Abstract
Aims
Urotensin-II (UII) is a vasoactive peptide that promotes vascular smooth muscle cells (VSMCs) proliferation and is involved in the pathogenesis of atherosclerosis, restenosis, and vascular remodelling. This study aimed to determine the role of calcium (Ca2+)-dependent signalling and alternative signalling pathways in UII-evoked VSMCs proliferation focusing on store-operated Ca2+ entry (SOCE) and epithelium growth factor receptor (EGFR) transactivation.
Methods and results
We used primary cultures of VSMCs isolated from Wistar rat aorta to investigate the effects of UII on intracellular Ca2+ mobilization, and proliferation determined by the 5-bromo-2-deoxyuridine (BrdU) assay. We found that UII enhanced intracellular Ca2+ concentration ([Ca2+]i) which was significantly reduced by classical SOCE inhibitors and by knockdown of essential components of the SOCE such as stromal interaction molecule 1 (STIM1), Orai1, or TRPC1. Moreover, UII activated a Gd3+-sensitive current with similar features of the Ca2+ release-activated Ca2+ current (ICRAC). Additionally, UII stimulated VSMCs proliferation and Ca2+/cAMP response element-binding protein (CREB) activation through the SOCE pathway that involved STIM1, Orai1, and TRPC1. Co-immunoprecipitation experiments showed that UII promoted the association between Orai1 and STIM1, and between Orai1 and TRPC1. Moreover, we determined that EGFR transactivation, extracellular signal-regulated kinase (ERK) and Ca2+/calmodulin-dependent kinase (CaMK) signalling pathways were involved in both UII-mediated Ca2+ influx, CREB activation and VSMCs proliferation.
Conclusion
Our data show for the first time that UII-induced VSMCs proliferation and CREB activation requires a complex signalling pathway that involves on the one hand SOCE mediated by STIM1, Orai1, and TRPC1, and on the other hand EGFR, ERK, and CaMK activation.
Keywords: Smooth muscle, Proliferation, Ion channels, EGFR
1. Introduction
Vascular smooth muscle cells (VSMCs) play a central role in controlling vascular tone and maintaining the integrity of vessel wall. Under physiological conditions, VSMCs contraction and relaxation are regulated by biologically active mediators which are synthesized and secreted to modulate the vascular tone. Many of these mediators might play also a pathological role, and induce abnormal cellular proliferation during disease-related vascular remodelling.1 Urotensin-II (UII) has emerged as a potent vasoconstrictor in different mammal species, including humans, and has been related to several cardiovascular diseases.2,3 The particular interest of the UII system relies into its ‘quasi-irreversible’ binding to a G-protein-coupled receptor (GPCR), known as a urotensin receptor (UTS2R).4 UTS2R is functionally linked to Gq and phospholipase C (PLC), and its activation promotes long-term effects such as VSMCs proliferation.4,5 UII mediates VSMCs proliferation and remodelling through different signalling molecules such as ERK1/2 and RhoA/Rho kinase,6 or via Ca2+/calmodulin-dependent kinase (CaMK).7 Moreover, a recent study has implicated epidermal growth factor receptor (EGFR) transactivation and ERK phosphorylation in UII-induced rat aortic VSMCs proliferation.8
Recently, we have determined that UII-induced rat coronary artery contraction involves [Ca2+]i increase through the store-operated Ca2+ entry (SOCE) pathway, which depends on the activation of the Ca2+-sensing regulatory protein stromal interaction molecule 1 (STIM1), and Orai1, the pore forming subunit of store-operated calcium channel (SOCC).9 Only in recent years, the activation of the SOCE pathway has been associated with VSMCs proliferation.10–12 VSMCs switch from a ‘contractile’ to a ‘synthetic’ proliferative phenotype is regulated by a rise in [Ca2+]i and by up or down-regulation of several SOCE-associated proteins as STIM1 and Orai1.12,13 Similar findings have been shown for some TRPC channels (TRPC1/3/5/6) that are activated by the sarcoplasmic reticulum depletion and suggested to be part of the endogenous SOCC.14
Several reports have also demonstrated that SOCE is important for transcription factors activation that regulate the expression of many proliferating genes. Particularly, nuclear factor of activated T-cell (NFAT), or Ca2+/cAMP response element-binding protein (CREB) that are activated by Ca2+-induced phosphorylation.15–18 Additionally, CREB can be targeted by different signalling pathways, such as EGFR and ERK1/2, which promote VSMCs proliferation and/or hypertrophy.18
Given the increasing importance of SOCE in VSMC proliferation and neointima formation,13 this study sought to unveil the role of SOCE and the transcription of CREB in UII-mediated proliferation, taking in consideration the increasing interest regarding UII signalling mechanism and its relationship with cardiovascular disease.3
2. Methods
All the procedures of this study were approved by the Bioethical Committee of the Institute of Biomedicine of Seville (IBiS, Spain) in accordance with the animal care guidelines of the European Communities Council (86/609/EEC). An expanded Materials and Methods section detailing the protocols and techniques used in this study can be found in the Supplementary material online.
2.1. Primary aortic smooth muscle cell preparation
Adult male Wistar rats weighing 250–350 g were heparinized (4 IU/g i.p.) and anaesthetized by i.p. administration of pentobarbital sodium overdose. Primary culture of aortic VSMCs was prepared following the same protocol as described previously.19,20
2.2. Intracellular Ca2+ measurement
Changes in [Ca2+]i were measured in Fura-2AM-loaded cells. Fluorescence images of 15–30 cells were recorded and analysed with a digital fluorescence imaging system (InCyt Basic Im2, Intracellular Imaging, Inc., Imsol, UK) as described previously.9,21
2.3. Patch clamp study
Whole-cell patch clamp recordings were performed to register ICRAC in VSMCs. The patch clamp technique was carried out using an Axopatch 200B and Digidata 1440A (Axon Instruments) as described previously.10,13
2.4. BrdU immunofluorescence
Cell proliferation was estimated by the 5-bromo-2-deoxyuridine (BrdU) incorporation assay. Briefly, cells were grown until they reached 70% confluence, then culture medium was replaced with serum-free DMEM, supplemented with 0.1% foetal bovine serum. Cells were then incubated with UII and grown for additional 48 h. After different treatments, cells were immunostained following the protocol described in Supplementary material online.
