Abstract
Background
Trans-translation mediated by SsrA (tmRNA) and its associated protein SmpB plays an important role in rescuing stalled ribosomes and detoxifying toxic protein products under stress conditions. However, the role of SsrA and SmpB in bacterial persister survival has not been studied. The recent finding that pyrazinamide as a unique persister drug inhibits trans-translation in Mycobacterium tuberculosis prompted us to examine the role of trans-translation in persister survival.
Methods
Using Escherichia coli as a model, we constructed SsrA and SmpB mutants and assessed the susceptibility of the mutants to various antibiotics and stress conditions in MIC/MBC and persister assays.
Results
We found that mutations in SsrA and SmpB caused a defect in persister survival as shown by their increased susceptibility to a variety of antibiotics, including gentamicin, streptomycin, amikacin, norfloxacin, trimethoprim and tetracycline, and also stresses, such as acid, weak acid salicylate, heat and peroxide. Additionally, the SsrA and SmpB mutants were 2–8-fold more susceptible than the parent strain to various antibiotics in MIC and MBC tests. The SmpB mutant was more susceptible to antibiotics and stresses than the SsrA mutant. A particularly interesting finding is the hypersusceptibility of the SmpB mutant and the SsrA mutant to trimethoprim. The defect of various SsrA and SmpB mutant phenotypes could be complemented by functional ssrA and smpB, respectively.
Conclusions
We conclude that SsrA and SmpB are important for persister survival and may serve as a good target for developing new antibiotics that kill persister bacteria for improved treatment of persistent bacterial infections.
Keywords: persisters, persister mechanisms, persistence, drug tolerance, drug target
Introduction
Persisters are hypothesized to be non-replicating or slowly growing bacteria that are not killed by antibiotics and can revert to growing forms upon antibiotic removal and become susceptible to the same antibiotics.1 Persisters may be medically important and pose a significant challenge to the treatment of many bacterial infections, such as tuberculosis and Lyme disease, and may be a cause of latent and persistent infections, lengthy treatment and post-treatment relapse.2,3
The mechanisms of persister formation or survival are complex and not well understood. However, recent studies have identified several pathways that are involved in persister formation or survival.2,3 These include toxin–antitoxin modules (HipAB),4 energy production (SucB, UbiF),5 stringent response (RelA),6 SOS response/DNA repair (LexA),7 the phosphate and cellular metabolism PhoU-mediated pathway8 and efflux/transporters.2 Despite the above progress, our current understanding of persister mechanisms is still incomplete and requires further studies.
Bacteria have evolved various survival mechanisms under stress conditions. One such mechanism is trans-translation, which is a process that degrades potentially toxic protein products and mutated mRNA template and allows recycling of ribosomes trapped on damaged mRNA under stress conditions, such as heat and oxidative stress, acid pH and amino acid starvation.9 Trans-translation is mediated by a hybrid tmRNA encoded by ssrA, which is expressed as a short peptide tag added to incomplete and potentially toxic protein products so that the tagged toxic protein products produced under stress conditions are sent for degradation by proteases.9 tmRNA is bound by a protective protein called SmpB and works together with EF-Tu and RpsA (ribosomal S1 protein) as a complex during the process of trans-translation.10 Although the trans-translation pathway is known to be involved in stress survival and virulence in Helicobacter pylori,11 Yersinia pestis12 and Francisella tularensis,13 its role in the phenomenon of bacterial persisters is unknown.
Recently, we found that the unique front-line tuberculosis drug pyrazinamide, which specifically kills persisters and shortens tuberculosis therapy,14 inhibits the trans-translation process in Mycobacterium tuberculosis.15 This finding prompted us to explore the association of trans-translation and bacterial persisters. In this study, we used Escherichia coli as a model to address whether trans-translation is important for persister survival by constructing mutants defective in SsrA and SmpB and assessing their susceptibilities to various antibiotics and stresses. Our results demonstrate that trans-translation is indeed involved in persister survival and tolerance to a diverse range of antibiotics and stresses.
Methods
Construction of SsrA and SmpB knockout mutants and complementation of the mutants
The SsrA and SmpB knockout mutants were constructed as described previously.16 Linear DNA fragments of smpB and ssrA amplified by PCR using primers A, B, C and D (Table S1, available as Supplementary data at JAC Online) were electroporated into E. coli for mutant construction. Further details of the construction of SsrA and SmpB knockout mutants, complementation of the mutants and bacterial culture conditions are shown in the Supplementary data available at JAC Online.
MIC and MBC determination
The MIC (lowest concentration that prevents visible growth) and MBC (lowest concentration killing 99.9% of initial inoculum) of different antibiotics for the SsrA and SmpB mutants and wild-type E. coli W3110 were determined in LB broth as described previously17 (see Supplementary data, available at JAC Online, for detailed antibiotic concentrations used).
