Abstract
Background
The effects of olmesartan (OLM) on blood pressure and kidney function in Japanese patients with chronic kidney disease (CKD) were compared between 20 mg twice daily (BID) and 40 mg once daily (QD) treatments.
Methods
The subjects were Japanese CKD patients with concurrent hypertension who had been treated with OLM 20 mg BID for at least 3 months on an outpatient basis (n=39). After a change in the treatment regimen to 40 mg OLM QD (after breakfast), blood pressure (BP) (n=39), morning home BP (n=13), estimated glomerular filtration rate (n=39), and urinary albumin-to-creatinine ratio (n=17) were monitored for 2 months.
Results
No significant change in office (mean ± standard deviation [SD] [mmHg], 143.9 ± 18.8/75.7 ± 12.0 to 141.6 ± 16.1/74.7 ± 11.7, not significant [ns]) or early morning home (mean ± SD [mmHg], 133.8 ± 15.9/71.2 ± 11.5 to 133.8 ± 13.9/74.5 ± 10.5, ns) BP was observed 2 months after the change in dose. The estimated glomerular filtration rate increased significantly (mean ± SD, 49.0 ± 28.0 to 51.8 ± 27.0, P<0.05), whereas urinary albumin-to-creatinine ratio did not change significantly (mean ± SD, 0.551 ± 0.445 to 0.364 ± 0.5194, ns).
Conclusion
High-dose OLM administered BID and QD had similar effects on outpatient and early morning home BP in CKD patients, suggesting that the BID regimen can be safely changed to a QD regimen. For CKD patients with hypertension requiring continuous long-term treatment, the possibility that the QD regimen might bring a greater therapeutic effect was suggested. However, recognizing the best blood pressure control level for a CKD patient is still a matter of debate, and should ideally be personalized.
Keywords: high-dose angiotensin receptor blocker, hypertension, chronic kidney disease, compliance, olmesartan
Introduction
The renin–angiotensin–aldosterone system is activated in patients with chronic kidney disease (CKD), increasing the risk of cardiovascular events1 and accelerating the transition to end-stage renal failure, in addition to being closely associated with hypertension.2,3 Therefore, strict blood pressure (BP) control and treatment of underlying diseases is important.
However, strict BP control for 24 hours is challenging, even with once daily (QD) administration of an antihypertensive drug, and two divided doses are favorable in some cases. Because CKD is associated with decreased sodium excretion during the day and increased sodium excretion during the night in correlation with reduced kidney function, many patients have nondipper type hypertension in which nocturnal control of BP is frequently difficult.4 Therefore, to achieve a more stable efficacy of angiotensin receptor blockers (ARBs), many patients with CKD receive drugs twice daily (BID), in the morning and in the evening.
On the other hand, in patients with hypertension who require long-term continuous treatment, a smaller number of tablets and lower daily dosing frequency lead to good compliance,2 which improves the therapeutic efficacy and continuity of treatment. Therefore, drugs certain to control hypertension with a simple prescription are ideal.
In Japan, few studies have compared the effects of QD versus BID drug regimens on compliance, and no studies have compared the effects of QD versus BID olmesartan (OLM) administration.
OLM administered QD provides high continuity of treatment using ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring as compared with other ARBs,5–8 making the QD regimen the most promising.
In the present study, we examined the effects of QD (morning) versus BID (morning and evening) OLM treatment on office BP, morning home BP, and renal function in patients with CKD complicated by hypertension.
Materials and methods
Patients and protocol
Thirty-nine consecutive Japanese outpatients (Tables 1 and 2) with CKD complicated by hypertension, treated with OLM 20 mg BID (after breakfast and supper) for more than 3 months, were switched to OLM 40 mg QD in the morning. Office BP and morning home BP were assessed, and renal function was examined by measuring serum creatinine levels to calculate estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (UACR) to estimate proteinuria. The follow-up period for assessment of the stable antihypertensive efficacy of OLM was set at 2 months.9 Antihypertensive drugs were not added during the follow-up period. This work was purely an observational study, and written informed consent was obtained from all patients.