3. Results
3.1. UII activates store-operated Ca2+ entry and ICRAC-like currents in VSMCs through UTS2R
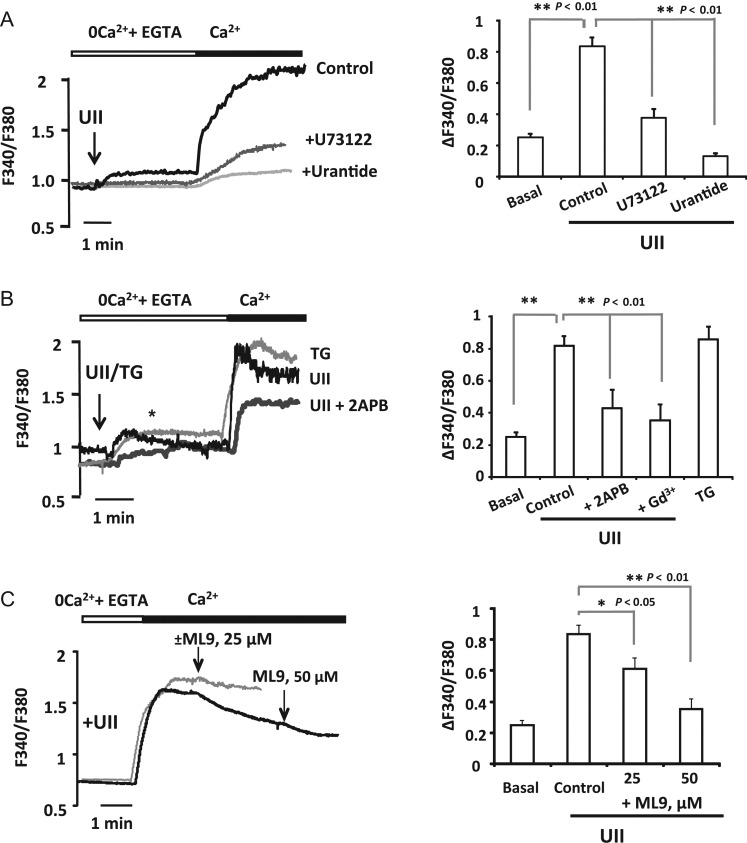
First, we studied the increase of [Ca2+]i stimulated by UII in aortic VSMCs. Figure 1A shows that UII (100 nM) evoked a Ca2+ response with two components: a [Ca2+]i increase in free Ca2+ solution corresponding to Ca2+ release from intracellular stores followed by a sustained enhancement in [Ca2+]i after Ca2+ (2 mM) re-addition, which corresponds to Ca2+ influx from extracellular medium. Next, we examined UTS2R and PLC inhibition with urantide22 and U73122, respectively..As illustrated in Figure 1A, both urantide (100 nM) and U73122 (50 µM) inhibited UII-induced Ca2+ release and extracellular Ca2+ influx.
Figure 1.
Urotensin-II activates UTS2R and induces store-operated Ca2+ influx. (A) Representative traces presented as fura-2 ratio (F340/F380) in aortic VSMCs and summary data of UII induced [Ca2+]i mobilization in isolated VSMCs. UII (100 nM) was applied 3–4 min in the absence of extracellular Ca2+ and then Ca2+ (2 mM) was added as indicated. Traces are for VSMCs treated with UII (control), for cells pre-incubated 10 min with Urantide (100 nM), or with PLC inhibitor (U73122, 50 µM). (B) Left panel illustrates representative recordings of the changes in [Ca2+]i. UII (100 nM) or thapsigargin (TG, 2 μM) were applied as indicated in (A). Traces are for VSMCs treated with UII or TG, and for cells incubated with UII and treated with 2APB (+2APB, 50 μM) 1–2 min before Ca2+ addition as indicated by ‘*’. Right panel shows summary data of experiments illustrated in left. ‘basal’ is for Ca2+ influx in untreated VSMCs, and ‘+Gd3+’ is for Ca2+ influx recorded in cells pre-incubated 3 min with gadolinium (5 μM). (C) Representative recordings (black trace) and summary data of the effects of ML9 (25 and 50 μM respectively) applied after Ca2+ influx induced by UII (100 nM). The addition of vehicule (grey trace) rather than ML9 had no effect on Ca2+ influx. The summary data in (A, B, and C) correspond to large number of cells (n = 60–220 cells) from 4–12 primary cultures. Data are means ± SEM.
Recently, we have demonstrated that UII-induced coronary artery vasoconstriction involves Ca2+ entry through SOCC.9 Here, we examined whether these channels participate in UII-mediated Ca2+ entry in aortic VSMCs. Figure 1B shows that UII (100 nM) evoked a Ca2+ influx, after re-addition of extracellular Ca2+ (2 mM), similar to that typically induced by passive depletion of the intracellular store with thapsigargin (2 µM). As summarized in Figure 1B, UII-evoked Ca2+ entry was blocked with classical inhibitors of SOCE, Gd3+ (5–10 µM) and 2-aminoethoxydiphenyl borate (2APB) (75 µM). Furthermore, the addition of ML9 (25 and 50 µM) after Ca2+ restoration also inhibited significantly the Ca2+ influx elicited by UII, as shown in Figure 1C.
