Abstract
Aims
African trypanosomiasis, caused by Trypanosoma brucei species, leads to both neurological and cardiac dysfunction and can be fatal if untreated. While the neurological-related pathogenesis is well studied, the cardiac pathogenesis remains unknown. The current study exposed isolated ventricular cardiomyocytes and adult rat hearts to T. brucei to test whether trypanosomes can alter cardiac function independent of a systemic inflammatory/immune response.
Methods and results
Using confocal imaging, T. brucei and T. brucei culture media (supernatant) caused an increased frequency of arrhythmogenic spontaneous diastolic sarcoplasmic reticulum (SR)-mediated Ca2+ release (Ca2+ waves) in isolated adult rat ventricular cardiomyocytes. Studies utilising inhibitors, recombinant protein and RNAi all demonstrated that this altered SR function was due to T. brucei cathepsin-L (TbCatL). Separate experiments revealed that TbCatL induced a 10–15% increase of SERCA activity but reduced SR Ca2+ content, suggesting a concomitant increased SR-mediated Ca2+ leak. This conclusion was supported by data demonstrating that TbCatL increased Ca2+ wave frequency. These effects were abolished by autocamtide-2-related inhibitory peptide, highlighting a role for CaMKII in the TbCatL action on SR function. Isolated Langendorff perfused whole heart experiments confirmed that supernatant caused an increased number of arrhythmic events.
Conclusion
These data demonstrate for the first time that African trypanosomes alter cardiac function independent of a systemic immune response, via a mechanism involving extracellular cathepsin-L-mediated changes in SR function.
Keywords: Sarcoplasmic reticulum, Cardiomyocyte, Calcium, Trypanosomiasis, Trypanosome
1. Introduction
Human African trypanosomiasis (HAT) is a neglected disease caused by subspecies of Trypanosoma brucei, which are blood-borne extracellular protozoa transmitted by tsetse fly (Glossina ssp). HAT is fatal if untreated. In early HAT, parasites are intravascular (haemolymphatic/Stage I disease) but as infection progresses, parasites cross endothelia and invade extravascular tissue within different organs. When parasites traverse the blood brain barrier (BBB), neuropsychiatric disturbances develop (‘sleeping sickness’/Stage II disease). Whilst central nervous system (CNS) involvement is the clinical focus of patient screens, cardiac disturbances are now recognized as significant symptoms in HAT. A recent field study observed high prevalence of cardiac electrical abnormalities in HAT (∼55% of Stage I, ∼70% of Stage II patients).1,2 Of note was the increased proportion of HAT patients experiencing palpitations.1,2 Other reported cardiac-related abnormalities include conduction block, low voltage abnormalities, ventricular dilatation and heart failure;1–7 23% of HAT patients have NT-proBNP levels (N-terminal pro b-type natriuretic peptide; a biomarker of excessive cardiomyocyte stretching) consistent with left ventricular dysfunction.2 Both experimentally infected animals and between 70–100% of human autopsies show clear heart pathology including pancarditis.8–10 Experimental animal models demonstrate significant trypanosome numbers within myocardial interstitium, with or without a mononuclear cellular infiltrate.9 Despite the large number of studies demonstrating heart involvement in African trypanosomiasis, the appreciation of cardiac dysfunction as a clinical feature, and consequently our understanding of the basic cardiac pathogenesis, is very limited. In contrast, a significant body of recent work has focused on the neuro-pathogenesis of the disease showing that both an inflammatory response and trypanosome interaction with BBB cells are important.11,12 Therefore, one inference is that the cardiac-related clinical signs in African trypanosomiasis result from the inflammatory response. Whilst this may indeed play a role, an alternative hypothesis of parasites interacting with cardiomyocytes and altering heart function is untested.
Sarcoplasmic reticulum (SR)-mediated Ca2+ release during excitation–contraction coupling causes cardiomyocyte and whole heart contraction (systole). Cardiomyocytes relax (diastole) by lowering intracellular Ca2+ concentration ([Ca2+]i,) predominantly by SR-mediated Ca2+ uptake via SERCA and sarcolemmal extrusion via the Na+/Ca2+ exchanger (NCX). Under certain circumstances (e.g. heart failure), SR-mediated Ca2+ release can also occur spontaneously without electrical excitation, as propagating Ca2+ waves. These events are linked to impaired contraction, abnormal electrical activity, ventricular premature complexes (VPC) (which can cause palpitations), and the triggering of fatal arrhythmias.13
Previous in vitro studies on trypanosome interaction with brain microvascular endothelial cells (BMECs) demonstrated that trypanosomes induce changes in [Ca2+]i dynamics which correlated with the parasite's ability to cross the BMEC monolayer.12 Given that trypanosomes affect host cell [Ca2+]i dynamics, and the pivotal role of Ca2+ in cardiomyocyte and heart function, the aim of this study was to utilize isolated cardiomyocytes and whole hearts to investigate the hypothesis that African trypanosomes alter intra-cardiomyocyte Ca2+ handling and whole heart function.
2. Methods
2.1. Adult cardiomyocyte isolation
Adult male Wistar rats (200–300 g) were euthanized by schedule one procedure (concussion followed by cervical dislocation) in accordance with the UK Animals (Scientific Procedures) Act 1986, Directive 2010/63/EU of the European Parliament and University of Glasgow ethical review panel. Cardiomyocytes were isolated as previously described14 (see Supplementary material online). Cardiomyocytes were re-suspended in a Modified Isolation Krebs–Henseleit (MIKH), 1.8 mM [Ca2+]o.
2.2. Preparation of trypanosomes, media, and supernatant
Parasites were cultured in HMI-9, 20% v/v Serum Plus® (SAFC Biosciences) (37°C, 5% CO2; see Supplementary material online); referred to as live trypanosomes. Parasite number was equivalent to that found during human and livestock infections (∼5.0×105 parasites mL−1). Supernatant was prepared by centrifugation of live trypanosome suspension (857 g, 10 min) and subsequent removal of supernatant. To ensure no trypanosome contamination, supernatant was filtered (0.2 µm filter, Sartorius Stedim). HMI-9 with 20% v/v Serum Plus® without trypanosomes is referred to as media and was treated identically to supernatant (i.e. centrifuged and filtered).
2.3. Spontaneous contractile event measurements in isolated rat cardiomyocytes
Isolated adult cardiomyocytes were incubated for 30 min [room temperature (RT)] with live trypanosomes, supernatant, or media and then placed in a cell bath containing the corresponding solution. Cardiomyocytes were viewed under a light microscope and contractile events measured (see Supplementary material online).
2.4. Confocal imaging of spontaneous SR-mediated Ca2+ release
Intact cardiomyocytes in MIKH, 1.0 mM [Ca2+]o were loaded with Ca2+-sensitive fluorophore (5.0 μM Fluo-3AM, Biotium;10 min incubation). MIKH was removed and cardiomyocytes re-suspended in media or supernatant for a further 30 min to ensure complete de-esterification. Cardiomyocytes were then perfused with media or supernatant and field stimulated (1.0 Hz, 45 s, RT) to allow for the steady-state SR Ca2+ content. Field stimulation was terminated and a 45 s rest period enabled spontaneous Ca2+ release event visualization and measurement (see Supplementary material online).