Susceptibility to antibiotics and various stresses in exposure assays
The susceptibilities of stationary phase SsrA and SmpB mutants, complemented strains and the parent strain W3110 to various antibiotics, including ampicillin (100 mg/L), norfloxacin (4 mg/L), gentamicin (20 mg/L), trimethoprim (64 mg/L), tetracycline (50 mg/L) and streptomycin (50 mg/L), were evaluated in drug exposure experiments in LB medium. The stationary phase cultures (diluted 1 : 100 with LB) were exposed to different antibiotics except for gentamicin and norfloxacin, where undiluted cultures were used for incubation without shaking at 37°C for various times, after which the cultures were plated for cfu determination on LB plates. The conditions for stress susceptibility of the SsrA mutant and the SmpB mutant are listed in the Supplementary data available at JAC Online.
Results and discussion
Susceptibility of the ssrA and smpB mutants and the complemented strains to various antibiotics in MIC and MBC tests
To assess the susceptibility of SsrA and SmpB mutants to various antibiotics, including ampicillin, norfloxacin, gentamicin, tetracycline, streptomycin, amikacin and trimethoprim, the MIC and MBC experiments were carried out with the wild-type strain W3110 as a control. The results showed that both ssrA and smpB mutants were more susceptible to most antibiotics than W3110 (Table 1). The MIC/MBC values for the ssrA mutant and the smpB mutant were about 2-fold and 4-8-fold lower than those for the wild-type strain W3110, respectively. However, the ssrA mutant or smpB mutant did not show significant changes in the MIC and MBC of ampicillin. Complementation of the SsrA and SmpB mutants with their respective functional genes overall restored the wild-type level of susceptibility to the antibiotics in the MIC and MBC tests (Table 1).
Table 1.
MIC and MBC determination for SsrA and SmpB mutants and their complemented strains and the parent strain E. coli W3110 for different antibiotics
| Antibiotic | MIC/MBC (mg/L) |
||||
|---|---|---|---|---|---|
| W3110 | W3110-SsrA−-pBAD202 | W3110-SsrA−-pBAD202-SsrA+ | W3110-SmpB−-pBAD202 | W3110-SmpB−-pBAD202-SmpB+ | |
| Ampicillin | 6.25/12.5 | 6.25/6.25 | 6.25/6.25 | 6.25/6.25 | 6.25/12.5 |
| Gentamicin | 2.5/2.5 | 1.25/2.5 | 2.5/5 | 0.125/0.625 | 2.5/5 |
| Norfloxacin | 0.125/0.125 | 0.063/0.125 | 0.125/1 | 0.063/0.125 | 0.125/0.125 |
| Trimethoprim | 0.5/32 | 0.25/1 | 0.25/>4 | 0.125/0.25 | 0.25/1 |
| Tetracycline | 2/>32 | 0.5/>32 | 1/>32 | 1/>32 | 1/>32 |
| Streptomycin | 5/10 | 5/5 | 5/10 | 1.25/2.5 | 5/10 |
| Amikacin | 6.25/12.5 | 1.56/12.5 | 3.125/12.5 | 0.78/1.56 | 6.25/12.5 |
Reduced persister levels of the SsrA and SmpB mutants in antibiotic exposure assays
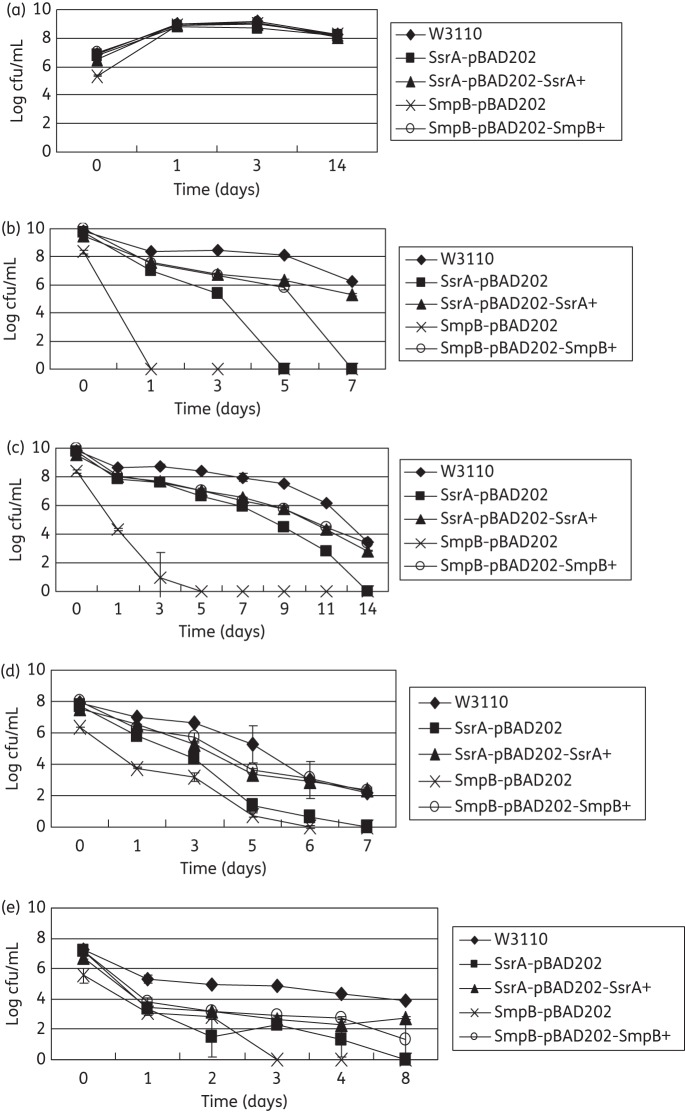