Next, to characterize the current mediating UII-induced SOCE in VSMCs, we tested if UII could activate CRAC channels in VSMCs. VSMCs have been demonstrated to start expressing whole-cell currents through CRAC channels (ICRAC-like current) after several passages in culture medium.10 Whole-cell current was measured in a bath solution containing 20 mM Ca2+ to determine Ca2+-ICRAC and was amplified using short pulses of standard divalent-free (DVF; Na+-ICRAC) solutions. Figure 2A shows that cell dialysis with 150 nM free-Ca2+ solution did not activate any inward current. Meanwhile, as shown in Figure 2B and summarized in Figure 2C and D, UII (1 µM) addition to the bath solution stimulated a significant inward Ca2+ current that was amplified in DVF solution. Both developed inward currents, Ca2+-ICRAC and Na+-ICRAC, were sensitive to Gd3+ (5 μM, Figure 2B). The analysis of the current–voltage (I–V) curve for Ca2+-ICRAC (Figure 2E) and Na+-ICRAC (Figure 2F) confirms the inward rectification of UII-induced current, one of the features of ICRAC. Altogether, these data show for the first time that UII activates SOCE and ICRAC-like current in aortic VSMCs.
Figure 2.
Urotensin-II stimulates ICRAC-like current in VSMCs. (A and B) Representative time course of the whole-cell CRAC current that developed during cell dialysis with pipette solution containing a buffered-free Ca2+ concentration of 150 nM. Whole-cell currents were measured in the presence of 20 mM Ca2+ extracellular solution and after applying pulses of divalent-free bath solutions (DVF) to amplify ICRAC. Small inwardly rectifying ICRAC developed after applying 1 µM UII (B) compared with control (A). Low concentrations of lanthanides (5 µM, Gd3+), a specific inhibitor of ICRAC, abolished UII-activated ICRAC (B). (C and D) Summary of Ca2+ ICRAC and Na+ ICRAC current densities (pA/pF) activated by UII in VSMCs. Ca2+ ICRAC and Na+ ICRAC were developed in seven and five VSMCs. (E and F) The current–voltage (I–V) relationships for Ca2+ ICRAC (E) (grey trace) and Na+ ICRAC (F) (grey trace) were taken from (A and B), respectively, where indicated with ‘+’ and ‘*’. In all sweeps represented in (E and F), background currents were subtracted. Data are means ± SEM.
3.2. UII promotes VSMCs proliferation associated with a significant rise in SOCE
The effect of UII on VSMCs proliferation was assessed by BrdU incorporation. Figure 3A shows that VSMCs incubation with UII (100 nM) during 48 h promoted significant increase in BrdU positive marked cells. However, cell pre-treatment with SOCE blockers, ML9 (5 µM) and 2APB (50 µM), inhibited significantly VSMCs proliferation, which confirms SOCE role in UII proliferative effects. Cell proliferation has been associated with several intracellular Ca2+ alterations with a great implication of SOCE.10,14 So, we examined whether long-term incubation with UII could potentiate the rise in [Ca2+]i mediated by SOCE. Thapsigargin-activated SOCE was evaluated in serum-starved VSMCs treated 48 h with UII (100 nM) to mimic the same condition as in Figure 3A. As illustrated in Figure 3B, thapsigargin (2 μM) induced significantly higher responses in VSMCs incubated with UII comparing with untreated cells, which indicates that VSMCs proliferation is accompanied with a significant increase in SOCE. Next, we checked the expression of key proteins related to SOCE in UII-mediated proliferating VSMCs. Figure 3C shows that serum-starved cells treated with UII (100 nM during 48 h) presented significant increase in mRNA expression of STIM1 and Orai1 but not Orai3, whereas Orai2 expression was slightly decreased. In addition, TRPC1 that is believed to participate in the SOCE signalling pathway was also up-regulated in VSMCs treated with UII. These data confirm that proliferating VSMCs stimulated with UII show high degree of SOCE due apparently to the up-regulation of STIM1, Orai1, and TRPC1.
Figure 3.
Urotensin-II stimulates VSMCs proliferation associated with an increase in SOCE and STIM1, Orai1 and TRPC1 expression. (A) Left panel, representative images showing the immunostaining with anti-BrdU antibody (upper images) and DAPI (to detect nuclei, lower images) of VSMCs. UII (100 nM) was applied during 48 h in control VSMCs, or in cells pre-incubated 5 min with ML9 (10 μM) or with 2APB (50 μM) to inhibit SOCE. Right panel, summary data showing the per cent of BrdU positive cells in experiments done as in left panel (n = 3–4). (B) Representative traces of Ca2+ changes and summary data showing that thapsigargin (TG, 2 μM) activated higher SOCE in growth-arrested VSMCs treated 48 h with 100 nM UII (0.1%FBS+UII) than in untreated quiescent VSMCs (0.1%FBS). n = 40–80 cells from three independent cultures. (C) VSMCs treated 48 h with UII (100 nM) expressed higher levels of STIM1, Orai1, and TRPC1 mRNA compared with untreated quiescent VSMCs (0.1% FBS). Orai2 mRNA expression decreased, meanwhile Orai3 mRNA levels remained unchanged (n = 4–5). ‘*’ and ‘†’ indicate significance at P < 0.05 comparing with untreated VSMCs. Data are means ± SEM.