2.5. Epifluorescence measurements of field stimulated Ca2+ transients
Intact cardiomyocytes (1.0 mM [Ca2+]o) were loaded with Ca2+-sensitive fluorophore (5.0 μM Fura-4FAM, Invitrogen). Cardiomyocytes were incubated (30 min) in media or supernatant and allowed to settle in a cell bath (Cell Microcontrols) followed by superfusion with media/supernatant (37°C) and field stimulation (1.0 Hz, 2.0 ms, voltage set to 1.5× threshold). In separate experiments, cardiomyocytes were field stimulated and perfused firstly with media (60 s) and then supernatant (60 s). Caffeine (10 mM, 20 s; without field-stimulation) was applied before the protocol, after perfusion with media and after supernatant. The three caffeine and two solution changes were all performed on the same isolated cardiomyocyte, enabling accurate paired-assessment of the SR Ca2+ content between media and supernatant (see Supplementary material online).
2.6. Epifluorescence measurements of diastolic [Ca2+]i during SR inhibition
Fura-2AM loaded cardiomyocytes were incubated with thapsigargin (1 μM; Merckmillipore) and ryanodine (1 μM; Merck Millipore) for 30 min to inhibit SR function and then perfused with media and/or supernatant for 5 min in the continued presence of inhibitors. Changes in diastolic [Ca2+]i were then measured (see Supplementary material online).
2.7. RNA interference of T. brucei cathepsin-L (TbCatL)
TbCatL RNAi (see Supplementary material online) was induced with 1 µg mL−1 tetracycline and triplicate growth curves performed for induced/uninduced cultures, initiated with 1.0 × 105 cells mL−1 and counted at 24, 48, and 72 h post-induction. Quantitative PCR was performed to measure relative TbCatL gene expression (see Supplementary material online).
2.8. Langendorff perfused rat hearts
Isolated hearts were cannulated via the aorta, perfused with media or supernatant and pseudo-ECG measurements performed (iworx; see Supplementary material online).
2.9. Statistical analysis
Data expressed as means ± SEM. For Ca2+ transient amplitude and Ca2+ wave parameters paired Student's t-test was used, with ANOVA for multiple comparisons. Multiple linear regression analysis was used to compare nominal categorical data with continuous variables. Normal distribution was assessed by plotting of residuals. Differences were considered significant when P < 0.05.
3. Results
3.1. Trypanosomes increase spontaneous contractile events in isolated cardiomyocytes
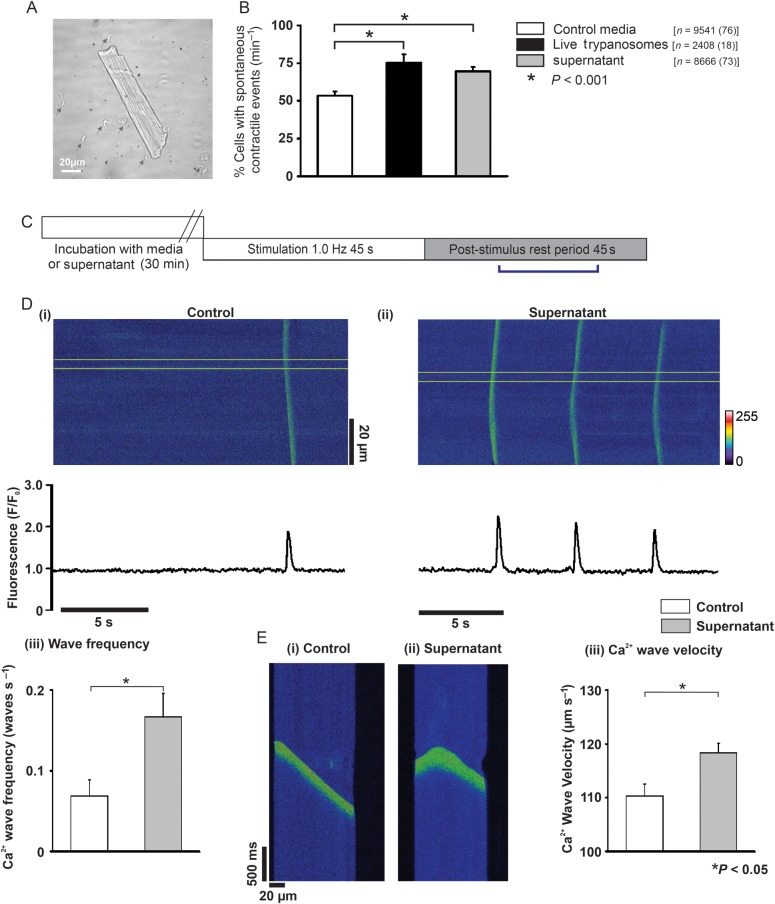
To investigate whether Trypanosoma brucei altered cardiomyocyte function, spontaneous contractile events were quantified. When cardiomyocytes were incubated with live trypanosomes (Figure 1A) the cardiomyocyte percentage producing spontaneous contractile events increased to 145% of media levels (52.1 ± 1.6 vs. 75.4 ± 5.3% cardiomyocytes exhibiting at least one spontaneous contractile event min−1; media vs. live trypanosomes; P < 0.001; Figure 1B). Interestingly, this effect was maintained when cardiomyocytes were incubated with supernatant, which increased spontaneous contractile events to 130% (52.1 ± 1.6 vs. 67.5 ± 1.9% of cardiomyocytes exhibiting at least one spontaneous contractile event min−1; media vs. supernatant; P < 0.001; Figure 1B). Separate experiments assessed this effect with reduction of external [Ca2+]o by equivalent amounts in both media and supernatant. Under these conditions, the supernatant effect was maintained with an increased percentage of cardiomyocytes demonstrating spontaneous contractile events above media levels (17.4 ± 3.2 vs. 34.5 ± 2.5% of cardiomyocytes exhibiting at least one spontaneous contractile event min−1; media vs. supernatant; P < 0.05; data not shown). Extracellular [Ca2+]o and pH were not significantly different between media or supernatant. If supernatant was heated to 80°C prior to incubation, the spontaneous contractile event increase was abolished (data not shown).