To determine the persister levels of the SsrA and SmpB mutants, the stationary phase cultures of the mutants and wild-type strain W3110 were exposed to no antibiotics as control (Figure 1a) and various antibiotics, including gentamicin (20 mg/L) (Figure 1b), norfloxacin (4 mg/L) (Figure 1c), ampicillin (100 mg/L), trimethoprim (64 mg/L) (Figure 1d) and tetracycline (50 mg/L) (Figure 1e), and the survival of the bacteria was monitored at different timepoints. Overall, the results showed that both the SsrA and SmpB mutants were more susceptible than the wild-type strain W3110 to different antibiotics and that complementation of the SmpB and SsrA mutants restored the level of persisters to close to wild-type levels in the antibiotic exposure assay (Figure 1). It is worth noting that, in general, the SmpB mutant was more susceptible to the antibiotics than the SsrA mutant and that the effect of complementation was more obvious for the SmpB mutant than for the SsrA mutant.
Figure 1.
SsrA and SmpB mutants have defective persister levels in antibiotic exposure assays. Stationary phase cultures of SsrA and SmpB mutants, their complemented strains and the parent strain W3110 were exposed to no antibiotic (a), gentamicin (20 mg/L) (b), norfloxacin (4 mg/L) (c), trimethoprim (64 mg/L) (d) and tetracycline (50 mg/L) (e) for various times. Aliquots of cultures were taken at different timepoints and the dilutions were plated for cfu determination on LB plates. The vertical axis represents cfu values on a log scale and the horizontal axis represents time of antibiotic exposure in days.
An interesting observation of this study is the hypersusceptibility of the SmpB mutant and the SsrA mutant to trimethoprim in both MIC/MBC tests (Table 1) and persister assays (Figure 1d). Trimethoprim is generally considered a bacteriostatic antibiotic and had a high MBC (32 mg/L) for wild-type E. coli W3110 (Table 1). However, it is quite surprising that trimethoprim had a very impressive bactericidal activity against the SsrA mutant and the SmpB mutant, with the MBC being 32- and 128-fold lower than that for the wild-type W3110, respectively (Table 1). Thus, mutations in the trans-translation pathway could potentiate or enhance the bactericidal activity of trimethoprim. Trimethoprim is known to inhibit the synthesis of folic acid, which is required for making the thymidine triphosphate (dTTP) needed for the synthesis of DNA, glycine, methionine and purines in bacteria. Inhibition or starvation of dTTP can cause thymine-less death in bacteria.18 The remarkable bactericidal activity of trimethoprim against the SmpB mutant and the SsrA mutant suggests that folic acid could be critical for bacteria lacking a functional trans-translation pathway, and inhibition of folic acid synthesis in SmpB or SsrA mutants might lead to thymine-less death more easily than in wild-type cells. However, the mechanism by which the defect in trans-translation potentiates the bactericidal activity of trimethoprim remains to be identified. Nevertheless, this observation may allow future chemotherapeutic approaches to be developed based on targeting both trans-translation and folic acid synthesis pathways for improved treatment of bacterial infections.