3.3. UII-stimulated VSMCs proliferation requires STIM1 and Orai1-dependent SOCE
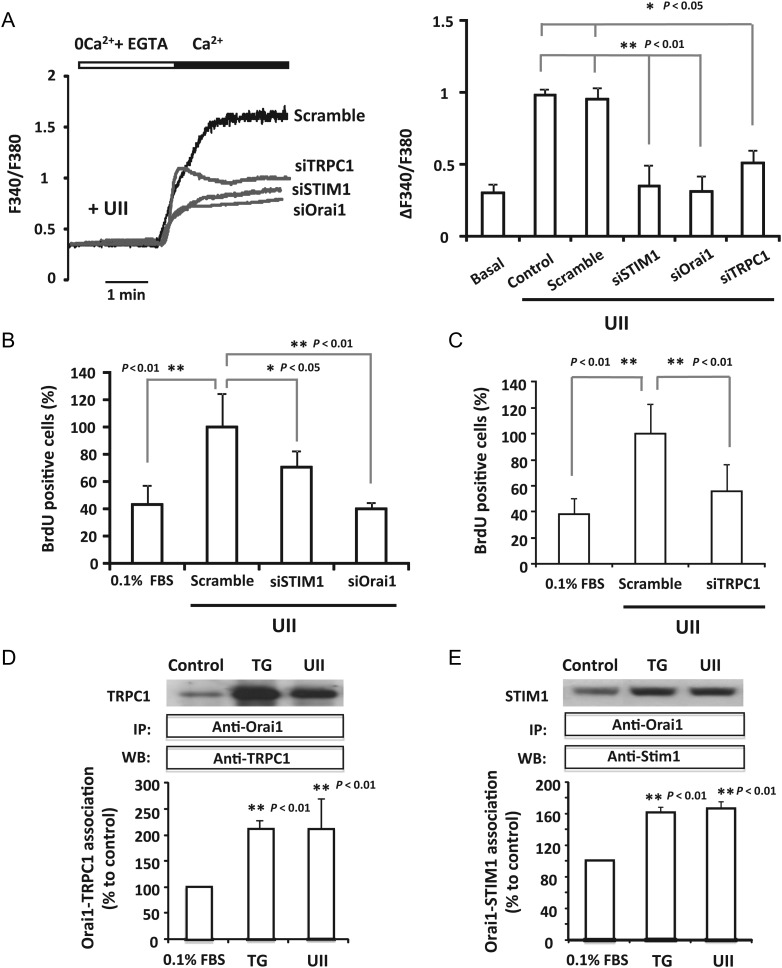
STIM1 and Orai1 are key proteins for SOCE activated by UII in the coronary artery.9 Thus, we investigated the role of STIM1 and Orai1 in cells transfected with siRNA in UII-induced Ca2+ increase and proliferation of aortic VSMCs. Supplementary material online, Figure SIA confirms that knockdown of STIM1 and Orai1 was successfully achieved in VSMCs transfected with siRNA. The study of [Ca2+]i mobilization showed that UII induced a sustained Ca2+ influx in cells transfected with scrambled siRNA similar to that recorded in non-transfected cells (Figure 4A). Meanwhile, STIM1 and Orai1 knockdown with siRNA significantly decreased UII-induced Ca2+ influx (Figure 4A). Moreover, Figure 4B and Supplementary material online, Figure SIB show that Orai1 and STIM1 down-regulation prevented VSMCs proliferation. These results confirm that STIM1 and Orai1 are implicated in UII-induced SOCE and VSMCs proliferation.
Figure 4.
STIM1, Orai1, and TRPC1 participate in Urotensin-II evoked SOCE and proliferation of VSMCs. (A) Representative traces and summary data showing [Ca2+]i changes recorded in VSMCs treated 5 min with UII (100 nM) in experiments similar to those shown in Figure 1B. Data are from VSMCs transfected 72 h prior to the experiments, with scramble siRNA (scramble), STIM1 siRNA (siSTIM1), Orai1 siRNA (siOrai1), or TRPC1 siRNA (siTRPC1). ‘Basal’ is for Ca2+ influx in untreated VSMCs, and ‘control’ is for non-transfected cells treated with UII. Data are from four to seven different transfections. (B and C) Data summary indicating the per cent of BrdU positive cells in VSMCs transfected with scrambled siRNA (scramble), siRNA against STIM1 (siSTIM1), Orai1 (siOrai1), or TRPC1 (siTRPC1). UII (100 nM) was applied during 48 h before the BrdU assay. n = 3–4 independent experiments. (D and E) Summary data representing by immunoprecipitation and western blotting, the quantification of TRPC1–Orai1 and STIM1–Orai1 association in non-stimulated (0.1% FBS), UII-(100 nM) treated cells, and TG-(2 μM) treated VSMCs. Cells were treated 5 min with either UII or TG. Data are means ± SEM, n = 5–6 independent cultures.
3.4. Evidence of TRPC1 participation in UII stimulation of calcium entry and VSMCs proliferation
Several reports have investigated the role of TRPC1 in SOCE in excitable and non-excitable cells. Here, we explored the potential participation of TRPC1 in Ca2+ influx and VSMCs proliferation induced by UII. Figure 4A and C confirm that siRNA-mediated TRPC1 down-regulation in VSMCs significantly decreased UII-stimulated Ca2+ entry and inhibited VSMCs proliferation, which suggests the participation of TRPC1 in this pathway. To understand the interaction between STIM1, Orai1, and TRPC1 in this complex signalling pathway, we studied the association between these proteins in VSMCs stimulated with either UII or thapsigargin to activate specifically SOCE. Figure 4D and E show that UII (100 nM) evoked a potent association between TRPC1 and Orai1, and between Orai1 and STIM1, respectively. Conversely, treatment of VSMCs with thapsigargin (2 µM) also promoted the interaction between TRPC1 and Orai1, and between Orai1 and STIM1. Altogether, these data indicate that UII activates a functional interaction between key SOCE proteins, STIM1, Orai1, and TRPC1, that allows Ca2+ entry with consequent VSMCs proliferation.