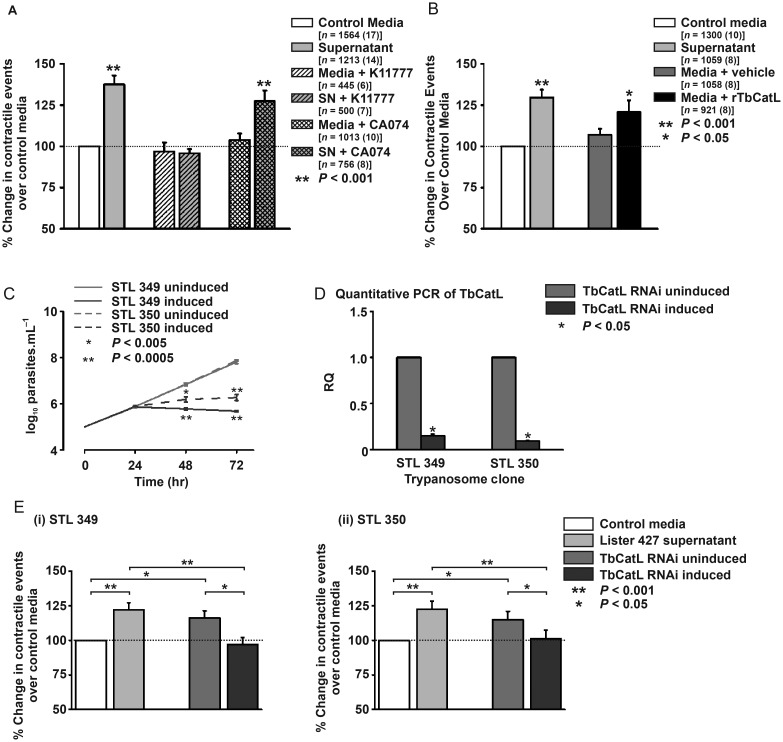
Figure 1.
(A) Isolated rat cardiomyocyte incubated with Trypanosoma brucei (grey arrowheads). (B) % cardiomyocytes with spontaneous contractile events (n = cardiomyocytes with number of hearts in parentheses). (C) Protocol used in separate confocal experiments. (D) Upper (i and ii) confocal line-scan images of cardiomyocytes from bracketed region in (C). Lower (i and ii) respective line profile trace taken from a 20 pixel region (denoted by yellow lines in upper image). (iii) Mean Ca2+ wave frequency; media (n = 18) and supernatant (n = 21). (Ei and ii) Individual Ca2+ waves, (iii) mean Ca2+ wave velocity in media (n = 53 waves from 18 cells) and supernatant (n = 158 waves from 21 cells).
3.2. Spontaneous contractile events are due to Ca2+ waves
Confocal imaging was performed to characterize the cardiomyocyte [Ca2+]i dynamics underpinning the spontaneous contractile events (Figure 1C). The line-scan mode produced images of time (x) vs. distance (y) across the cardiomyocyte (Figure 1Di and ii). Spontaneous contractile events (denoted by an inward deflection in the y-axis of the image, Figure 1E) were preceded by a fluorescence and therefore [Ca2+]i rise, which propagated from one region of the cell to another (Figure 1Di and ii). These events were characteristic of spontaneous SR-mediated Ca2+ release (Ca2+ waves). Ca2+ wave frequency in cardiomyocytes incubated with supernatant increased to 243% of media levels (0.07 ± 0.02 vs. 0.17 ± 0.03waves s−1; media vs. supernatant; P < 0.05; Figure 1Diii). Ca2+ wave velocity, calculated as the Ca2+ wave propagation gradient (Figure 1Ei and ii), increased in supernatant to 107% of media (110.2 ± 2.2 vs. 118.4 ± 1.7 µm s−1; media vs. supernatant; P < 0.05; Figure 1Eiii).
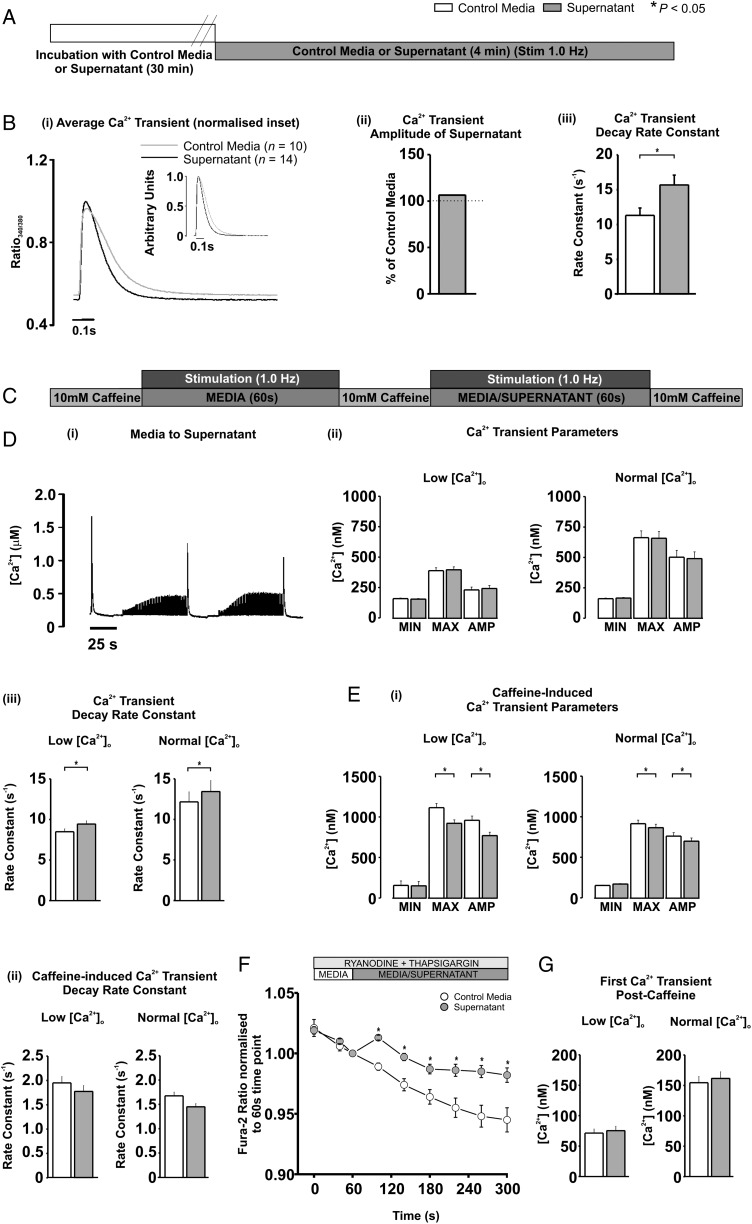
3.3. Supernatant increases the decay rate constant of the stimulated Ca2+ transient
Cardiomyocytes loaded with Fura-4F were used to determine the electrically stimulated systolic Ca2+ transient characteristics without movement artefact (Figure 2A). The mean Ca2+ transient maximum and minimum ratios were unaltered by supernatant perfusion (data not shown), therefore, resulting in no significant change in Ca2+ transient amplitude (106.42% of media; P > 0.05; Figure 2Bi and ii). Similarly, the Ca2+ transient mean maximum rates of rise and fall were unaltered by supernatant (data not shown), although the rate of fall tended to be faster (127.8% of media), albeit not significantly (P = 0.09). In contrast, supernatant significantly increased Ca2+ transient decay rate constant to 138.6% of media levels (11.3 ± 1.06 vs. 15.68 ± 1.41 s−1; media vs. supernatant; P < 0.05; Figure 2Biii).
Figure 2.
