Abstract
Objectives
There is no pharmacokinetic interaction between tenofovir and nevirapine, but a higher emergence rate of resistance mutations has been reported when these drugs are coadministered. We sought to examine if there is a potential intracellular interaction that may account for the emergence of resistant virus.
Methods
Primary CD4+ and CD14+ cells were isolated from healthy volunteer blood. Monocyte-derived macrophages were differentiated from CD14+ cells. Accumulation of radiolabelled tenofovir and nevirapine was then assessed in these cells.
Results
We show here that tenofovir and nevirapine immune cell intracellular concentrations are lower when coincubated in CD4+ cells and monocyte-derived macrophages, but not in CD14+ cells.
Conclusions
These data indicate a potential intracellular drug–drug interaction between these drugs that warrants further investigation.
Keywords: influx drug transporters, HIV, drug–drug interactions
Introduction
Most HIV drugs are substrates for drug transporters (both influx and efflux), but drug–drug interactions (DDIs) between antiretrovirals (ARVs) that impact on their intracellular accumulation are poorly studied, with the possible exception of ABCB1.1 The expression of efflux and influx drug transporters in peripheral immune cells has been described.2,3 However, uptake transporters have been less well studied and their substrate profiles are relatively unclear. Therefore, DDIs involving uptake transporters in the target cells are poorly understood.
Tenofovir and nevirapine are widely used ARVs and are likely to remain as WHO first-line regimens for the foreseeable future. The active component of tenofovir is a diphosphate metabolite produced via cytoplasmic kinases. Therefore, the penetration of the parent compound into the target cell is vital to its function. The clearance of tenofovir does not involve cytochrome P450 enzymes and therefore the potential for DDIs through this system is low.4 Tenofovir has been demonstrated to be a substrate of the uptake transporters OAT1 (SLC22A6) and OAT3 (SLC22A8), which are expressed in peripheral immune cells.5 Nevirapine has been demonstrated not to be transported by the uptake transporters SLCO1B1, SLCO1B3 and SLCO1A2, leaving the mechanisms underlying its intracellular accumulation unclear.6 Tenofovir and nevirapine have also been demonstrated to be substrates of ABCC10 in primary immune cells.7,8
DDIs at the level of efflux drug transporters have been described previously. A number of ARVs have been shown to significantly inhibit the efflux transporters ABCG2,9,10 ABCB111 and ABCC proteins,11 which may contribute to reported DDIs. The level of interaction between ARVs and influx transporters is less well understood. Recently, it was shown that coadministration of lopinavir/ritonavir significantly increased the intracellular AUC of tenofovir diphosphate and nevirapine decreased intracellular tenofovir diphosphate.12 There are conflicting data within the literature with respect to the presence of a DDI between tenofovir and nevirapine. The DAUFIN study showed early incidence of virological failure associated with the emergence of K65R and, to a lesser extent, M184V resistance mutations,13 but another study showed no evidence of a DDI.14 Given that there is no pharmacokinetic DDI between these drugs,15 we hypothesized that there may be an intracellular DDI that could compromise concentrations within target cells.
Materials and methods
Materials
Radiolabelled nevirapine and tenofovir were purchased from Moravek Biochemicals (CA, USA). Healthy volunteer blood, from six volunteers, was obtained from the National Blood Service (Liverpool, UK). Experiments were conducted in triplicate. CD4+ and CD14+ magnetic beads, macrophage colony-stimulating factor (M-CSF) and transforming growth factor (TGF)-β were purchased from Miltenyi Biotec (Germany). Centrifree ultrafiltration devices were purchased from Millipore (Watford, UK). All other reagents were from Sigma–Aldrich unless otherwise stated.