3.5. Role of EGFR transactivation, ERK phosphorylation, and CaMKII in UII signalling
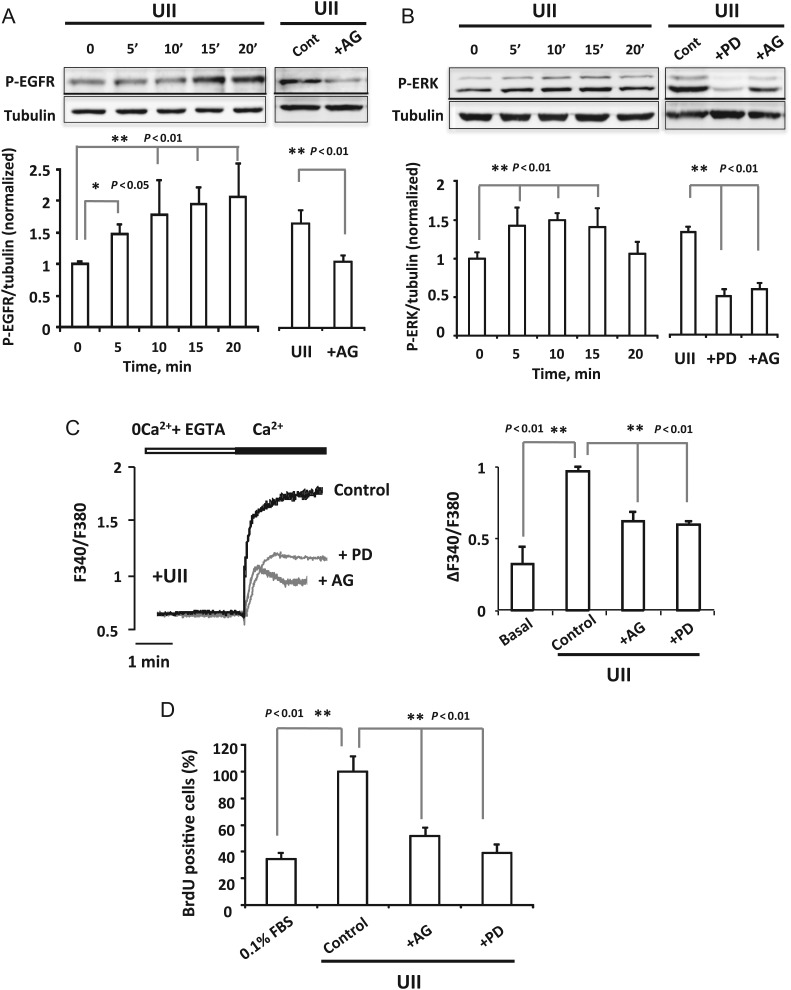
UII effects on [Ca2+]i increase and proliferation have been related to other signalling pathways as EGFR, ERK, or CaMK.6–8 Figure 5A shows that UII (100 nM) activated EGFR phosphorylation 5 min after its addition which was sustained through 20 min of cells exposure to UII. EGFR phosphorylation was efficiently reduced in cells pre-treated with EGFR inhibitor AG1478 (100 nM, Figure 5A right). Furthermore, ERK activation is important for the EGFR signalling pathway; therefore, we determined ERK phosphorylation by UII. Figure 5B shows that ERK phosphorylation increased within 5 min of UII (100 nM) exposure and was sustained through 15 min of cells treatment. As shown in Figure 5B, the phosphorylation of ERK was potently inhibited by AG1478 (100 nM) and by ERK inhibitor PD98059 (10 µM). Next, we examined the role of SOCE in EGFR and ERK phosphorylation. As shown in Supplementary material online, Figure SII, 2APB (50 µM) and ML9 (10 µM) reduced significantly EGFR and ERK phosphorylation. Furthermore, we tested the implication of EGFR and ERK activation in UII-mediated [Ca2+]i increase and cells proliferation, respectively. As shown in Figure 5C and D, the pre-incubation of VSMCs with AG1478 (100 nM) and PD98059 (10 µM) reduced significantly [Ca2+]i increase and VSMCs proliferation.
Figure 5.
Role of EGFR and ERK phosphorylation in UII-induced Ca2+ influx and proliferation. (A and B) Left panels, western blot and summary data showing the time course of UII activation of EGFR and ERK, respectively. (A) Right panel, western blot and data showing the amount of activation of EGFR in cells treated 10 min with Urotensin-II (100 nM; Cont) and in cells pre-treated 15 min with the EGFR inhibitor, AG1478 (100 nM; +AG) before the addition of Urotensin-II (100 nM, 10 min). (B) Right panel shows the effect of the ERK inhibitor, PD98059 (10 μM; +PD) and the EGFR inhibitor, AG1478 (100 nM; +AG) in cells pre-treated 15 min with the inhibitors before the addition of Urotensin-II (100 nM, 10 min). Results are mean ± SEM (n = 4). (C) Representative traces and summary data showing [Ca2+]i changes recorded in VSMCs treated 5 min with UII (100 nM). Data are from control VSMCs; and for cells pre-incubated 15 min with PD98059 (10 μM; +PD) and AG1478 (100 nM; +AG) before Urotensin-II (100 nM). n = 65–170 cells from four to five different culture. (D) Data summary indicating the per cent of BrdU positive cells in quiescent VSMCs (0.1%FBS); in cells treated 48 h with Urotensin-II (100 nM; control) and in cells pre-incubated 15 min with AG1478 (100 nM; +AG) or with PD98059 (10 μM; +PD) before Urotensin-II (100 nM). n = 5 cultures.
Moreover, a previous study has implicated CaMK in UII-induced pulmonary artery VSMCs proliferation.7 Hence, we explored CaMKII implication in UII effects in aortic VSMCs using the specific inhibitor KN93. As shown in Supplementary material online, Figure SIIIA, pre-treatment of cells with KN93 (2 µM) to block CaMKII inhibited significantly intracellular Ca2+ influx induced by UII, and potently prevented VSMCs proliferation (Supplementary material online, Figure SIIIB). These data confirm that UII effects in rat aorta VSMCs involve the transactivation of EGFR, ERK phosphorylation, and CaMKII signalling pathways.