(A) Protocol used in epifluorescence Ca2+ measurements. (Bi) Average trace from last 12 stimulated Ca2+ transients in media (n = 10; grey) or supernatant (n = 14; black); (inset) normalized traces overlayed. (Bii) mean Ca2+ transient amplitude and (iii) decay rate constant. (C) Protocol used in separate epifluorescence experiments. (D) Typical trace of [Ca2+]i during protocol in (C). (Dii) Ca2+ transient parameters and (iii) decay rate constant at low [Ca2+]o (left panel; n = 8) and normal [Ca2+]o (right panel; n = 13). (Ei) Caffeine-induced Ca2+ transient parameters and (ii) decay rate constant at low [Ca2+]o (left panel) and normal [Ca2+]o (right panel). (F) Fura-2AM ratio during SR inhibition with media (n = 10) and supernatant (n = 11). (G) First Ca2+ transient amplitude post-caffeine during protocol C at low [Ca2+]o (left panel) and normal [Ca2+]o (right panel).
3.4. Supernatant reduces SR Ca2+ content
The SR Ca2+ content significantly influences Ca2+ transient characteristics. The SR Ca2+ content was determined by the assessment of the Ca2+-induced Ca2+ release amplitude, induced by 10 mM caffeine application (20 s) once after perfusion of cardiomyocytes with media and then again after supernatant perfusion in the same cell (Figure 2C). This paired protocol permitted accurate determination of small SR Ca2+ content changes between media and supernatant. As in Figure 2B, the Ca2+ transient minimum, maximum, and amplitude between media and supernatant were not significantly different (Figure 2Di and ii right panel), yet the Ca2+ transient decay rate constant was significantly increased (12.2 ± 1.2 vs. 13.4 ± 1.4 s−1; media vs. supernatant; P < 0.05; Figure 2Diii right panel). The same results were obtained when Ca2+ transient amplitude was reduced by lowering extracellular [Ca2+]o, i.e. no change in Ca2+ transient amplitude but an increased decay rate constant induced by supernatant (8.5 ± 0.4 vs. 9.4 ± 0.4 s−1; media vs. supernatant; P < 0.05; Figure 2Diii left panel). The amplitude of the caffeine-induced Ca2+ release was reduced by supernatant at both normal [Ca2+]o (760.9 ± 42.9 vs. 698.3 ± 39.4 nM; media vs. supernatant; P < 0.05; Figure 2Ei right panel) and lower [Ca2+]o (958.7 ± 52.2 vs. 769.4 ± 41.9 nM; media vs. supernatant; P < 0.05; Figure 2Ei left panel) suggesting that supernatant significantly reduced the SR Ca2+ content. In the continued presence of caffeine, the SR cannot re-accumulate Ca2+ and removal from the cell is predominantly by sarcolemmal extrusion. The caffeine-induced Ca2+ transient time constant of decline was not significantly altered by supernatant at either normal or lower extracellular [Ca2+]o (Figure 2Eii).
3.5. Supernatant increases diastolic [Ca2+]i via a non-SR dependent route
To assess whether supernatant could alter diastolic [Ca2+]i in the absence of SR-mediated Ca2+ release or uptake, the higher affinity dye Fura-2AM was utilized in cardiomyocytes where SR function was inhibited by thapsigargin and ryanodine (Figure 2F). In the absence of SR-mediated Ca2+ release, supernatant caused a persistent, significant elevation of diastolic [Ca2+]i over the 4 min period compared with media (Figure 2F). One possible route through which diastolic [Ca2+]i may have increased was through the L-type Ca2+ channel. To assess this possibility, the amplitude of the first Ca2+ transient after 10 mM caffeine application (an index of Ca2+ influx via the L-type Ca2+ channel) was measured during media or supernatant perfusion (Figure 2Di). No significant difference was detected in the first Ca2+ transient amplitude between media and supernatant (Figure 2G).
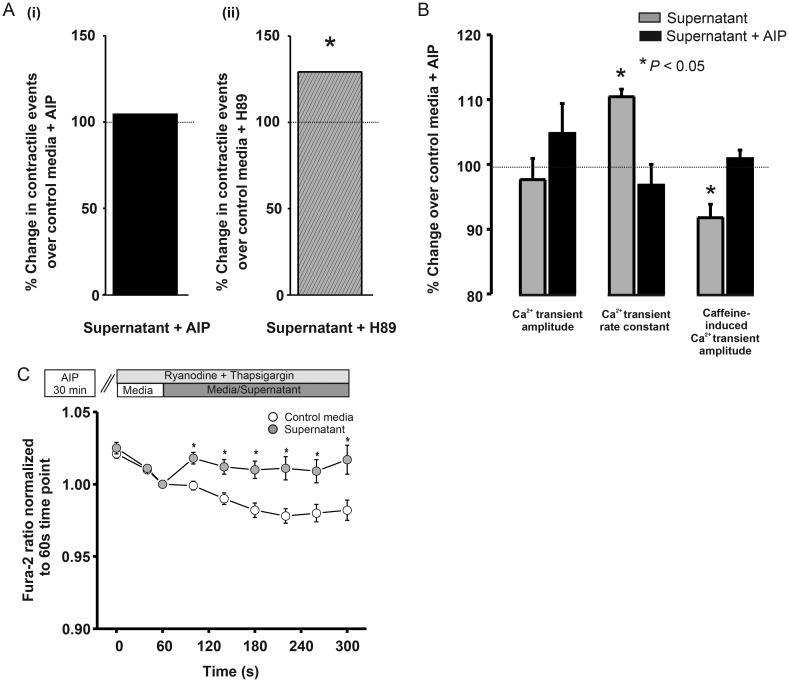
3.6. Alteration of SR function by supernatant is CaMKII dependent
To examine whether CaMKII or PKA is involved in the supernatant action on SR function, inhibitors were utilized in separate experimental cohorts (as in Figure 1B). The increase in spontaneous contractile events was abolished by the CaMKII inhibitor autocamtide-2 related inhibitory peptide (AIP) (to 104% of media + AIP levels; Figure 3Ai) but not by the PKA inhibitor H89 (N-[2-(p-bromocinnamylamino)ethyl]-5-iso-quinolinesulphonamide) (Sigma-Aldrich) (to 129% of media + H89 levels; Figure 3Aii). An additional cohort of cells pre-incubated in AIP underwent field stimulation during the same protocol shown in Figure 2C. No change in Ca2+ transient amplitude was apparent. However, AIP abolished the supernatant-induced Ca2+ transient rate decay constant increase and SR Ca2+ content decrease (Figure 3B). To assess whether the non-SR dependent diastolic [Ca2+]i elevation was CaMKII-dependent as shown in Figure 2F, the same protocol was used but in cardiomyocytes pre-incubated with AIP. The CaMKII inhibitor did not abolish the supernatant-induced diastolic [Ca2+]i elevation (Figure 3C).
Figure 3.
(A) % Change in cardiomyocytes with spontaneous contractile events supernatant + AIP (n = 307) vs. media + AIP (n = 121) or (ii) H89 + supernatant (n = 377) vs. media + H89 (n = 305). (B) % change in Ca2+ transient and caffeine-induced parameters with AIP. (C) Fura-2AM ratio during SR inhibition + AIP with media (n = 25) and supernatant (n = 21).