Isolation of peripheral blood mononuclear cell (PBMC) subsets
Blood samples, from the National Blood Service, were used to isolate PBMCs through density gradient centrifugation. Ten millilitres of a whole blood sample was layered upon 5 mL of Ficoll-Paque and centrifuged at 300 g for 30 min at 4°C. PBMCs were counted and resuspended to an appropriate cell density. Individual cell subsets were then separated from PBMCs using MACS beads. MACS buffer (PBS, 0.5% BSA and 2 mM EDTA) was added to lymphocyte or monocyte samples and centrifuged at 300 g for 10 min. The supernatant fraction was removed and the pellet resuspended in MACS buffer (80 μL) with MACS beads (specific for CD4, CD8, CD14 or CD56; 20 μL). Samples were vortexed and incubated for 15 min at 4–8°C. MACS buffer was then added, samples were centrifuged at 300 g for 10 min and the supernatant fraction was removed to wash the samples. The cells were then resuspended in 500 μL of MACS buffer. Once placed into a magnetic field, the MACS LS columns were primed with 3 mL of MACS buffer. The cells were then added and washed through the column three times with MACS buffer (3 mL). The columns were then removed from the magnetic field and MACS buffer was added to flush the columns. Positively selected cells were collected in a universal tube and the total cell count was obtained using a Countess automated cell counter. Monocytes were differentiated into monocyte-derived macrophages (MDMs) using Iscove's modified Dulbecco's medium containing 20% fetal calf serum (FCS), 10 ng/mL M-CSF and 10 ng/mL TGF-β over a period of 12 days. The medium was replaced with fresh medium every 3 days.
Cellular accumulation of radiolabelled drug in CD4 and MDM cells
CD4 and MDM cells were counted and resuspended in medium (2.5 × 106 cells/mL). Radiolabelled drug (tenofovir or nevirapine; 0.3 μCi/mL, 10 μM) was added to the cells, mixed and incubated for 30 min at 37°C. The volume and constitution of the coincubation was identical to that of the single incubations. Samples were centrifuged (7800 g, 1 min) and an extracellular aliquot sample (100 μL) taken and placed into a scintillation vial. Cell pellets were then washed three times (7800 g, 1 min, 4–8°C) in 1 mL of ice-cold Hanks balanced salt solution and the supernatant fraction was discarded. The pellet was resuspended in 100 μL of deionized water and transferred into another scintillation vial. Scintillation fluid (4 mL) was added to each vial and placed in the scintillation counter. Data were expressed as the ratio of intracellular drug to extracellular drug (cellular accumulation ratio).
Cellular accumulation of tenofovir and nevirapine in PBMCs over time
PBMCs (1 × 106) were incubated with either tenofovir (0.3 μCi, 10 μM) or nevirapine (0.3 μCi, 10 μM) for 0, 30, 60, 120 and 180 min (37°C, 5% CO2). The cellular accumulation ratio was then derived as described previously.
Assessment of the extent of protein binding of tenofovir and nevirapine
Tenofovir and nevirapine (0.3 μCi, 10 μM) were incubated separately in the presence of 20% FCS for 30 min. Following incubation, samples were centrifuged at 2000 g for 30 min. An aliquot of the resultant unbound drug was then collected and expressed as a percentage of the starting concentration.
Statistical analysis
The distribution of the data was determined using a Shapiro–Wilk test. An unpaired t-test was used to determine statistical significance between intracellular accumulations in primary immune cells. A P value <0.05 was considered statistically significant.
Results
Intracellular accumulation of tenofovir and nevirapine in CD4+, CD14+ and MDM cells
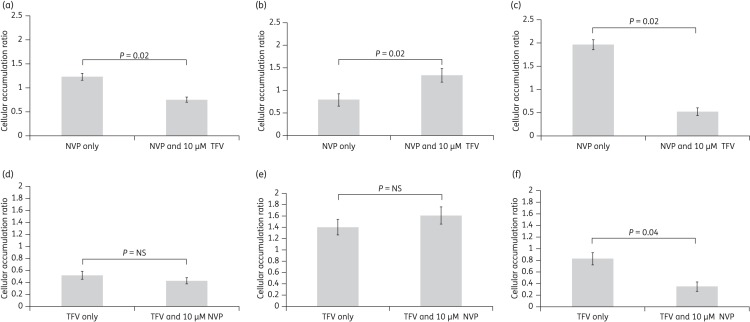
Both tenofovir and nevirapine accumulated in CD4+, CD14+ and MDM cells. When coincubated with tenofovir, there was a 39% reduction in the accumulation of nevirapine in CD4+ cells (Figure 1a, P = 0.02) and a 73% reduction in MDMs (Figure 1c, P = 0.02). However, in CD14+ cells, tenofovir caused a 68% increase in nevirapine accumulation (Figure 1b, P = 0.02). Nevirapine caused a 57% decrease in the accumulation of tenofovir in MDMs (Figure 1f, P = 0.04).
