthen this view, we performed immunocytochemistry to clarify the expression of cGKI in isolated aortic endothelium cells. Co-staining of ECs with anti-cGKI and anti-CD31 showed that cGKI is present in ECs from Ctr but not in those from cGKI−/− mice (see Supplementary material online, Figure S11).
Figure 6.
Intracellular Ca2+ levels in SMCs and ECs from the aorta. Intracellular Ca2+ levels are expressed as the ratio of the fluorescence intensity (at 510 nm) at alternating 350 (F350) and 380 nm (F380) excitation wavelengths. Please note that corresponding original recordings are presented in see Supplementary material online, Figure S7. (A) Intracellular Ca2+ levels in vascular SMCs from Ctr, Trpc3−/−, and Trpc6−/− mice under control conditions, in the presence of phenylephrine (PE, 30 µM), and in the presence of PE and 8-Br-cGMP (300 µM). (B) Intracellular Ca2+ levels in ECs from Ctr, Trpc3−/−, and Trpc6−/− mice under control conditions, in the presence of carbachol (CCh, 30 µM), and in the presence of CCh and 8-Br-cGMP (300 µM). Columns represent means ± SEM. Ca2+ levels represent the maximum in the presence of the respective agonist (reached within 2 min) and the minimum in the presence of 8-Br-cGMP (reached within 5 min). Numbers represent the number of cells. Cells were obtained from 5 to 9 mice. Data were analysed using ANOVA followed by the Bonferroni post hoc test. Asterisks on top of the columns represent statistically significant differences of the values in the presence of PE and CCh to the control values, respectively, or of the values in the presence of 8-Br-cGMP to the values in the presence of the respective agonist. ***P < 0.001; n.s., non-significant.
To further support the hypothesis that Trpc6 channels are involved in the generation of an endothelial-mediated relaxing tone, we assessed the effects of CCh on PE-induced contraction in intact aortic rings. Stimulation of muscarinic receptors on the endothelium induces partially relaxation by the synthesis of nitric oxide.23 Contractions by 3 µM PE were relaxed by CCh to a smaller extent in intact aortic rings from Trpc6−/− and Trpc3−/−/6−/− mice than in those from Ctr and Trpc3−/− mice (see Supplementary material online, Figure S12). This result suggests that endothelial Trpc6 channels are partially involved in the CCh-induced relaxation of vascular SM.
4. Discussion
The present study shows that α-adrenergic-induced contraction and relaxation of this contraction by cGMP/cGKI signalling does not depend on the presence of Trpc1,3,6 channels in vascular SM. The data support the notion that cGKI and Trpc3 or Trpc6 channels are not functionally linked in the aorta and hind limb smooth muscles, because cGMP/cGKI-mediated relaxation in the aorta and hind limb vessels was not impaired in Trpc3−/−, Trpc6−/−, and Trpc3−/−6−/− mice. Instead, our data suggest that Trpc6 channels are involved in the maintenance of the NO-derived vasodilator tone in intact vessels. This notion is supported by the following findings: (i) contraction after α-adrenergic stimulation in intact aorta from Trpc6−/− mice was larger than that observed in Ctr mice but identical if NO production was blocked by l-NAME; (ii) immunocytochemistry revealed no significant signal for Trpc6 channels in aortic SMCs but in aortic ECs; (iii) PCR analysis showed no significant signal for Trpc6-coding mRNA in endothelium-denuded aorta; (iv) hormone-induced Ca2+ signalling was diminished in endothelial but not in SMCs from Trpc6−/− mice; (v) muscarinic-induced relaxation was attenuated in PE-contracted intact aorta from Trpc6−/− mice; (vi) cGMP/cGKI did not attenuate the intracellular Ca2+ level in endothelial cells from Trpc6−/− mice. Thus, we propose that, at least in aorta, endothelial Trpc6 channels are involved in the generation of the NO-mediated vasodilator tone. A similar involvement has been described for the endothelial Trpc4 channel.25 In the latter study, ACh-induced Ca2+ levels were attenuated in cultured mouse vascular EC from Trpc4−/− mice as well as ACh-induced vasorelaxation of aortic rings from Trpc4−/− mice. Thus, it seems possible that the remaining muscarinic-induced vasorelaxation and Ca2+ signals in EC from Trpc6−/− are due to the activity of Trpc4 channels.