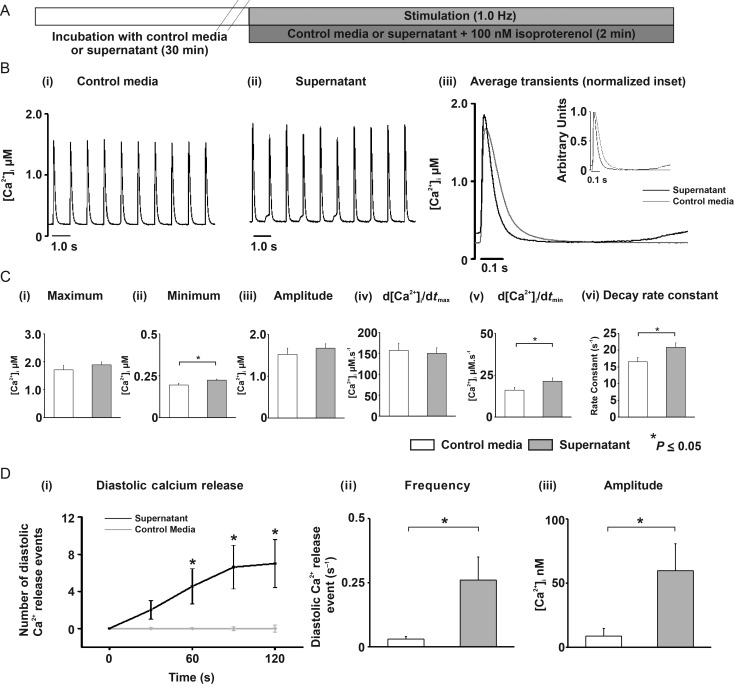
3.7. Supernatant effects on the stimulated Ca2+ transient during β-adrenergic stimulation
The sympathetic nervous system and β-adrenergic signalling pathway activate in response to physiological and pathophysiological stresses. To examine supernatant effects under β-adrenergic stimulation, isoproterenol was added (ISO,100 nM; Figure 4A). Under these conditions, supernatant did not significantly alter the mean maximum [Ca2+]i or Ca2+ transient amplitude, but did increase the minimum [Ca2+]i between field stimulations to 115% of control levels (0.20 ± 0.010 vs. 0.22 ± 0.006 μM; media vs. supernatant; P < 0.05; Figure 4Bi–iii, and Cii). The maximum rate of rise of the Ca2+ transient was not altered by supernatant but supernatant increased the maximum rate of fall to 133% of control levels (16.0 ± 1.7 vs. 21.4 ± 2.0 μM ms−1; media vs. supernatant; P < 0.05; Figure 4Cv). This latter result is confirmed by the significant increase in Ca2+ transient decay constant by supernatant to 126% of control values; 16.54 ± 1.34 vs. 20.86 ± 1.31 s−1; media vs. supernatant; P < 0.05; Figure 4Cvi).
Figure 4.
(A) Protocol with 100 nM isoproterenol (ISO) (B) Typical traces of cells superfused with (i) media(n = 13) or (ii) supernatant(n = 12); (iii) Average trace from last 12 transients; media(grey), supernatant(black); (inset) normalized traces overlayed. (Ci–vi) Mean Ca2+ transient parameters. (Di) Number of diastolic Ca2+ releases in supernatant (black) normalized to control (grey); (ii) mean Ca2+ wave frequency and (iii) amplitude.
As shown in Figure 4Bi vs. ii, supernatant increased the appearance of Ca2+ waves between stimulated Ca2+ transients. Cardiomyocytes superfused with supernatant and ISO demonstrated a Ca2+ wave frequency increase over time, plateauing at 90 s; (Figure 4Di). The mean Ca2+ wave frequency (measured over 90–120 s) increased to 866% of control levels during perfusion with supernatant compared with media (0.03 ± 0.01 vs. 0.26 ± 0.09 Ca2+ wave s−1; media vs. supernatant; P < 0.05; (Figure 4Dii). The Ca2+ wave amplitude also increased to 632% of control levels (9.4 ± 6.4 vs. 59.7 ± 21.0 nM [Ca2+]i; media vs. supernatant; P < 0.05; Figure 4Diii).
3.8. Ca2+ wave increase is abolished by inhibition of parasite cathepsin-L
The trypanosome factor associated with BMEC cytosolic Ca2+ rises was T. brucei cathepsin-L (TbCatL).12 Trypanosoma brucei expresses two Clan CA, Family C1 cysteine proteases; TbCatL and TbCatB.15,16 Given these data, cardiomyocytes were incubated with/without TbCatL-specific inhibitor K1177712 (10 µM) and spontaneous contractile events assessed within 60 s. Supernatant increased the contractile events to 137% of media levels (100.0 vs. 137.5 ± 5.4% cardiomyocytes with contractile events relative to media; media vs. supernatant; P < 0.05; Figure 5A). K11777 inhibited this effect (96.8 ± 5.4 vs. 95.8 ± 2.5% cardiomyocytes with contractile events relative to media; media + K11777 vs. supernatant + K11777; P > 0.05; Figure 5A). When repeated with CA074 (10 µM; TbCatB-specific inhibitor) the percentage increase of cardiomyocytes producing contractile events was maintained (103.8 ± 4.0 vs. 127.4 ± 6.4% cardiomyocytes with contractile events relative to media; media + CA074 vs. supernatant + CA074; P < 0.001; Figure 5A). These data suggest that the increase in spontaneous contractile events (due to Ca2+ waves; Figure 1) is TbCatL mediated.
Figure 5.
(A and B) Mean % change in contractile events over media levels in isolated cardiomyocytes (rTbCatL = 2 nM Recombinant T. brucei cathepsin-L + 5 μM dithiothreitol; n = number of cells from number of hearts). (C) Growth curves of biological duplicates (STL349 and STL350) with inducible TbCatL RNAi. (D) Relative TbCatL gene expression to the uninduced culture; triplicate cultures for STL 349 (n = 3) and STL 350 (n = 3). (Ei) % change in spontaneous contractile events in cardiomyocytes for clone STL349. Media [n = 747 (7)], supernatant [n = 817 (7)], uninduced culture [n = 858 (7)], and induced culture [n = 841 (7)]; (ii) % change in spontaneous contractile events in cardiomyocytes for clone STL350. Media [n = 1018 (11)], supernatant [n = 1427(12)], uninduced culture [n = 1597 (12)], or induced culture [n = 1564 (12)].
3.9. Recombinant TbCatL increases Ca2+ wave frequency
To confirm the importance of TbCatL in increasing Ca2+ waves, recombinant TbCatL (rTbCatL, 2.0 nM) was added to the media (Figure 5B). Similar to supernatant, rTbCatL significantly increased the contractile events to 113% of media (106.9 ± 3.7 vs. 120.8 ± 7.1% cardiomyocytes with contractile events relative to media; media with vehicle vs. media + rTbCatL; P < 0.05; Figure 4B). These results demonstrate that rTbCatL replicates the supernatant effect of cardiomyocyte SR function alteration.