Figure 1.
Interaction between tenofovir (TFV) and nevirapine (NVP) in primary immune cells. Accumulation of NVP (a, b and c) and TFV (d, e and f) was assessed in primary CD4+, CD14+ and MDM cells, respectively. Interactions between TFV and NVP resulted in significantly lower intracellular accumulation in CD4+ and MDM cells. Data are expressed as mean ± SD (n = 6). NS, not significant.
Accumulation of tenofovir and nevirapine in PBMCs over time
Accumulation of tenofovir reached equilibrium at 60 min (Figure 2a), whereas nevirapine accumulation was similar over the 3 h period.
Figure 2.
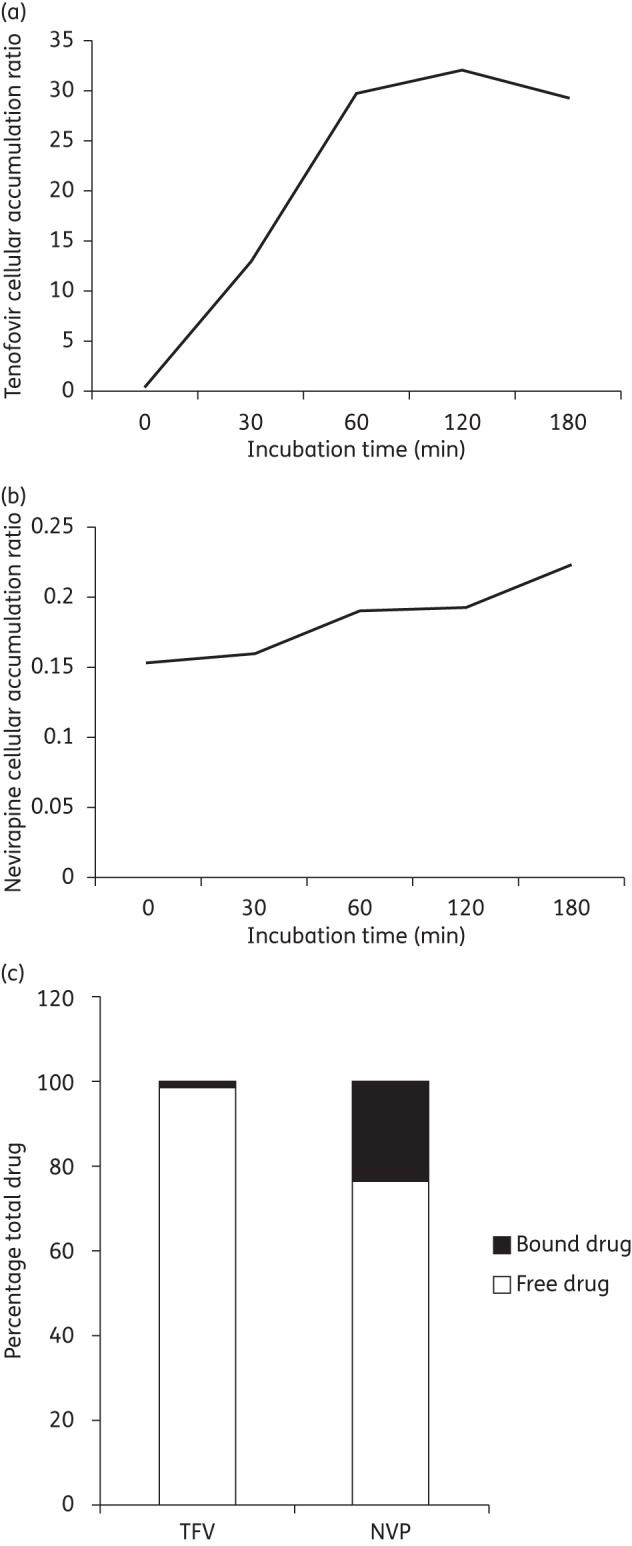
Accumulation of tenofovir (TFV) and nevirapine (NVP) in PBMCs over time. Accumulation of TFV (a) and NVP (b) in PBMCs over a period of 3 h. Accumulation of each drug was determined at five timepoints during the 3 h period. (c) Percentage bound and free tenofovir and nevirapine in the presence of 20% FCS.