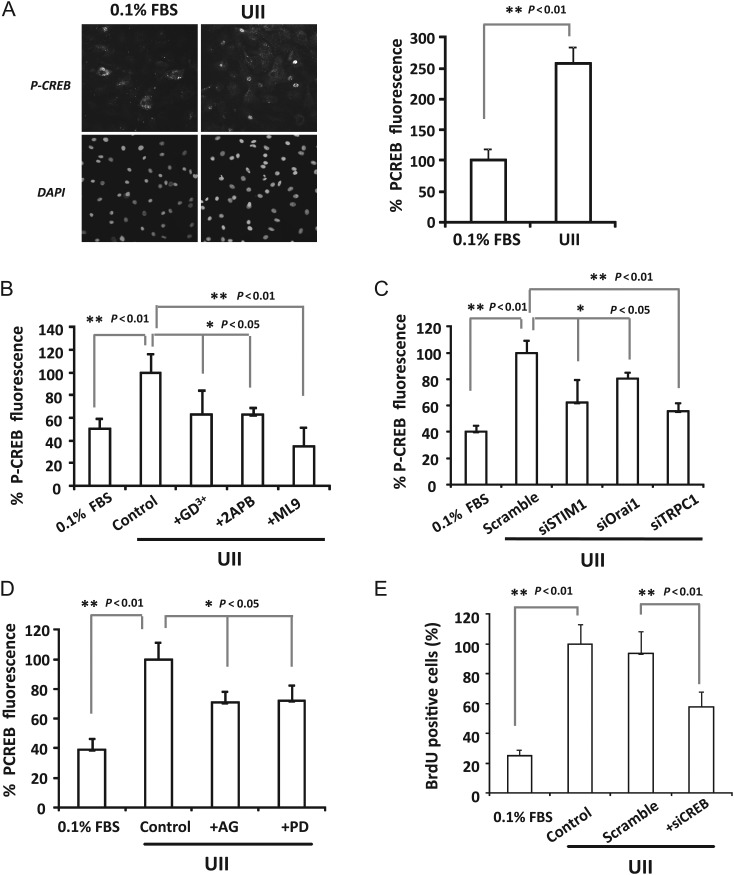
3.6. UII activates CREB phosphorylation through SOCE and EGFR transactivation
To highlight downstream pathways involved in UII-mediated SOCE of VSMCs proliferation and to elucidate the link between [Ca2+]i increase and its effects on VSMCs proliferation, we focused on CREB contribution in UII effects. Figure 6A and Supplementary material online, Figure SIV show that VSMCs exposure during 5 min with different concentrations of UII significantly activated CREB. Next, SOCE role in CREB activation was examined. We found that SOCE pharmacological inhibition (Figure 6B), and knockdown of STIM1, Orai1, or TRPC1 (Figure 6C) significantly prevented UII-induced CREB activation, which confirms the important role of SOCE. Furthermore, we studied the implication of EGFR, ERK, and CaMKII in CREB phosphorylation. Experiments shown in Figure 6D and Supplementary material online, Figure SIIIC demonstrate that all the inhibitors of these ones reduced significantly UII-mediated CREB activation, which indicates that UII activation of CREB involves SOCE and other signalling pathways. Finally, we explored the CREB role in VSMCs proliferation in cells transfected with siRNA against CREB. Figure 6E demonstrates that CREB silencing inhibited significantly UII-induced VSMCs proliferation suggesting the important role of CREB transcription in VSMCs proliferation.
Figure 6.
Urotensin-II stimulates CREB phosphorylation through SOCE and EGFR activation. (A) Representative images and summary data showing CREB phosphorylation detected by immunofluorescence (upper panel) and DAPI (lower panel) in non-treated cells (0.1%FBS) and in VSMCs incubated 5 min with Urotensin-II (100 nM; UII). n = 6 cultures. (B and D) Summary data of CREB phosphorylation in control VSMCs treated 5 min with UII (100 nM, control), or in VSMCs pre-incubated 5 min with Gd3+ (5 μM; +Gd3+), 2APB (50 μM; +2APB), ML9 (20 μM; +ML9), AG1478 (100 nM; +AG) or with PD98059 (10 μM; +PD) then treated with Urotensin-II. n = 3–6 experiments. (C) Bar graph summarizing CREB phosphorylation induced by UII (100 nM). Urotensin-II was applied 5 min in VSMCs transfected 72 h with scramble siRNA, siSTIM1, siOrai1, or siTRPC1 (n = 3–4). (E) Data summary indicating the per cent of BrdU positive cells in quiescent VSMCs (0.1%FBS); in cells treated with Urotensin-II (100 nM; control); in VSMCs transfected with scrambled siRNA (scramble) and siRNA against CREB (siCREB). Urotensin-II (100 nM) was applied during 48 h (n = 3). Values are means ± SEM.
4. Discussion
Several lines of evidence have demonstrated a major contribution of the UII system to cardiovascular diseases, metabolic syndrome, diabetes, or renal disease.3,23 UII acts as a chronic vasoactive regulator thanks to its ‘pseudo-irreversible’ binding and its slow-rate dissociation from the receptor, which leads to prolonged activation of UTS2R allowing long-term effects such as VSMCs proliferation.5,6 Here, we provide pharmacological and molecular data demonstrating the critical role of SOCE key proteins, such as STIM1, Orai1, and TRPC1, in UII-mediated VSMC proliferation. Moreover, our data confirm that UII effects are more complex and involve several proliferative signalling pathways, such as EGFR transactivation, ERK, CaMKII, and CREB stimulation.