3.10. TbCatL RNA interference abolishes the Ca2+ wave increase
Two independent T. brucei clones (STL349 and STL350) with inducible RNAi TbCatL knockdown were assessed in cardiomyocyte spontaneous contractile event measurements. TbCatL is necessary for parasite survival.17 Consequently, RNAi induction inhibited growth after 48 h (Figure 5C). Quantitative PCR on cDNA from mRNA extracted at 24 h (i.e. before affected growth) show that RNAi induction reduced TbCatL expression by 85–90% (0.15 ± 0.02 for STL349 and 0.1 ± 0.005 for STL350 relative quantification to uninduced cultures; P < 0.05; Figure 5D).
Induced TbCatL RNAi for STL349 significantly reduced the cardiomyocyte percentage producing spontaneous contractile events to media levels, compared with the same clone uninduced (97.0 ± 5.1 vs. 116.2 ± 5.0% cardiomyocytes with contractile events relative to media levels; induced vs. uninduced; P < 0.05; Figure 5Ei). The same occurred with STL350 (102.0 ± 5.7 vs. 116.5 ± 5.6% cardiomyocytes with contractile events relative to media levels; induced vs. uninduced; P < 0.05; Figure 5Eii). In each experiment subset (Figure 5Ei and ii), supernatant increased spontaneous contractile events to 123% of media levels (100.0 vs. 122.7 ± 5.4% cardiomyocytes with contractile events relative to media levels; media vs. supernatant; P < 0.05). These data show that the supernatant-induced increased Ca2+ waves are abolished if TbCatL gene expression is reduced to ∼10% of control levels.
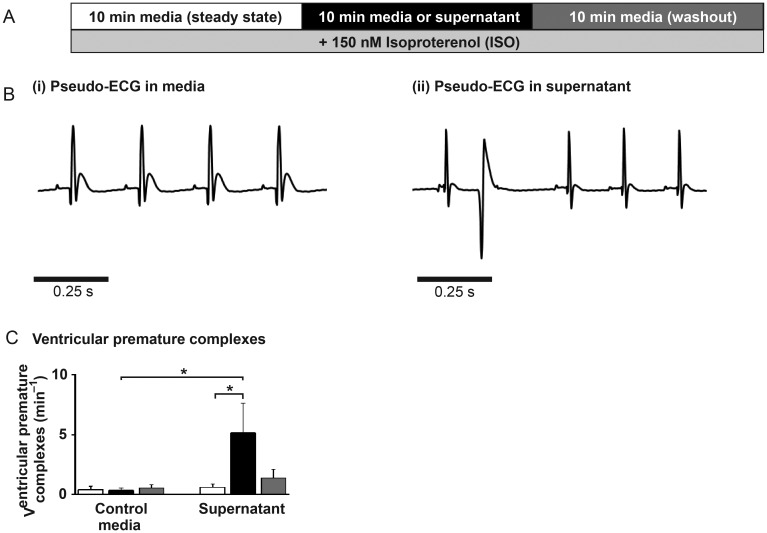
3.11. Supernatant leads to arrhythmic events in whole hearts
Given the established link between spontaneous SR-mediated Ca2+ release and arrhythmias, the arrhythmogenic potential of supernatant was investigated in ex vivo whole hearts. The pseudo-ECG was examined for VPCs (Figure 6A and B), known to be associated with abnormal diastolic Ca2+ release. Each VPC was counted as one arrhythmic event. There was no significant difference in mean arrhythmic frequency between the different time periods of the protocol in the time-control group; (0.40 ± 0.28 vs. 0.33 ± 0.19 vs. 0.53 ± 0.27 VPCs min−1; first, second, and third 10 min period, respectively; P > 0.05; Figure 6C). However, in hearts perfused with supernatant there was a significant increase in mean arrhythmic event frequency which then reduced during the washout period (0.60 ± 0.26 vs. 5.15 ± 2.49 vs. 1.36 ± 0.73 PCs min−1; first, second, and third 10 min period, respectively; P < 0.05 between first and second period; Figure 6C). There was no significant difference in heart rate between hearts perfused with media (282 ± 17 b.p.m.) or supernatant (278 ± 12 b.p.m.).
Figure 6.
(A) Langendorff perfused heart protocol. (B) typical pseudo-ECG recordings. (C) Mean number of ventricular premature complexes (VPCs) per min for time control (n = 6) and supernatant-perfused hearts (n = 12) in media (first 10 min; white bar), switch to media or supernatant (second 10 min; black bar), and media washout (third 10 min; grey bar).
4. Discussion
Using isolated cardiomyocytes and whole hearts, we have demonstrated for the first time that Trypanosoma brucei can alter cardiac function without a systemic immune response or autonomic nervous system effect. Prior to this study, the only suggested mechanism for cardiac-related clinical symptoms has been the immune/inflammatory response to the parasite. Our data demonstrate that this is not the sole mechanism and present a new paradigm for trypanosome-induced cardiac dysfunction.
4.1. Alteration of spontaneous SR-mediated Ca2+ release in isolated cardiomyocytes
Isolated cardiomyocytes show increased spontaneous contractile activity when exposed to both parasites and supernatant (Figure 1). Such spontaneous contractile activity can be associated with spontaneous SR-mediated Ca2+ release in the form of Ca2+ waves, which was demonstrated in this study (Figure 1). A similar effect has been observed in BMECs, where [Ca2+]i spontaneous releases occurred despite the removal of the parasite from the media.12 It is clear therefore that in both cardiomyocytes and BMECs, the [Ca2+]i alteration is mediated by a trypanosome-derived (secreted/excreted) factor.
4.2. Ca2+ wave velocity and frequency are increased by supernatant
Altered Ca2+ wave characteristics indicate altered SR function.18 In this study, two notable effects on Ca2+ wave characteristics occurred post-field stimulation. In agreement with spontaneous contractile event measurements (Figure 1A), an increased Ca2+ wave frequency was observed in cardiomyocytes incubated with supernatant. Ca2+ waves are linked to both arrhythmias and contractile dysfunction and increased Ca2+ wave frequency is likely to increase whole heart arrhythmias.13 Ca2+ wave frequency is determined by the time taken to reach a SR Ca2+ threshold.13 SERCA stimulation by a direct or indirect effect of a supernatant factor would increase the SR Ca2+accumulation rate and hence decrease the time between spontaneous events.13,19 O'Neill et al.19 also showed that Ca2+ wave velocity was directly related to SERCA activity, and thus the increased Ca2+ wave velocity observed in the current study may be an expected additional effect of enhanced SERCA activity.