SsrA and SmpB mutants are more susceptible to a variety of stresses
To assess the effect of SsrA and SmpB mutations on the susceptibility of the mutants to various stress conditions, we subjected the SsrA and SmpB mutants and their complemented strains to acid pH (Figure S1, available as Supplementary data at JAC Online, a and b) and weak acid salicylate (5 mM, pH 5.0) (Figure S1, c), starvation (saline) (Figure S1, d), oxidative stress (peroxide, 25 mM) (Figure S1, e) and heat (57°C) (Figure S1, f). Overall, the SsrA and SmpB mutants, besides being more susceptible to various antibiotics as above, were also more susceptible to various stress conditions than the wild-type strain W3110; the SmpB mutant was more susceptible than the SsrA mutant with the exception of the starvation condition, in which the SsrA mutant seemed to be more susceptible than the SmpB mutant (Figure S1). In addition, complementation of the SsrA and SmpB mutants with their respective wild-type genes rendered the complemented strains close to wild-type persister levels. Nevertheless, the ssrA gene tended to complement the SsrA mutant strain less well (Figure S1). This is possibly due to the presence of alternative trans-translation genes that compensate for the SsrA defect. These findings are consistent with the previous observations of a role of trans-translation in amino acid starvation,19 oxidative stress (paraquat)11 and heat shock.9
Conclusions
The very diverse phenotypes of the SsrA and SmpB mutants suggest that various antibiotics and stresses may trigger a common mechanism of toxic protein product accumulation that relies on detoxification by the trans-translation pathway for survival under stress conditions. Further studies are needed to address this hypothesis. Since SsrA and SmpB are involved in persister and stress survival in E. coli, as shown in this study, and SsrA and SmpB are present in virtually all bacteria, SsrA and SmpB are likely to be involved in persistence in other bacteria. In fact, we found that mutations in SmpB and SsrA in M. tuberculosis have a similar persister defect (W. Shi and Y. Zhang, unpublished results). Our findings support the idea that trans-translation could serve as a novel target for the development of new antibiotics that target persister bacteria for improved treatment of persistent bacterial infections.
Funding
The work was partially supported by NIH grant AI099512. J. L. and L. J. were supported by the China Scholarship Council.
Transparency declarations
None to declare.
Supplementary data
References
- 1.Bigger JW. Treatment of staphylococcal infections with penicillin. Lancet. 1944;244:497–500. [Google Scholar]
- 2.Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother. 2012;56:2223–30. doi: 10.1128/AAC.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–72. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 4.Moyed HS, Bertrand KP. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol. 1983;155:768–75. doi: 10.1128/jb.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma C, Sim S, Shi W, et al. Energy production genes sucB and ubiF are involved in persister survival and tolerance to multiple antibiotics and stresses in Escherichia coli. FEMS Microbiol Lett. 2010;303:33–40. doi: 10.1111/j.1574-6968.2009.01857.x. [DOI] [PubMed] [Google Scholar]
- 6.Korch SB, Henderson TA, Hill TM. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol. 2003;50:1199–213. doi: 10.1046/j.1365-2958.2003.03779.x. [DOI] [PubMed] [Google Scholar]
- 7.Debbia EA, Roveta S, Schito AM, et al. Antibiotic persistence: the role of spontaneous DNA repair response. Microb Drug Resist. 2001;7:335–42. doi: 10.1089/10766290152773347. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Zhang Y. PhoU is a persistence switch involved in persister formation and tolerance to multiple antibiotics and stresses in Escherichia coli. Antimicrob Agents Chemother. 2007;51:2092–9. doi: 10.1128/AAC.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keiler KC. Biology of trans-translation. Annu Rev Microbiol. 2008;62:133–51. doi: 10.1146/annurev.micro.62.081307.162948. [DOI] [PubMed] [Google Scholar]
- 10.Barends S, Karzai AW, Sauer RT, et al. Simultaneous and functional binding of SmpB and EF-Tu-TP to the alanyl acceptor arm of tmRNA. J Mol Biol. 2001;314:9–21. doi: 10.1006/jmbi.2001.5114. [DOI] [PubMed] [Google Scholar]
- 11.Thibonnier M, Thiberge JM, De Reuse H. Trans-translation in Helicobacter pylori: essentiality of ribosome rescue and requirement of protein tagging for stress resistance and competence. PLoS One. 2008;3:e3810. doi: 10.1371/journal.pone.0003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okan NA, Mena P, Benach JL, et al. The smpB-ssrA mutant of Yersinia pestis functions as a live attenuated vaccine to protect mice against pulmonary plague infection. Infect Immun. 2010;78:1284–93. doi: 10.1128/IAI.00976-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svetlanov A, Puri N, Mena P, et al. Francisella tularensis tmRNA system mutants are vulnerable to stress, avirulent in mice, and provide effective immune protection. Mol Microbiol. 2012;85:122–41. doi: 10.1111/j.1365-2958.2012.08093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCune RM, Feldmann FM, McDermott W. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966;123:469–86. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi W, Zhang X, Jiang X, et al. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333:1630–2. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray PR. Manual of Clinical Microbiology. Washington, DC: ASM Press; 1999. [Google Scholar]
- 18.Kwon YK, Higgins MB, Rabinowitz JD. Antifolate-induced depletion of intracellular glycine and purines inhibits thymineless death in E. coli. ACS Chem Biol. 2010;5:787–95. doi: 10.1021/cb100096f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Yagi M, Morita T, et al. Cleavage of mRNAs and role of tmRNA system under amino acid starvation in Escherichia coli. Mol Microbiol. 2008;68:462–73. doi: 10.1111/j.1365-2958.2008.06167.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.