Table 1.
Baseline characteristics of the study population (n=39)
| Parameter | Twice daily dosing n (%) or mean ± SD |
|---|---|
| Number of patients | 39 |
| Age (years) | 69.7 ± 11.7 |
| Women | 16 (42.1) |
| Medical history | |
| Chronic kidney disease | 39 (100) |
| Diabetic kidney | 18 (46.1) |
| Benign nephrosclerosis | 11 (28.2) |
| IgA kidney | 4 (10.2) |
| Gouty kidney | 4 (10.2) |
| Nephropathy of pregnancy | 1 (2.5) |
| Hepatorenal syndrome | 1 (2.5) |
| eGFR (ml/min/1.73 m2) | 49.0 ± 28.3 |
| G1 | |
| ≥90 | 1 (2.5) |
| G2 | |
| 60–89 | 12 (30.7) |
| G3a | |
| 45–59 | 10 (25.6) |
| G3b | |
| 30–44 | 5 (12.8) |
| G4 | |
| 15–29 | 6 (15.3) |
| G5 | |
| <15 | 5 (12.8) |
Abbreviations: eGFR, estimated glomerular filtration rate; G, stages of chronic kidney disease; IgA, immunoglobulin A; SD, standard deviation.
Table 2.
Antihypertensive treatment
| n (%) | |
|---|---|
| Olmesartan | 39 (100) |
| Number of antihypertensive drugs | |
| 1 | 9 (23.0) |
| 2 | 17 (43.5) |
| 3 | 8 (20.5) |
| ≥4 | 5 (12.8) |
| Drugs | |
| Calcium channel blocker | 27 (71) |
| ACE inhibitor | 8 (21) |
| Renin inhibitor | 3 (7.9) |
| Aldosterone antagonist | 2 (5.2) |
| Alpha blocker | 1 (2.6) |
| Beta blocker | 1 (2.6) |
| Diuretic | 1 (2.6) |
Abbreviation: ACE, angiotensin converting enzyme inhibitor.
Blood pressure measurement
Office systolic and diastolic BP was measured every 4 weeks with the patient in a sitting position after 5 minutes of rest. Each BP measurement was determined using a sphygmomanometer, and the average of two measurements was calculated. Home BP was also calculated as the average of two measurements. We had instructed patients to maintain a home BP diary. Home BP was measured before the oral administration of OLM and within 1 hour after waking in the morning. We followed the Japanese guidelines for the treatment of hypertension10 when assessing the patients’ BP using the auscultation method and a mercury or automatic sphygmomanometer.
Renal function
Renal function was evaluated on the basis of the eGFR, which was calculated using an equation developed for the Japanese population, as recommended by the Japanese Society of Nephrology11 as,
| (1) |
where sCrn is serum creatinine. The serum potassium concentration (K) was measured. Urinary samples and blood samples were collected at approximately the same time. UACR was expressed as g/gCr.
Statistical analysis
The changes in BP were expressed as mean ± standard deviation (SD) and analyzed using one-way analysis of variance. Serum creatinine and eGFR were expressed as mean ± SD. The changes in serum creatinine and eGFR were analyzed using the paired t-test. UACR (g/gCr) was expressed as mean ± standard error of the mean and analyzed using the paired t-test. All P-values <0.05 were considered statistically significant.
Results
Patient characteristics
Table 1 shows the background characteristics of the patients who received OLM 20 mg BID. Table 2 shows the antihypertensive drug treatment regimens in these patients. A total of 39 patients with CKD complicated by hypertension, ranging in age from 41–90 years, were included in the study. Thirty patients used other antihypertensive drugs in addition to OLM.
Blood pressure
After 2 months of QD therapy, there was a small reduction in office BP, but the difference did not reach statistical significance. We had instructed all 39 patients to maintain a home BP diary, but only 13 of them adhered to this instruction during the follow-up period. Morning home BP did not differ significantly between QD and BID treatments (Figures 1 and 2; Table 3). Assessment of changes in BP up to 2 months after administration of OLM 40 mg QD revealed no significant difference between the baseline value and that obtained at month 1 or 2. No significant change in BP from baseline to that at month 1 or 2 was detected in the 13 patients whose early morning home BP was measured.