Protein binding of tenofovir and nevirapine
Using centrifugation filters, the extent of protein binding of tenofovir and nevirapine was assessed. After 30 min, only 1% of the total amount of tenofovir was bound compared with 23% for nevirapine (Figure 2c).
Discussion
Many studies examining the pharmacology of ARVs investigate the concentrations of the drugs in plasma isolated from patients. However, in order to fully characterize the impact of drug exposure on pharmacodynamics, it may be necessary to examine the intracellular pharmacology of these drugs. The efficacy of most ARVs is dependent upon sufficient penetration into target cells in order that they may reach their targets and prevent HIV replication. DDIs involving efflux drug transporters may increase intracellular concentrations of their substrates, but DDIs involving influx transporters may serve to prevent sufficient accumulation within the target cells.
We examined the intracellular accumulation of tenofovir and nevirapine in immune cell subsets from healthy volunteers and assessed whether coincubation of these ARVs resulted in differences in cellular accumulation. There was significantly less tenofovir accumulation in MDM cells when coincubated with nevirapine, but no interaction was observed in CD4+ or CD14+ cells. However, when coincubated with tenofovir in CD4+ and MDM cells, there was a significant reduction in the accumulation of nevirapine. These data suggest that tenofovir and nevirapine may compete for transport by influx transporters and provides a putative mechanism for the emergence of viral resistance when regimens containing both these drugs are administered clinically.13 As the tenofovir utilized in the study was radiolabelled, we can assume that it would undergo the same steps of phosphorylation as non-radiolabelled tenofovir and that the values given represent the sum of the parent drug and its metabolites. Both nevirapine and tenofovir are substrates for ABCC10 and ABCC10 is expressed in immune cells.7,8 However, the difference in cellular accumulation is not consistent with the inhibition of an efflux pump. Additionally, the difference in interaction between the immune cell subsets may be due to differential expression of influx and efflux transporters between immune cell subsets.3,16 Therefore, the mechanism for this interaction requires further clarification, but is consistent with inhibition of an as yet unidentified influx transporter(s).
Despite the data presented, there are a number of limitations that must be considered when putting the data in a clinical context. Tenofovir accumulation was not at equilibrium at 30 min (Figure 2a) and therefore the true extent of the interaction may not have been realized. Additionally, nevirapine was found to be 23% protein bound in 20% FCS, conditions in which the coincubation data were generated, which again may potentially mask the true extent of the interaction. Additionally, even though the experiments were conducted within 1 h of isolation, the method of isolation of the immune cell subsets could potentially alter the expression of drug transporters and metabolic enzymes in its own right. However, this cannot be assessed without isolating the cells in this manner, making it difficult to reach a firm conclusion on this. Finally, whilst the concentrations of tenofovir and nevirapine used in this ex vivo study do not immediately match reported plasma concentrations of tenofovir and nevirapine, the interaction observed would not necessarily have been previously predicted.