UII binding to its receptor UTS2R stimulates the synthesis of IP3, which releases Ca2+ from internal stores known to activate SOCE similarly to other agonists of GPCR.2,9,24 We provide data showing that UII acts through UTS2R and PLC signalling cascade, and the induced Ca2+ entry displays classical pharmacological features of SOCE. The electrophysiological study confirms that at least part of the UII-induced Ca2+ influx depends on ICRAC-like current. We show for the first time that UII activates a small current in the presence of external Ca2+, that is amplified in DVF bath solutions. The developed current presents similar biophysical properties of classical ICRAC current, such as strong inward rectification, and inhibition by low concentrations of lanthanides. Similar characteristics of ICRAC currents have been previously recorded in synthetic rat aorta VSMCs activated by cell dialysis with 20 mM BAPTA or with PDGF.10,13 Previously, we have shown that STIM1 and Orai1 are necessary for UII-induced Ca2+ entry and vasoconstriction in the rat coronary artery.9 Here, we determine that UII addition to growth-arrested VSMCs stimulates their proliferation that is associated with an enhancement of thapsigargin-activated SOCE owing certainly to the up-regulation of STIM1, Orai1, and TRPC1. These results agree with previous studies which demonstrated that VSMCs switch from a ‘contractile’ to a ‘synthetic’ proliferative phenotype was associated with changes in the expression of proteins related to Ca2+ homeostasis, such as CaV 2.1 channels, Orai1, TRPCs, or intracellular receptors.14,25 Our data confirm the relevant role of STIM1 and Orai1, in both UII-induced Ca2+ influx and VSMCs proliferation, consistent with earlier studies that established the essential role of Orai1 and STIM1 for SOCE and the consequent VSMCs proliferation activated by different stimulus in rat aortic VSMCs.10–13
Importantly, we also demonstrate the involvement of TRPC1 in UII-stimulated Ca2+ influx and VSMCs proliferation. The implication of TRPC1 in SOCE still remains under debate.26–28 Several groups have shown different interaction between proteins to form the SOCE signalling macromolecular complex (see reviews29,30). Recent studies have shown that SOCE is independent of TRPC1,10,11 but others have suggested that mammalian SOCC might be heterotetramers and can be formed by different isoforms of TRPC, or by the association between TRPC and Orai.27–30 This discrepancy remains unresolved and might be due to the experimental approaches and/or agonists used to stimulate VSMCs. Here, we show that UII can activate TRPC1 and Orai1-dependent Ca2+ entry, and under Ca2+ buffering only CRAC current is developed, which might be amplified by TRPC1 association with Orai1. The functional and/or physical association between SOCE key proteins have been barely investigated in VSMCs. Previous studies in VSMCs have demonstrated that STIM1 functionally associates to TRPC1,26 L-type Ca2+ channels,31 and ion transporters.14 Our co-immunoprecipitation experiments demonstrate that passive store depletion with thapsigargin or UII addition promotes TRPC1 association with Orai1 and the association between STIM1 and Orai1, which confirms such results determined in other cell lines as HEK cells,27 platelets,28 and recently in pulmonary arterial VSMCs.32 Therefore, we believe that one of the most important findings in the present study is that upon UII application, Orai1 functionally and physically associates with STIM1 and TRPC1 in aortic VSMCs, which apparently form a macrocomplex signalling pathway that mediates [Ca2+]i enhancement and further cell proliferation.
Considerable interest has been focused towards the influence of ion channels plasticity and gene expression in VSMCs. It is well known that the sustained Ca2+ influx through SOCC is crucial for transcriptional activation of CREB, which drives cellular proliferation through several proliferating genes.15 In this study, we show that UII promotes CREB phosphorylation through SOCE which depends on STIM1, Orai1, and TRPC1 in accordance with previous studies,11,17 although TRPC1 appeared not involved in angiotensin-II-induced VSMCs proliferation.11 Importantly, we demonstrate that cells transfection with siRNA against CREB significantly inhibited cells proliferation, confirming the essential role of CREB activation for VSMCs proliferation.
Moreover, in this report we confirm the implication of other signalling molecules in UII effects. We demonstrate that UII promotes EGFR transactivation, ERK activation, and CaMKII, similar to other GPCR agonists. We demonstrate that UII-evoked Ca2+ entry, CREB activation, and VSMCs proliferation are partially dependent on these signalling pathways in agreement with data shown in previous studies.6–8 Transactivation of EGFR seems a critical signalling step for some GPCR.33 Similar results have been determined in VSMCs stimulated with hydrogen peroxide (H2O2) which promotes EGFR transactivation and ERK1/2 stimulation followed by CREB phosphorylation.34 Furthermore, we determine significantly diminished UII-mediated phosphorylation of EGFR and ERK due to SOCE inhibition. The role of extracellular Ca2+ entry by SOCE has been linked to activation of the proliferative kinase ERK1/2 that is involved in cell proliferation and migration.35 In addition, recents studies have demonstrated that epithelium growth factor (EGF) activation of EGFR stimulates SOCE in human mesangial cells,36 or in cancer cell line,37 which confirms the relevance and the need of further investigation to highlight the role of EGFR transactivation in VSMCs proliferation.
As summarized in the schematic model in Supplementary material online, Figure SV, we have shown that UII promotes CREB activation and VSMCs proliferation through the multi-complex signalling pathway that involves SOCE, EGFR transactivation, ERK1/2, and CaMKII pathways. Our results shed more light on the functional role of UII in the arterial remodelling processes that occur during cardiovascular diseases,23 and confirm different molecular players of SOCE as possible targets for therapeutic strategies to improve vascular occlusive diseases.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This study was supported by Spanish Ministry of Science and Innovation (BFU-2010-21043-C02-01; BFU-2010-21043-C02-02); Instituto Carlos III and Cardiovascular Network (RD12/0042/0041, PI12/00941); and from The Andalusian Government (P10-CVI-6095; PI-0108-2012). M.T. group is supported by grant form NIH (HL097111). N.D. was supported by a fellowship from The Extremadura Government (PRE09020).