Contraction of vascular SM has been proposed to depend partially on Trpc channels that are either mediating receptor-operated currents and/or store-operated currents to provide Ca2+ for contraction and/or refilling of intracellular Ca2+ stores, respectively.26 Receptor-activated currents mediated by Trpc1, 3, and 6 channels have been recorded in some vascular SMs including mesenteric artery myocytes,27 cerebral artery myocytes,28 and portal vein myocytes.29 However, some of these results were not repeated in studies using knockout mice. PE-induced contraction of aortic SM was normal in Trpc1−/− mice.30 PE-induced contraction of aortic SM was not reduced but enhanced in Trpc6−/− mice.15 We did not observe a reduced but a normal contractile response in PE-stimulated aortic rings and hind limb vessels from Trpc3−/−, Trpc6−/−, Trpc3−/−/6−/−, and Trpc1−/−/3−/−/6−/− mice in conditions in which the vasodilator tone, induced by endothelial NO production, was inhibited. A vasodilator tone was only observed in PE-stimulated aortic rings but not in the PE-stimulated murine hind limb. This difference may be related to the experimental design: contractility in aortic rings is usually studied at the maximum of the length–tension relation to obtain maximal responses to agonist stimulation but may also provide mechanical stretch that stimulates endothelial NO production.31 In agreement with the hind limb perfusion results, it was reported that an endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of WT mice.32
This study supports the notion that Trpc1, Trpc3, and Trpc6 channels do not significantly participate in α-adrenergic-induced contraction of vascular SM in aorta and hind limb vessels. We cannot exclude the possibility that overexpression of an additional TRP channel may functionally substitute the deleted Trpc channels in SM from Trpc3−/−, Trpc6−/−, Trpc3−/−/6−/−, and Trpc1−/−/3−/−/6−/− mice although no up-regulation of Trpc2–7 channels was observed in Trpc1−/− mice.33 In addition, we cannot exclude effects of cGMP/cGKI signalling on Trpc channel activity in cell culture of SMCs in which the pattern of Trpc channel expression may have been changed. For example, Trpc1 expression is up-regulated in cerebral arteries following organ culture.34
Endothelium-derived NO contributes to a vasodilator tone that counteracts hormone-induced contraction of vascular SM.3,23 In aortic rings with intact endothelium, e.g. in the presence of the NO-mediated vasodilator tone, α-adrenergic stimulation induced larger contractions in the rings from sm-cGKI−/− mice than in rings from Ctr mice. Similar results were reported for the α-adrenergic stimulation in the rings from eNOS−/− mice.35These findings confirm that NO and SM cGKI are the main mediators of the vasodilator tone.
A number of studies showed that the activity of Trpc channels is controlled by cGMP/cGKI signalling in SM.8,10,11,15 We challenged this mechanism in a more physiological setting by measuring the effects of cGMP on α-adrenergic stimulated aorta and hind limbs from Trpc knockout mice. We found no differences in the relaxant effects of cGMP in preparations from Trpc3−/−, Trpc6−/−, Trpc3−/−/6−/−, or Trpc1−/−/3−/−/6−/− mice. Thus, we suggest that the relaxant effects of cGMP/cGKI signalling on vascular SM do not depend on Trpc1, Trpc3, or Trpc6 channels, supporting no functional coupling between cGKI and these Trpc channels in vascular SM. However, cGMP/cGKI did attenuate CCh-induced Ca2+ levels in ECs of Ctr but not of Trpc6−/− mice, supporting a functional link between Trpc6 and cGKI in the endothelium. Another endothelial TRP channel, TRPV4, has likewise been proposed to participate in endothelial-dependent muscarinic receptor signalling.25,36
In summary, the present study suggests that the absence of Trpc1,3,6 does not influence α-adrenergic-mediated contraction and attenuation of this contraction by cGMP/cGKI signalling in vascular SM, indicating no physiological link between cGKI and Trpc6 channels in this murine tissue. Instead, the data indicate that the absence of cGKI impairs the vasodilator tone by disrupting signalling in SMCs, whereas the absence of Trpc6 impairs the vasodilator tone by interfering with endothelial Ca2+ signalling and probably NO production.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
The work was funded by grants from Deutsche Forschungsgemeinschaft, Fond der Chemischen Industrie, and by the Intramural Research Program of the NIH (Project Z01-ES-101684 to L.B.).