Figure 1.
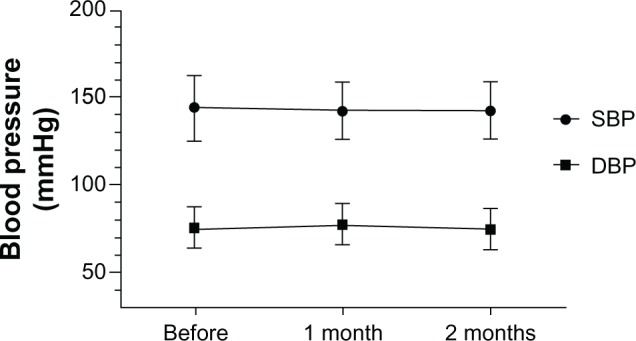
Changes in blood pressure during the course of treatment with high-dose olmesartan administered once daily.
Notes: One-way analysis of variance was used; results are expressed as mean ± standard deviation.
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Figure 2.
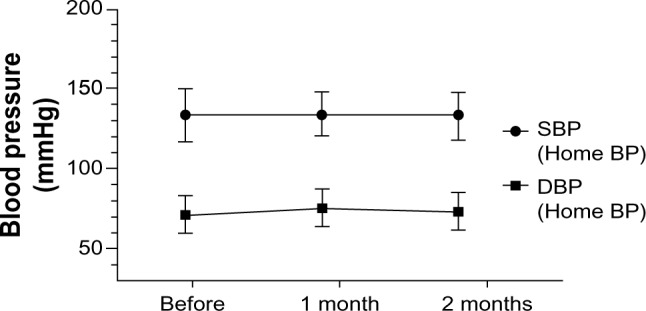
Changes of morning blood pressure during the course of treatment with high-dose olmesartan administered once daily.
Notes: One-way analysis of variance was used; results are expressed as mean ± standard deviation.
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 3.
Changes in office and home systolic/diastolic blood pressure (SBP/DBP) from baseline to after 1 and 2 months of once daily high-dose olmesartan treatment
| Parameter | Baseline | 1 month | 2 months | P-value |
|---|---|---|---|---|
| Office SBP (n=39) | 143.9 ± 18.8 | 142.0 ± 16.3 | 141.6 ± 16.1 | 0.826 |
| Office DBP (n=39) | 75.7 ± 12.0 | 77.3 ± 12.7 | 74.7 ± 11.7 | 0.650 |
| Home SBP (n=13) | 133.8 ± 15.9 | 134.0 ± 13.5 | 133.8 ± 13.9 | 0.999 |
| Home DBP (n=13) | 71.2 ± 11.5 | 75.6 ± 12.1 | 74.5 ± 10.5 | 0.621 |
Notes: One-way analysis of variance was used; results are expressed as mean ± standard deviation. Measurements mmHg.
Renal function
Table 4 shows the changes in eGFR, serum creatinine, K, and UACR after 2 months of QD treatment.
Table 4.
Renal function and potassium during once daily highdose olmesartan treatment for 2 months
| Parameter | Baseline Mean ± SD | 2 months Mean ± SD | p-value |
|---|---|---|---|
| eGFR mL/min/1.73 m2 (n=39) | 49.0 ± 28.0 | 51.8 ± 27.0* | 0.0424 |
| Creatinine mg/dL (n=39) | 1.53 ± 1.48 | 1.48 ± 1.38 | 0.279 |
| K mEq/L (n=39) | 4.48 ± 0.47 | 4.42 ± 0.46 | 0.686 |
| Parameter | Baseline Mean ± SD | 2 month Mean ± SD | p-value |
|
| |||
| UACR g/gCr (n=17) | 0.551 ± 0.445 | 0.364 ± 0.5194 | 0.119 |
Note:
P<0.05 paired t-test.
Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation; UACR, urinary albumin-to-creatinine ratio.