Supplementary Material
References
- 1.Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis. 2002;45:173–202. doi: 10.1053/pcad.2002.130041. [DOI] [PubMed] [Google Scholar]
- 2.Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 3.Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1156–R1172. doi: 10.1152/ajpregu.00706.2009. [DOI] [PubMed] [Google Scholar]
- 4.Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, et al. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song W, McDonald J, Camarda V, Calo G, Guerrini R, Marzola E, et al. Cell and tissue responses of a range of urotensin II analogs at cloned and native urotensin II receptors: evidence for coupling promiscuity. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:148–157. doi: 10.1007/s00210-006-0057-2. [DOI] [PubMed] [Google Scholar]
- 6.Sauzeau V, Le Mellionnec E, Bertoglio J, Scalbert E, Pacaud P, Loirand G. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ Res. 2001;88:1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- 7.Iglewski M, Grant SR. Urotensin II-induced signaling involved in proliferation of vascular smooth muscle cells. Vasc Health Risk Manag. 2010;6:723–734. doi: 10.2147/vhrm.s11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai CS, Loh SH, Liu JC, Lin JW, Chen YL, Chen CH, et al. Urotensin II-induced endothelin-1 expression and cell proliferation via epidermal growth factor receptor transactivation in rat aortic smooth muscle cells. Atherosclerosis. 2009;206:86–94. doi: 10.1016/j.atherosclerosis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez-Rodríguez A, Díaz I, Rodríguez-Moyano M, Calderón-Sánchez E, Rosado JA, Ordóñez A, et al. Urotensin-II signaling mechanism in rat coronary artery: role of STIM1 and Orai1-dependent store operated calcium influx in vasoconstriction. Arterioscler Thromb Vasc Biol. 2012;32:1325–1332. doi: 10.1161/ATVBAHA.111.243014. [DOI] [PubMed] [Google Scholar]
- 10.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo RW, Yang LX, Li MQ, Pan XH, Liu B, Deng YL. Stim1- and Orai1-mediated store-operated calcium entry is critical for angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc Res. 2012;93:360–370. doi: 10.1093/cvr/cvr307. [DOI] [PubMed] [Google Scholar]
- 12.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK. Orai1-mediated ICRAC is essential for neointima formation after vascular injury. Circ Res. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–C790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulver RA, Rose-Curtis P, Roe MW, Wellman GC, Lounsbury KM. Store-operated Ca2+ entry activates the CREB transcription factor in vascular smooth muscle. Circ Res. 2004;94:1351–1358. doi: 10.1161/01.RES.0000127618.34500.FD. [DOI] [PubMed] [Google Scholar]
- 16.Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I, et al. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol. 2011;50:621–633. doi: 10.1016/j.yjmcc.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi Y, Watanabe H, Murakami M, Ono K, Munehisa Y, Koyama T, et al. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun. 2007;361:934–940. doi: 10.1016/j.bbrc.2007.07.096. [DOI] [PubMed] [Google Scholar]
- 18.Ichiki T, Tokunou T, Fukuyama K, Iino N, Masuda S, Takeshita A. Cyclic AMP response element-binding protein mediates reactive oxygen species-induced c-fos expression. Hypertension. 2003;42:177–183. doi: 10.1161/01.HYP.0000079791.26014.04. [DOI] [PubMed] [Google Scholar]
- 19.Smani T, Patel T, Bolotina VM. Complex regulation of store-operated Ca2+ entry pathway by PKC-ɛ in vascular SMCs. Am J Physiol Cell Physiol. 2008;294:C1499–C1508. doi: 10.1152/ajpcell.00365.2007. [DOI] [PubMed] [Google Scholar]
- 20.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 21.Smani T, Domínguez-Rodríguez A, Hmadcha A, Calderón-Sánchez E, Horrillo-Ledesma A, Ordóñez A. Role of Ca2+-independent phospholipase A2 and store-operated pathway in urocortin-induced vasodilatation of rat coronary artery. Circ Res. 2007;101:1194–1203. doi: 10.1161/CIRCRESAHA.107.159053. [DOI] [PubMed] [Google Scholar]
- 22.Patacchini R, Santicioli P, Giuliani S, Grieco P, Novellino E, Rovero P, et al. Urantide: an ultrapotent urotensin II antagonist peptide in rat aorta. Br J Pharmacol. 2003;140:1155–1158. doi: 10.1038/sj.bjp.0705555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsoukas P, Kane E, Giaid A. Potential clinical implications of the urotensin ii receptor antagonists. Front Pharmacol. 2011;2:38. doi: 10.3389/fphar.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saetrum-Opgaard O, Nothacker H, Ehlert FJ, Krause DN. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur J Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz E, Valero RA, Quintana A, Hoth M, Núñez L, Villalobos C. Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/orai channels normally prevented by mitochondria. J Biol Chem. 2011;286:16186–16196. doi: 10.1074/jbc.M110.198952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, et al. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008;103:e97–e104. doi: 10.1161/CIRCRESAHA.108.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galán C, Dionisio N, Smani T, Salido GM, Rosado JA. The cytoskeleton plays a modulatory role in the association between STIM1 and the Ca2+ channel subunits Orai1 and TRPC1. Biochem Pharmacol. 2011;82:400–410. doi: 10.1016/j.bcp.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Vaca L. SOCIC: the store-operated calcium influx complex. Cell Calcium. 2010;47:199–209. doi: 10.1016/j.ceca.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Berna-Erro A, Redondo PC, Rosado JA. Store-operated Ca2+ entry. Adv Exp Med Biol. 2012;740:349–382. doi: 10.1007/978-94-007-2888-2_15. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, et al. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng LC, O'Neill KG, French D, Airey JA, Singer CA, Tian H, et al. TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca2+ entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2012;303:C1156–C1172. doi: 10.1152/ajpcell.00065.2012. [DOI] [PubMed] [Google Scholar]
- 33.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 34.Ichiki T, Tokunou T, Fukuyama K, Iino N, Masuda S, Takeshita A. Cyclic AMP response element-binding protein mediates reactive oxygen species-induced c-fos expression. Hypertension. 2003;42:177–183. doi: 10.1161/01.HYP.0000079791.26014.04. [DOI] [PubMed] [Google Scholar]
- 35.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, et al. Orai3 is an estrogen receptor α-regulated Ca2+ channel that promotes tumorigenesis. FASEB J. 2013;27:63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li WP, Tsiokas L, Sansom SC, Ma R. Epidermal growth factor activates store-operated Ca2+ channels through an inositol 1,4,5-trisphosphate-independent pathway in human glomerular mesangial cells. J Biol Chem. 2004;279:4570–4577. doi: 10.1074/jbc.M304334200. [DOI] [PubMed] [Google Scholar]
- 37.Wang JY, Chen BK, Wang YS, Tsai YT, Chen WC, Chang WC, et al. Involvement of store-operated calcium signaling in EGF-mediated COX-2 gene activation in cancer cells. Cell Signal. 2012;24:162–169. doi: 10.1016/j.cellsig.2011.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.