4.3. Effects of supernatant on SR function
The proposal that exposure to supernatant increases cardiac SERCA activity is consistent with the supernatant effects on the stimulated Ca2+ transient (Figures 2 and 4), which showed an increased decay rate constant. SERCA activity (KSERCA) was estimated by measuring the rate constant of decay of the electrically stimulated Ca2+ transient. However, sarcolemmal efflux from the cell also contributes to this rate constant of decay. When the rate constant of decay of the caffeine-induced Ca2+ transient (which includes sarcolemmal efflux but no SR uptake contribution) is subtracted from that of the electrically stimulated Ca2+ transient (which includes the combined SR uptake and sarcolemmal efflux)20 the % change in KSERCA induced by the supernatant was 114.3 ± 2.2% of media control. This compares well with the value (110.4 ± 1.2; Figure 2Diii) when using the rate decay constant of the electrically induced transient alone. Therefore, both values are very similar and we conclude that SERCA activity is significantly increased by the supernatant by 10–15%. An alternative explanation for the increased decay time constant is alteration of myofilament Ca2+ responsiveness. However, the supernatant had no effect on myofilament sensitivity to [Ca2+]i (Supplementary material online). If enhanced SERCA activity was the exclusive action of the supernatant on SR function then the SR Ca2+ content may be expected to have increased. However, Figure 2 clearly shows that the supernatant decreases the SR Ca2+ content. This decrease cannot be explained by increased sarcolemmal efflux, as the caffeine-induced Ca2+ transient decay rate constant and diastolic [Ca2+]i were unaltered. Therefore, we hypothesize that in addition to mild (10–15%) stimulation of SERCA activity, TbCatL also enhances SR-mediated Ca2+ leak; the net effect being reduction in the SR Ca2+ content. An increased SR-mediated Ca2+ leak could potentially occur via the ryanodine receptor (RyR). A large body of work has confirmed that increased RyR open probability may lead to: (i) unaltered stimulated Ca2+ transient amplitude, (ii) reduction in the SR Ca2+ content, (iii) increased Ca2+ wave frequency in resting cells, and (iv) sustained and increased Ca2+ waves during electrical stimulation in the presence of β-adrenergic stimulation (reviewed in13). The data presented demonstrate the above features and therefore support the hypothesis that TbCatL induces SR-mediated Ca2+ leak with parallel SERCA activity increase. CaMKII (but not PKA) inhibition abolished the supernatant effect on SR Ca2+ uptake and content. Importantly, this in turn led to the abolishment of the expected increased in Ca2+ waves (Figure 3). Enhanced SR Ca2+ leak and a subsequent reduction of SR Ca2+ content can be prevented by AIP in cardiomyocytes overexpressing CaMKII without significantly altering the Ca2+ transient amplitude.21 Furthermore, SR-localized AIP can also inhibit CaMKII-dependent regulation of SERCA-mediated Ca2+ uptake.22 Therefore, these data support the proposed mechanism of a parallel effect of the supernatant on abnormal SR Ca2+ release and uptake that leads to the increased Ca2+ wave frequency observed.
4.4. Effects of supernatant on diastolic [Ca2+]i
Isolated cardiomyocyte experiments where SR-mediated Ca2+ release and uptake was inhibited (Figure 2F) revealed that supernatant elevated [Ca2+]i compared with control media. Our experiments do not discriminate between reduced Ca2+ efflux or enhanced Ca2+ influx, but increased diastolic [Ca2+]i may favour the production of abnormal SR Ca2+ release. One potential mechanism for increased diastolic [Ca2+]i is reduced NCX activity, the main sarcolemmal Ca2+ extrusion process. However, Ca2+ extrusion rate in the presence of caffeine was unaltered by supernatant (Figure 2), suggesting NCX activity was unaffected. Alternatively, supernatant may have enhanced Ca2+ entry into cardiomyocytes, but in quiescent cells this is unlikely to be via the L-type Ca2+ channel (see Figure 2G). Interestingly, this effect was not AIP-sensitive (Figure 3). The changes in basal and store-based Ca2+ signalling observed in cardiomyocytes parallel those seen in BMECs,12 an effect mediated by TbCatL action on a GPCR mediated pathway.23 Activation of this or a similar pathway in cardiomyocytes may underlie the supernatant effect on the heart. Overall, it is possible that supernatant elevates diastolic [Ca2+]i (which could be substantial within subcellular compartments) causing CaMKII activation, which then increases SR-mediated Ca2+ leak and uptake with a net effect being an increase in Ca2+ waves and reduced SR Ca2+.
4.5. Ca2+ waves are caused by TbCatL
The cathepsin-L inhibitor K11777 prevented the supernatant-mediated increase in Ca2+ waves. This demonstrated for the first time that TbCatL increases spontaneous Ca2+ release from the cardiomyocyte SR. These results are consistent with rTbCatL increasing Ca2+ waves (gain of function) and RNAi reducing TbCatL gene expression and decreasing Ca2+ waves (loss of function). The combined pharmacological, recombinant protein, and RNAi approaches confirmed that trypanosome-produced extracellular TbCatL causes the increased arrhythmogenic SR-mediated Ca2+ release in adult rat ventricular cardiomyocytes, independent of an immune/inflammatory response. TbCatL is essential to parasite survival (hence RNAi use rather than gene knockout, which is lethal).17 Current HAT drugs are difficult to administer in the field and cause severe toxic side effects in 5–10% of cases (average case-fatality rate of 50%).24 Cysteine protease peptide inhibitors are currently being tested as novel trypanocides.17 Our study suggests that in addition to being trypanocidal, drugs that target TbCatL may limit both BBB pathology and cardiac dysfunction.
4.6. Supernatant causes arrhythmic events in whole hearts
Field studies report a range of ECG abnormalities and palpitations in HAT patients that can result from VPCs.2 The link is established between Ca2+ waves, delayed after-depolarization and subsequent VPC production in the whole heart which may induce fatal arrhythmias (reviewed in13). Supernatant is able to increase VPCs independent of an immune response under β-adrenergic stimulation conditions. This is the first time that trypanosomes have been shown to contribute directly to whole heart arrhythmias. It is unclear to what degree HAT patients under stress (e.g. exercise/pathological states) would have an increased arrhythmia risk, but from our findings we hypothesize that HAT patients would be more prone to arrhythmic events under stress conditions. Indeed, there are reports of sudden death in HAT patients before full progression of the disease, and in these cases sudden arrhythmic-induced cardiac death cannot be ruled out.5,6
4.7. Study implications and conclusions
Extracellular cathepsin-L not only disrupts Ca2+ release from intracellular stores in the brain but as we have shown for the first time, also in the heart. This common pathogenic mechanism provides a platform to support future investigations that centre on normalization of the effects of cathepsin-L via drugs that act on SR/ER-mediated Ca2+ signalling in order to address the neurological and cardiovascular pathology associated with HAT. Previous studies have shown that arrhythmias associated with disrupted SR function can be treated with flecainide and K201(JTV-519)14 and this suggests a potential therapeutic approach for treatment of cathepsin-L induced cardiac dysfunction.