The eGFR significantly increased from the baseline value (Figure 3 and Table 4), whereas no significant change in creatinine or UACR was detected (Figure 4 and Table 4). These results indicated that the high-dose OLM BID regimen could be safely switched to the QD regimen in CKD patients with hypertension.
Figure 3.
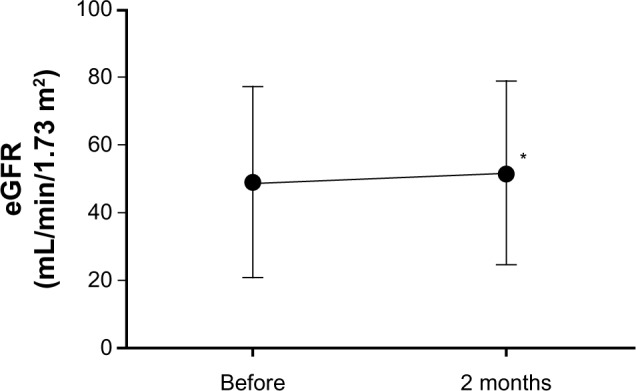
Changes of eGFR during administration of once daily high-dose of olmesartan.
Notes: Results are expressed as mean ± standard deviation; paired t-test.
Abbreviation: eGFR, estimated glomerular filtration rate. *P<0.05.
Figure 4.
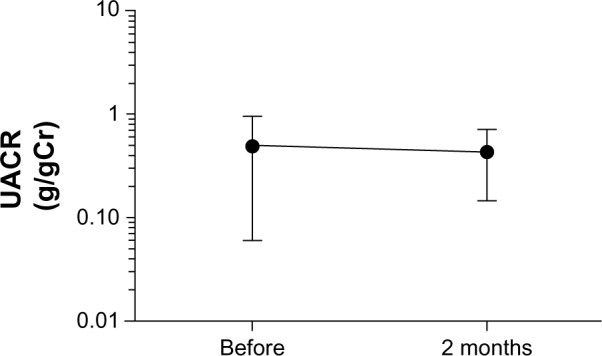
Changes of UACR during administration of once daily high-dose of olmesartan.
Notes: Results are expressed as mean ± standard deviation; paired t-test.
Abbreviation: UACR, urinary albumin-to-creatinine ratio.
Adverse reactions
There was no withdrawal associated with hypotension or anemia while patients were on QD therapy, and no changes in laboratory values were observed. No significant change in K was detected (Table 4).
Discussion
In the present study, we showed that the high-dose OLM BID regimen could be safely switched to the QD regimen in CKD patients with hypertension. A high-dose BID regimen of ARBs is an effective approach for early-morning and evening control of BP and treatment-resistant hypertension in CKD patients with increased renin–angiotensin–aldosterone system activity.
Nonetheless, a reduction in the number of daily tablets and the dosing frequency may improve treatment compliance12–14 in CKD patients with hypertension requiring long-term continuous therapy, potentially improving the therapeutic effect in these patients.
On the basis of the meta analysis15 that revealed a difference in treatment compliance between QD and BID regimens and reports that treatment compliance can be improved by the measurement of home BP,16 it is plausible that switching to the QD regimen resulted in improved treatment compliance during the follow-up period. Although a home BP diary was maintained by 33% of the patients in this study, all patients were told of the importance of home BP measurements and instructed on how to measure home BP. Although there was no significant difference between regimens, a slight decrease in office BP was detected after switching to the QD regimen, indicating the possibility that the QD regimen might have contributed to decreased intraglomerular pressure and UACR (without any significant difference), and that increased GFR cannot be ruled out.
It is generally accepted that the inhibitory effect on proteinuria can be explained by the effects of ARB on decreasing intraglomerular pressure and reducing glomerular afterload. Although the results of this study did not show any significant difference, a slight decrease in office BP was detected, as is the case in decreased UACR. Undeniably, this decrease in office BP induced the decrease in UACR.