In conclusion, we have demonstrated interactions between tenofovir and nevirapine that restrict their accumulation in CD4+ and MDM cells. This study describes a pharmacodynamic interaction between nevirapine and tenofovir that is not explained by differences in plasma pharmacokinetics and highlights the importance of examining the intracellular pharmacology of ARVs, particularly at their site of action. The mechanisms behind these interactions and whether they occur clinically now warrants further examination.
Funding
This work was funded by the National Institute of Health Research (NIHR—Department of Health) and the Northwest Development Agency (NWDA).
Transparency declarations
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.Zhang L, Strong JM, Qiu W, et al. Scientific perspectives on drug transporters and their role in drug interactions. Mol Pharm. 2006;3:62–9. doi: 10.1021/mp050095h. [DOI] [PubMed] [Google Scholar]
- 2.Liptrott NJ, Penny M, Bray PG, et al. The impact of cytokines on the expression of drug transporters, cytochrome P450 enzymes and chemokine receptors in human PBMC. Br J Pharmacol. 2009;156:497–508. doi: 10.1111/j.1476-5381.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleasby K, Castle JC, Roberts CJ, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36:963–88. doi: 10.1080/00498250600861751. [DOI] [PubMed] [Google Scholar]
- 4.Bazzoli C, Jullien V, Le Tiec C, et al. Intracellular pharmacokinetics of antiretroviral drugs in HIV-infected patients, and their correlation with drug action. Clin Pharmacokinet. 2010;49:17–45. doi: 10.2165/11318110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Uwai Y, Ida H, Tsuji Y, et al. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2) Pharm Res. 2007;24:811–5. doi: 10.1007/s11095-006-9196-x. [DOI] [PubMed] [Google Scholar]
- 6.Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20:112–20. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liptrott NJ, Pushpakom S, Wyen C, et al. Association of ABCC10 polymorphisms with nevirapine plasma concentrations in the German Competence Network for HIV/AIDS. Pharmacogenet Genomics. 2012;22:10–9. doi: 10.1097/FPC.0b013e32834dd82e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pushpakom SP, Liptrott NJ, Rodriguez-Novoa S, et al. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011;204:145–53. doi: 10.1093/infdis/jir215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss J, Rose J, Storch CH, et al. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J Antimicrob Chemother. 2007;59:238–45. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- 10.Storch CH, Ehehalt R, Haefeli WE, et al. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J Pharmacol Exp Ther. 2007;323:257–64. doi: 10.1124/jpet.107.122994. [DOI] [PubMed] [Google Scholar]
- 11.Weiss J, Theile D, Ketabi-Kiyanvash N, et al. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug Metab Dispos. 2007;35:340–4. doi: 10.1124/dmd.106.012765. [DOI] [PubMed] [Google Scholar]
- 12.Pruvost A, Negredo E, Theodoro F, et al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother. 2009;53:1937–43. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clotet B. Once-daily dosing of nevirapine in HAART. J Antimicrob Chemother. 2008;61:13–6. doi: 10.1093/jac/dkm432. [DOI] [PubMed] [Google Scholar]
- 14.Droste JA, Kearney BP, Hekster YA, et al. Assessment of drug–drug interactions between tenofovir disoproxil fumarate and the nonnucleoside reverse transcriptase inhibitors nevirapine and efavirenz in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:37–43. doi: 10.1097/01.qai.0000191997.70034.80. [DOI] [PubMed] [Google Scholar]
- 15.Redfield RR, Morrow JS. Combination antiretroviral therapy with tenofovir, emtricitabine or lamivudine, and nevirapine. Clin Infect Dis. 2008;47:984–5. doi: 10.1086/591802. [DOI] [PubMed] [Google Scholar]
- 16.Skazik C, Heise R, Bostanci O, et al. Differential expression of influx and efflux transport proteins in human antigen presenting cells. Exp Dermatol. 2008;17:739–47. doi: 10.1111/j.1600-0625.2008.00745.x. [DOI] [PubMed] [Google Scholar]