Supplementary Material
References
- 1.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- 3.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006;176:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann F. The biology of cyclic GMP-dependent protein kinases. J Biol Chem. 2005;280:1–4. doi: 10.1074/jbc.R400035200. [DOI] [PubMed] [Google Scholar]
- 5.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, et al. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 6.Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med. 2003;9:1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- 7.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Crossland RF, Noorani MM, Marrelli SP. Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. Am J Physiol Heart Circ Physiol. 2009;297:H417–H424. doi: 10.1152/ajpheart.01130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich A, Kalwa H, Gudermann T. TRPC channels in vascular cell function. Thromb Haemost. 2010;103:262–270. doi: 10.1160/TH09-08-0517. [DOI] [PubMed] [Google Scholar]
- 10.Kwan HY, Huang Y, Yao X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc Natl Acad Sci USA. 2004;101:2625–2630. doi: 10.1073/pnas.0304471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi S, Lin H, Geshi N, Mori Y, Kawarabayashi Y, Takami N, et al. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol. 2008;586:4209–4223. doi: 10.1113/jphysiol.2008.156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voets T, Nilius B. TRPCs, GPCRs and the Bayliss effect. EMBO J. 2009;28:4–5. doi: 10.1038/emboj.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn M. Endothelial actions of atrial and B-type natriuretic peptides. Br J Pharmacol. 2012;166:522–531. doi: 10.1111/j.1476-5381.2012.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukowski R, Weinmeister P, Bernhard D, Feil S, Gotthardt M, Herz J, et al. Role of smooth muscle cGMP/cGKI signaling in murine vascular restenosis. Arterioscler Thromb Vasc Biol. 2008;28:1244–1250. doi: 10.1161/ATVBAHA.108.166405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 20.Geiselhoringer A, Gaisa M, Hofmann F, Schlossmann J. Distribution of IRAG and cGKI-isoforms in murine tissues. FEBS Lett. 2004;575:19–22. doi: 10.1016/j.febslet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Ruth P, Wang GX, Boekhoff I, May B, Pfeifer A, Penner R, et al. Transfected cGMP-dependent protein kinase suppresses calcium transients by inhibition of inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci USA. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 23.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 24.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 25.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 26.Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond) 2010;119:19–36. doi: 10.1042/CS20090641. [DOI] [PubMed] [Google Scholar]
- 27.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol. 2006;577:479–495. doi: 10.1113/jphysiol.2006.119305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2055–H2061. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- 29.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K, Dubrovska G, Nielsen G, Fesus G, Uhrenholt TR, Hansen PB, et al. Amplification of EDHF-type vasodilatations in TRPC1-deficient mice. Br J Pharmacol. 2010;161:1722–1733. doi: 10.1111/j.1476-5381.2010.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 32.Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, et al. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, et al. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- 34.Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, et al. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005;288:C872–C880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- 35.Kojda G, Laursen JB, Ramasamy S, Kent JD, Kurz S, Burchfield J, et al. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial cell nitric oxide synthase: contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc Res. 1999;42:206–213. doi: 10.1016/s0008-6363(98)00315-0. [DOI] [PubMed] [Google Scholar]
- 36.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, et al. Elementary Ca2+ Signals Through Endothelial TRPV4 Channels Regulate Vascular Function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.