The superior continuous blood pressure-lowering effect of OLM compared with other drugs has recently been reported,5–8 and the Japanese ABPM guidelines state that a sustained effect of OLM can be anticipated. In addition, a US study comparing the sustained effect of OLM administered BID using ABPM in outpatients with moderate to severe essential hypertension showed no significant difference in mean systolic or diastolic blood pressure during the final hour.14 Our study showed that the blood pressure-lowering and sustained effects of high-dose OLM administered QD and BID were similar. OLM administered in 40 mg tablets QD reduces the number of tablets prescribed to CKD patients and could therefore be expected to improve treatment compliance. Furthermore, we showed increased eGFR after 2 months of treatment. However, further examination is necessary to ascertain whether this improvement is attributable to mild decreases in blood pressure or whether it is associated with improved compliance.
Conclusion
OLM administered QD has a sustained effect and can control early morning blood pressure in CKD patients with hypertension. In addition, its effects are not likely to be influenced by clinical conditions or meals. Therefore, treatment with OLM can be adjusted to the lifestyles of patients to improve treatment compliance and enhance the antihypertensive effect in CKD patients with hypertension requiring long-term therapy. Recognizing the best blood pressure control level for a CKD patient is still a matter of debate and should ideally be personalized.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Sleight P, Redon J, Verdecchia P, et al. ONTARGET investigators Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27(7):1360–1369. doi: 10.1097/HJH.0b013e32832d7370. [DOI] [PubMed] [Google Scholar]
- 3.Ogihara T, Saruta T, Rakugi H, et al. CASE-J trial Group Relationship between the achieved blood pressure and the incidence of cardiovascular events in Japanese hypertensive patients with complications: a sub-analysis of the CASE-J trial. Hypertens Res. 2009;32(4):248–254. doi: 10.1038/hr.2008.34. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M, Munemura M, Usami T, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65(2):621–625. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama S, Watada H, Mita T, et al. Comparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertension. Hypertens Res. 2008;31(1):7–13. doi: 10.1291/hypres.31.7. [DOI] [PubMed] [Google Scholar]
- 6.Brunner HR, Arakawa K. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil in achieving 24-hour blood pressure reductions and ambulatory blood pressure goals. Clin Drug Investig. 2006;26(4):185–193. doi: 10.2165/00044011-200626040-00002. [DOI] [PubMed] [Google Scholar]
- 7.Smith DH, Dubiel R, Jones M. Use of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs. 2005;5(1):41–50. doi: 10.2165/00129784-200505010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Miyakawa M. Comparative efficacy of angiotensin II receptor blockers on early morning blood pressure in patients with essential hypertension: final report. Japanese. Therapeutic Research. 2009;30:1879–1882. [Google Scholar]
- 9.Mitsuyoshi N, Takanori T, Atsumi O, Shigeto K. Investigation of relationship between pharmacokinetics and antihypertensive effect of CS-866 (olmesartan medoxomil) in mild to moderate essential hypertensive patients. J Clin Ther Med. 2003;19(12):1397–1420. [Google Scholar]
- 10.Ogihara T, Kikuchi K, Matsuoka H, et al. Japanese Society of Hypertension Committee The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009) Hypertens Res. 2009;32(1):3–107. [PubMed] [Google Scholar]
- 11.Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164(7):722–732. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura K, Arima H, Tominaga M, et al. COMFORT Investigators Does a combination pill of antihypertensive drugs improve medication adherence in Japanese? A randomized controlled trial. Circ J. 2012;76(6):1415–1422. doi: 10.1253/circj.cj-11-1481. [DOI] [PubMed] [Google Scholar]
- 15.Iskedjian M, Einarson TR, MacKeigan LD, et al. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin Ther. 2002;24(2):302–316. doi: 10.1016/s0149-2918(02)85026-3. [DOI] [PubMed] [Google Scholar]
- 16.Ashida T, Sugiyama T, Okuno S, Ebihara A, Fujii J. Relationship between home blood pressure measurement and medication compliance and name recognition of antihypertensive drugs. Hypertens Res. 2000;23(1):21–24. doi: 10.1291/hypres.23.21. [DOI] [PubMed] [Google Scholar]