The findings of this study also have implications for other pathogens, such as Trypanosoma cruzi, which causes Chagas disease. Notable cardiac pathology is present in Chagas disease, and T. cruzi induces abnormal Ca2+ handling in cardiomyocytes.25 Another important implication concerns coronary heart disease (CHD). It is well established that patients with CHD have abnormal SR-mediated Ca2+ release, contractile dysfunction, increased arrhythmia risk, and elevated levels of endogenous cathepsin-L in serum, yet the effect of mammalian-derived extracellular cathepsins on SR function remains unknown.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the Royal College of Veterinary Surgeons Trust (GR000 652 to C.L. and L.M.); University of Glasgow Medical Fund (to C.L. and L.M.); Biotechnology and Biological Sciences Research Council (BBSRC) doctoral training grant (to D.M. and C.W.), the Wellcome Trust and the Royal Society (University Research Fellowship UF090083 to L.M.). The Wellcome Trust Centre for Molecular Parasitology is supported by core funding from the Wellcome Trust (085349) (to J.M.).
Supplementary Material
Acknowledgements
We thank Jim McKerrow and Ana Paula Lima for the kind gift of recombinant TbCatL.This work was supported by the Wellcome Trust. The Wellcome Trust Centre for Molecular Parasitology is supported by core funding from the Wellcome Trust [085349].
References
- 1.Blum JA, Schmid C, Burri C, Hatz C, Olson C, Fungula B, et al. Cardiac alterations in human African trypanosomiasis (T.b. gambiense) with respect to the disease stage and antiparasitic treatment. PLoS Negl Trop Dis. 2009;3:e383. doi: 10.1371/journal.pntd.0000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum JA, Burri C, Hatz C, Kazumba L, Mangoni P, Zellweger MJ. Sleeping hearts: the role of the heart in sleeping sickness (human African trypanosomiasis) Trop Med Int Health. 2007;12:1422–1432. doi: 10.1111/j.1365-3156.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 3.Hidron A, Vogenthaler N, Santos-Preciado JI, Rodriguez-Morales AJ, Franco-Paredes C, Rassi A., Jr Cardiac involvement with parasitic infections. Clin Microbiol Rev. 2010;23:324–349. doi: 10.1128/CMR.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones IG, Lowenthal MN, Buyst H. Electrocardiographic changes in African trypanosomiasis caused by Trypanosoma brucei rhodesiense. Trans R Soc Trop Med Hyg. 1975;69:388–395. doi: 10.1016/0035-9203(75)90194-7. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand E. Cardiac involvement in human African trypanosomiasis. Med Trop (Mars) 1987;47:91–93. [PubMed] [Google Scholar]
- 6.Chappuis F, Loutan L, Simarro P, Lejon V, Buscher P. Options for field diagnosis of human African trypanosomiasis. Clin Microbiol Rev. 2005;18:133–146. doi: 10.1128/CMR.18.1.133-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco-Paredes C, Rouphael N, Mendez J, Folch E, Rodriguez-Morales AJ, Santos JI, et al. Cardiac manifestations of parasitic infections. Part 2: parasitic myocardial disease. Clin Cardiol. 2007;30:218–222. doi: 10.1002/clc.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poltera AA, Owor R, Cox JN. Pathological aspects of human African trypanosomiasis (HAT) in Uganda. A post-mortem survey of fourteen cases. Virchows Arch A Pathol Anat Histol. 1977;373:249–265. doi: 10.1007/BF00432240. [DOI] [PubMed] [Google Scholar]
- 9.Morrison WI, Murray M, Sayer PD, Preston JM. The pathogenesis of experimentally induced Trypanosoma brucei infection in the dog. I. Tissue and organ damage. Am J Pathol. 1981;102:168–181. [PMC free article] [PubMed] [Google Scholar]
- 10.Poltera AA, Cox JN, Owor R. Pancarditis affecting the conducting system and all valves in human African trypanosomiasis. Br Heart J. 1976;38:827–837. doi: 10.1136/hrt.38.8.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grab DJ, Kennedy PG. Traversal of human and animal trypanosomes across the blood-brain barrier. J Neurovirol. 2008;14:344–351. doi: 10.1080/13550280802282934. [DOI] [PubMed] [Google Scholar]
- 12.Nikolskaia OV, de A Lima AP, Kim YV, Lonsdale-Eccles JD, Fukuma T, Scharfstein J, et al. Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J Clin Invest. 2006;116:2739–2747. doi: 10.1172/JCI27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77:285–292. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 14.Elliott EB, Hasumi H, Otani N, Matsuda T, Matsuda R, Kaneko N, et al. K201 (JTV-519) alters the spatiotemporal properties of diastolic Ca2+ release and the associated diastolic contraction during beta-adrenergic stimulation in rat ventricular cardiomyocytes. Basic Res Cardiol. 2011;106:1009–1022. doi: 10.1007/s00395-011-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdulla MH, O'Brien T, Mackey ZB, Sajid M, Grab DJ, McKerrow JH. RNA interference of Trypanosoma brucei cathepsin B and L affects disease progression in a mouse model. PLoS Negl Trop Dis. 2008;2:e298. doi: 10.1371/journal.pntd.0000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 17.Steverding D, Sexton DW, Wang X, Gehrke SS, Wagner GK, Caffrey CR. Trypanosoma brucei: chemical evidence that cathepsin L is essential for survival and a relevant drug target. Int J Parasitol. 2012;42:481–488. doi: 10.1016/j.ijpara.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Loughrey CM, Smith GL, MacEachern KE. Comparison of Ca2+ release and uptake characteristics of the sarcoplasmic reticulum in isolated horse and rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1149–H1159. doi: 10.1152/ajpheart.00060.2004. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill SC, Miller L, Hinch R, Eisner DA. Interplay between SERCA and sarcolemmal Ca2+ efflux pathways controls spontaneous release of Ca2+ from the sarcoplasmic reticulum in rat ventricular myocytes. J Physiol. 2004;559:121–128. doi: 10.1113/jphysiol.2003.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode EF, Briston SJ, Overend CL, O'Neill SC, Trafford AW, Eisner DA. Changes of SERCA activity have only modest effects on sarcoplasmic reticulum Ca2+ content in rat ventricular myocytes. J Physiol. 2011;589:4723–4729. doi: 10.1113/jphysiol.2011.211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, et al. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res. 2006;98:235–244. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 22.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007;42:196–205. doi: 10.1016/j.yjmcc.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grab DJ, Garcia-Garcia JC, Nikolskaia OV, Kim YV, Brown A, Pardo CA, et al. Protease activated receptor signaling is required for African trypanosome traversal of human brain microvascular endothelial cells. PLoS Negl Trop Dis. 2009;3:e479. doi: 10.1371/journal.pntd.0000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legros D, Ollivier G, Gastellu-Etchegorry M, Paquet C, Burri C, Jannin J, et al. Treatment of human African trypanosomiasis-present situation and needs for research and development. Lancet Infect Dis. 2002;2:437–440. doi: 10.1016/s1473-3099(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 25.Barr SC, Han W, Andrews NW, Lopez JW, Ball BA, Pannabecker TL, et al. A factor from Trypanosoma cruzi induces repetitive cytosolic free Ca2+ transients in isolated primary canine cardiac myocytes. Infect Immunity. 1996;64:1770–1777. doi: 10.1128/iai.64.5.1770-1777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.